Back to Journals » Journal of Hepatocellular Carcinoma » Volume 10
Comparable Clinical Outcomes Between Transarterial Chemoembolization or Hepatic Arterial Infusion Chemotherapy Combined with Tyrosine Kinase Inhibitors and PD-1 Inhibitors in Unresectable Hepatocellular Carcinoma
Authors Long T , Yang Z, Zeng H, Wu W , Hu Z, Yang Z, Hu D, Zhou Z, Chen M, Zhang Y
Received 12 September 2023
Accepted for publication 12 October 2023
Published 20 October 2023 Volume 2023:10 Pages 1849—1859
DOI https://doi.org/10.2147/JHC.S436211
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Mohamed Shaker
Teng Long,1,2,* Zhoutian Yang,1,2,* Huilan Zeng,1,2,* Weijie Wu,1,2 Zhiwen Hu,1,2 Zhenyun Yang,1,2 Dandan Hu,1,2 Zhongguo Zhou,1,2 Minshan Chen,1,2 Yaojun Zhang1,2
1State Key Laboratory of Oncology in South China, Sun Yat-sen University Cancer Center, Guangzhou, 510060, People’s Republic of China; 2Department of Liver Surgery, Sun Yat-sen University Cancer Center, Guangzhou, 510060, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Minshan Chen; Yaojun Zhang, Department of Liver Surgery, Sun Yat-Sen University Cancer Center, Dongfeng East Road 651, Guangzhou, Guangdong, 510000, People’s Republic of China, Tel +86-20-87343828, Fax +86-20-87343585, Email [email protected]; [email protected]
Purpose: To compare the treatment efficacy and safety of transarterial chemoembolization (TACE) or hepatic arterial infusion chemotherapy (HAIC) combined with tyrosine kinase inhibitors (TKIs) and programmed cell death protein-1 (PD-1) inhibitors for patients with unresectable hepatocellular carcinoma (HCC).
Patients and Methods: 81 unresectable HCC patients were retrospectively analyzed, including 30 or 51 patients treated with either TKIs and PD-1 inhibitors combined with TACE (TTP) or HAIC (HTP), respectively. Tumor response and survival outcomes were compared.
Results: The median overall survival (mOS) was 21.0 months in the TTP group and 15.0 months in the HTP group (P = 0.525; HR = 1.23; 95% CI 0.66– 2.29). The median progression-free survival (mPFS) was 6.7 months in the TTP group and 9.9 months in the HTP group (P = 0.160; HR = 0.70; 95% CI 0.42– 1.16). After Propensity Score Matching (PSM), the mOS was 21.0 months in the TTP group and 18.0 months in the HTP group (P = 0.644; HR = 1.20; 95% CI 0.56– 2.58). The mPFS was 6.4 months in the TTP group and 15.0 months in the HTP group (P = 0.028; HR = 0.49; 95% CI 0.26– 0.93). The disease control rate in overall response (90.2% vs 76.7%, P = 0.116, before PSM; 91.7% vs 75.0%, P = 0.121, after PSM) and intrahepatic response (94.1% vs 80.0%, P = 0.070, before PSM; 91.7% vs 79.2%, P = 0.220, after PSM) were higher in the HTP group than in the TTP group.
Conclusion: Though including more advanced tumors, the clinical outcomes of HAIC combined with TKIs and PD-1 inhibitors are comparable to TACE-based combination therapy for unresectable HCC. Nevertheless, HTP significantly improved the PFS benefits in HCC patients with with large tumor burden or vascular invasion.
Keywords: transarterial chemoembolization, hepatic arterial infusion chemotherapy, tyrosine kinase inhibitors, programmed cell death protein − 1, hepatocellular carcinoma
Introduction
Primary liver cancer is the sixth most common cancer and the third leading cause of cancer-related death worldwide in 2020, and 75–85% of the reported cases were hepatocellular carcinoma (HCC).1 Due to the uneventful onset of HCC, patients were mostly diagnosed at intermediate or advanced stage when they were impossible to receive surgical resection for radical therapies.2 Guided by several recommendations, transarterial chemoembolization (TACE) is recommended as first choice for HCC patients with intermediate stage, and sorafenib or lenvatinib was systemic therapy recommended as first-line treatment for patients with advanced liver cancer.3–6 However, the prognosis of these patients is beyond satisfaction. The 1-, 3-, and 5-year survival rates of TACE for intermediate- to advanced-stage patients were 54%, 24% and 16%, respectively.7 Another study reported that the 1-, 5-, and 10-year overall survival (OS) and progression-free survival (PFS) rates of patients receiving TACE were 84.5%, 39.6% and 27.0%; 64.3%, 40.5% and 22.7%, respectively.8 Consistently, the median overall survival (mOS) of patients treated with sorafenib was 6.5–10.7 months compared with 4.2–7.9 months in those who received placebo.6,9 Moreover, for patients treated with lenvatinib, the mOS, median progression-free survival (mPFS), 1-year OS rate, 1-year PFS rate, objective response rate (ORR), and disease control rate (DCR) were 11.36 months, 6.68 months, 56.0%, 27.0%, 36.0% and 75.0%, respectively.10
Recently, Hepatic artery infusion chemotherapy (HAIC) is attracting increasing attention for its high response rates and favorable survival as an emerging therapy for advanced HCC patients.11,12 The efficacy and safety of HAIC have been proved to be better than TACE for unresectable HCC.13–15 HAIC has been verified to significantly improved the mOS and mPFS than TACE in terms of unresectable HCC with a maximum diameter of more than 7 cm (23.1 vs 16.1 months, P<0.001; 9.6 vs 5.4 months, P<0.001, respectively).16
Currently, programmed cell death protein-1 (PD-1) inhibitors were the major focus in terms of cancer research. The median OS of PD-1 inhibitors in patients with unresectable HCC was 13.9–16.4 months and the treatment-related adverse events are relatively low.17 Moreover, the combinations of interventional therapies and PD-1 inhibitors with tyrosine kinase inhibitors (TKIs) have become trends to be safer and more effective for incurable HCC patients.18–20
Recently, the triple combination therapies have been reported to demonstrate superior clinical outcomes than the double combination therapies or monotherapy. Han et al reported that TACE plus TKI and PD-1 inhibitors showed a mOS of 24.1 months, a mPFS of 10.6 months, ORRs of 42% and DCRs of 80%.21 In addition, Guo et al demonstrated that the sequential combination of TACE with PD-1 inhibitors and TKIs for patients with advanced HCC significantly improved markers of treatment efficacy (mOS, 19.8 months; mPFS, 11.7 months; ORRs, 48.4%).22 The combination therapies of HAIC, TKIs and PD-1 inhibitors have also been reported to have improved response rates and survival outcomes for unresectable HCC patients. He et al reported the mOS (not reached), the mPFS (11.1 months) and the ORR (59.2%) of HCC patients treated with lenvatinib, toripalimab plus HAIC.20 In the same center, Mei et al also revealed the clinical outcomes of HAIC combined with PD-1 inhibitors plus lenvatinib in patients diagnosed with advanced HCC (mOS, 15.9 months; mPFS, 8.8; ORR, 40%; DCR, 77.6%).23
However, the comparison of the response rates and survival outcomes of the triple combination therapies containing TACE, TKIs and PD-1 inhibitors (TTP), or HAIC, TKIs and PD-1 inhibitors (HTP) for advanced HCC patients has never been reported. Thus, we conducted the present retrospective study to compare the therapeutic efficacy and safety of advanced HCC patients treated with TTP or HTP.
Materials and Methods
Patients
The medical records of intermediate-to-advanced HCC patients at the Sun Yat-sen University Cancer Center who received interventional therapies, including TACE or HAIC combined with PD-1 inhibitors and TKIs between March 2019 and August 2021 were retrospectively retrieved in this study. The specific criteria for inclusion were as follows: (a) patients were pathologically diagnosed with HCC based on the American Association for the Study of Liver Diseases practice guidelines;24 (b) patients aged from 18 to 80; (c) patients with Eastern Cooperative Oncology Group performance status (ECOG PS) of 0–1; (d) patients with Barcelona Clinic Liver Cancer (BCLC) intermediate or advanced stage;4 (e) patients received at least one cycle of TACE or HAIC combined with PD-1 inhibitors and TKIs; (f) patients with complete medical and follow-up data; and (g) patients with at least one measurable lesion. Patients were excluded based on the following criteria: (a) the time interval of interventional therapies and systemic therapies was beyond 1 month; (b) patients received therapies other than described above; (c) patients diagnosed with second primary malignant tumors; (d) Child-Pugh grade B or C. The patient characterization process was displayed in Figure 1.
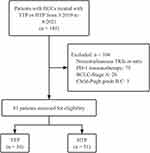 |
Figure 1 Flow diagram of the patient characterization process. |
Treatment Procedure
TACE and HAIC was performed according to our previously reported protocol.16,25 Patients received PD-1 inhibitors and TKIs within one week before or after the start of TACE or HAIC.
Diagnosis and Definitions
According to the modified Response Evaluation Criteria in Solid Tumors (mRECIST), tumor response included complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD).26 ORR and DCR were defined as the sum of CR and PR and the sum of CR, PR, and SD, respectively. OS was defined as the time interval from initial treatment to cancer-related death. PFS was defined as the time interval from initial treatment to disease progression or any cause of death. Treatment-related adverse events (AEs) were evaluated by National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0.
Statistical Analysis
In order to minimize the bias related to the baseline differences of tumor burdens and liver function between interested groups, patients were matched on their propensity score.27 Normally distributed continuous variables were expressed in terms of mean and standard deviation, and analyzed with Student’s t-test. Nonnormally distributed continuous variables were indicated as median and interquartile range, and analyzed with non-parameter test. Categorical variables were described using frequency and analyzed with χ2 test. Survival analysis was performed using the Kaplan-Meier method, and differences among the survival curves were analyzed with the Log rank test. Univariate and multivariate regression analyses were used to analyze the prognostic significance of the variables in predicting survival. All analyses were performed using SPSS 25.0 software (SPSS Inc., Chicago, IL, USA). P < 0.05 was considered statistically significant.
Results
Patient Characteristics
185 HCC patients who received TTP or HTP from March 2019 to August 2021 were analyzed. The following were excluded: 75 patients did not receive concurrent treatment of the combination of TACE or HAIC with TKIs and anti-PD-1 immunotherapy; 26 patients’ tumor grade were classified as BCLC/A; 3 patients’ Child-Pugh classification were B or C. Finally, a total of 81 patients were included and then divided into TTP group (n=30) or HTP group (n=51).
The clinicopathological characteristics and treatment are shown in Table 1. A total of 81 Asian patients were recruited, 71 (87.6%) of whom were male. A large proportion of the patients were with Child-Pugh score of 5 (75.3% in the two groups). There were higher proportions of patients with BCLC/C (80.4% vs 46.7%, P = 0.004), large tumor size (58.8% vs 30%, P = 0.023), vascular invasion (68.6% vs 33.3%, P = 0.004), high AST (73.5 vs 48.7, P = 0.049), and more cycles of interventional treatment and anti-PD-1 therapy (4.0 vs 2.0, P = 0.049; 6.0 vs 2.0, P = 0.034) in the HTP group than in the TTP group. Thus, the propensity score matching (PSM) was performed to avoid baseline differences. After PSM, no significant differences were among the two groups in regard to the variables described above as shown in Table S1 (all P >0.05).
 |
Table 1 Baseline Characteristics |
The categories of PD-1 inhibitors are summarized in Table S2. The median cycles of TACE in the TTP group were 2 [interquartile range (IQR), 2–4 cycles] and the median cycles of HAIC in the HTP group were 4 (IQR, 2–5 cycles). The median duration of TKI treatment was 4 months (IQR, 2–13 months) in the TTP group and was 6 months (IQR, 2–13 months) in the HTP group. The median cycles of PD-1 inhibitors treatment were 4 cycles (IQR, 2–10 months) in the TTP group and were 6 cycles (IQR, 4–10 cycles) in the HTP group. More patients in the TTP group received subsequent ablation than the HTP group (26.7% vs 0%, P<0.001), and fewer patients in the TTP group received subsequent TKIs therapy (20.0% vs 45.1%, P = 0.023). The follow-up treatment are shown in Table S3.
Survival
The median follow-up time was 15.9 months. There were no significant differences between the survival outcomes of patients in the TTP group and the HTP group. The 6-, 12- and 18-month OS rates were 77%, 67% and 55%, respectively, in the TTP group, and 90%, 63% and 49%, respectively, in the HTP group. The mOS was 21.0 months in the TTP group and 15.0 months in the HTP group (P = 0.525; HR = 1.23; 95% CI 0.66–2.29). The 6- and 12-, 18- PFS rates were 57%, 30% and 20% respectively, in the TTP group, and 69%, 43% and 27% respectively, in the HTP group. The mPFS was 6.7 months in the TTP group and 9.9 months in the HTP group (P = 0.160; HR = 0.70; 95% CI 0.42–1.16). However, the PFS of patients in the HTP group was significantly better than those in the TTP group after PSM. The OS rates and PFS rates of 6, 12 and 18 months for TTP group were 71%, 67% and 55% vs 92%, 71% and 50%, 54%, 25% and 17% vs 75%, 54% and 38% compared to HTP group, respectively. The mOS was 21.0 months in the TTP group and 18.0 months in the HTP group (P = 0.644; HR = 1.20; 95% CI 0.56–2.58). The mPFS was 6.4 months in the TTP group and 15.0 months in the HTP group (P = 0.028; HR = 0.49; 95% CI 0.26–0.93). The survival curves are displayed in Figure 2.
The subgroup analyses of OS and PFS are shown in the forest plot (Figure 3). The results of subgroup analyses were consistent with those in the whole patients. Generally, compared to TTP, HTP provided a clinical benefit for PFS in subgroups with age ≦ 50 years, ECOG score of 1, liver cirrhosis, AFP ≦ 400ng/mL, Child-Pugh score of 6, tumor size>10 cm, vascular invasion and no extrahepatic metastasis. Notably, HTP appeared to particularly benefit patients with liver cirrhosis (interaction P = 0.007), Child-Pugh score of 6 (interaction P = 0.001) and no extrahepatic metastasis (interaction P = 0.006) in terms of PFS.
Tumor Response
The treatment response is shown in Table 2. According to mRESIST, the ORR was 46.7% in the TTP group and 43.1% in the HTP group (P = 0.758), and the DCR was 76.7% in the TTP group and 90.2% in the HTP group (P = 0.116) for overall response; whereas the ORR was 46.7% in the TTP group and 47.1% in the HTP group (P = 0.973), and the DCR was 80.0% in the TTP group and 94.1% in the HTP group (P = 0.070) for intrahepatic response. Consistently, after PSM, the difference was not significant between the ORR and DCR of the TTP group and the HTP group in terms of overall response (50.0% vs 45.8%, P = 0.773; 75.0% vs 91.7%, P = 0.121, respectively) and intrahepatic response (50.0% vs 59.1%, P = 0.536; 79.2% vs 91.7%, P = 0.220, respectively).
 |
Table 2 Summary of Best Response |
Safety
All AEs were mild and treatable and no toxicity-related deaths occurred. The most common treatment-related AEs was pain and the most common laboratory-related change was decreased albumin in both groups. It was noteworthy that the frequencies of hypoalbuminemia (16 [53.3%] vs 45 [88.2%]; P<0.001) were significantly higher in the HTP group than in the TTP group. There was no significant difference in the others AEs rates. The AEs were shown in Table S4.
Prognostic Factor Analysis
The prognostic factors for survival according to univariate and multivariate analyses are shown in Table 3. The comparison of TTP to HTP could not be identified as an independent risk factor for either OS (HR = 0.91; 95% CI 0.47–1.75; P = 0.774) or PFS (HR = 0.68; 95% CI 0.38–1.20; P = 0.184) in multivariate analysis, which contained all the significant variables from the univariate analysis.
 |
Table 3 Univariate and Multivariate Analysis of Risk Factors for Overall Survival and Progression-Free Survival |
Discussion
Despite the emergence of interventional chemotherapy, targeted therapy and immunotherapy, patients with intermediate-advanced HCC still have a poor prognosis. Increasing research explored the combination of interventional treatment and systemic therapies, and proved the efficacy of triple-combination therapy in intermediate-to-advanced HCC patients. However, to date, there are no comparisons between the combination of TACE with TKIs and PD-1 inhibitors and the combination of HAIC with TKIs and PD-1 inhibitors. Here we demonstrated comparable survival benefits and tumor response rates achieved by the combination therapies based on TTP or HTP for unresectable HCC patients.
It has frequently been reported that TACE- and HAIC-based triple combination therapies are adopted as a treatment for advanced HCC patients. The reported mOS was 19.8 to 24.1 months for TTP and was 15.9 to not reached for HTP.20–23 Consistent with our study, TACE- and HAIC-based combination therapies brought favorable and satisfying survival benefits in the treatment of advanced HCC, though significant differences in tumor response and survival outcomes between the two groups were not reached. However, the differences should not be ignored for they might be caused by the limited number of cases.
There are rationales for the triple-combination therapy. First, interventional therapies result in hypoxia microenvironment and elevated expression of VEGF.28 TKIs can target VEGF and eliminate tumor angiogenesis after interventional therapies.29 In addition, the combination of TKIs and PD-1 inhibitors can reduce tumor volume by promoting normalization of blood vessels and breaking the hypoxic microenvironment.30 Second, due to the intrinsic immune tolerance of liver, the immune response to tumor is decreased.31 TACE and HAIC induce the release of tumor antigens through immunogenic cell death of tumor cells, and switch the immunosuppressive “cold” tumor to an immunogenic “hot” tumor by reshaping the immune microenvironment, thus enhance the efficacy of systemic therapies.29,30,32–36
Intriguingly, HTP significantly improved mPFS (10.0 months vs 6.8 months, P = 0.160 before PSM; 15.0 months vs 6.4 months, P = 0.028 after PSM) compared with TTP (Figure 2). In the subgroups analysis (Figure 3), patients accepted HTP treatment with age ≦ 50 years, ECOG score of 1, liver cirrhosis, AFP ≦ 400ng/mL, Child-Pugh score of 6, tumor size>10 cm, vascular invasion and no extrahepatic metastasis had better PFS than those who accepted TTP treatment (P < 0.05). Furthermore, a trend of superior DCR seemed to be demonstrated in the HTP group compared to the TTP group (90.2% vs 76.7%, P = 0.116 for overall response before PSM; 94.1% vs 80.0%, P = 0.070 for intrahepatic response before PSM; 91.7% vs 75.0%, P = 0.121 for overall response after PSM; 91.7% vs 79.2%, P = 0.220 for intrahepatic response after PSM) (Table 2). The main reason might be the continuous infusion of chemotherapeutic drugs, rather than embolization, can ensure the adequate local drug concentration and eliminate the micrometastasis in liver parenchyma, thus controlling intrahepatic lesions. However, compared with TTP, HTP did not prolonged OS in both the whole participants analysis and the subgroup analysis. This finding might be attributed to the unbalance of the baseline characteristics: higher proportions of patients with BCLC/C (80.4% vs 46.7%, P = 0.004), large tumor size (58.8% vs 30%, P = 0.023), vascular invasion (68.6% vs 33.3%, P = 0.004) erased the contribution of HTP treatment (Table 1). Even after PSM, there seemly were still higher proportions of patients with BCLC/C (66.7% vs 50.0%, P = 0.380), large tumor size (54.2% vs 37.5%, P = 0.385), vascular invasion (62.5% vs 37.5%, P = 0.149) (Table S1). Although TKIs and PD-1 inhibitors was combined, HAIC has limited ability to control the progression of extrahepatic lesions as a locoregional approach, and therefore might produce comparable benefit for survival in patients with very late-stage HCC.
As for safety, the AEs in the two groups were consistent with others studies. Li et al reported that the TACE group had significantly higher frequencies fever, elevated ALT, elevated AST, and hyperbilirubinemia, while more patients treated with HAIC had diarrhea, sensory neuropathy, and hypoalbuminemia.16 Guo et al reported that the most common AEs in the patients received TACE followed by systemic treatment was hepatic dysfunction.22 He et al reported that the following AEs were more frequent in the patients treated with HAIC, lenvatinib plus toripalimab than in the lenvatinib group: neutropenia, thrombocytopenia, nausea, elevated alanine aminotransferase, elevated aspartate aminotransferase, hyperbilirubinemia and hypoalbuminemia.20 In our study, patients treated with TTP had significantly higher frequencies of grade 3/4 elevated ALT related to embolization. Conversely, significantly higher frequencies of any grade hypoalbuminemia were observed in patients treated with HTP, which might be owing to the cytotoxic chemotherapeutic agents or the worse liver function before treatment. Of note, the above AEs were manageable and could be reduce by dose modification or treatment interruption. In general, the impaired liver function due to the embolization of hepatic arteries or continuous infusion of chemotherapeutics was similar.
Nonetheless, the present study had some limitations. First, this retrospective study was single-center and had limited cases. Second, there are many factors unbalance between TTP group and HTP group. In retrospective studies, baseline imbalances are often unavoidable and may be due to insufficient patient numbers. Moreover, patients enrolled in our study received different categories of TKIs and anti-PD-1 agents, which might lead to biases of the treatment procedure. In addition, the subsequent therapies after the combination therapies varied in both groups, which may also influence the survival outcomes. A multicenter and randomized controlled trials should be implemented to further investigate the comparison of these triple-combination therapies for higher level medical evidence in the future.
Conclusion
In conclusion, the clinical outcomes of HAIC combined with TKIs and PD-1 inhibitors are comparable to TACE-based combination therapy for intermediate-to-advanced HCC. Nevertheless, HTP significantly improved the PFS benefits with acceptable toxicities in HCC patients with large tumor burden or vascular invasion.
Data Sharing Statement
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
Ethics Approval and Consent to Participate
This study was conducted according to the ethical guidelines of the 1975 Declaration of Helsinki. This research was approved by the institutional review board of Sun Yat-sen University Cancer Center (Approval Number: B2020-236-01). The study used retrospective anonymous clinical data that were obtained after each patient agreed to treatment.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work is funded by the National Natural Science Foundation of China (No. 82103566).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Roayaie S, Jibara G, Tabrizian P, et al. The role of hepatic resection in the treatment of hepatocellular cancer. Hepatology. 2015;62(2):440–451. doi:10.1002/hep.27745
3. Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study Of Liver Diseases. Hepatology. 2018;68(2):723–750. doi:10.1002/hep.29913
4. Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76(3):681–693. doi:10.1016/j.jhep.2021.11.018
5. Rizzo A, Ricci AD, Gadaleta-Caldarola G, Brandi G. First-line immune checkpoint inhibitor-based combinations in unresectable hepatocellular carcinoma: current management and future challenges. Expert Rev Gastroenterol Hepatol. 2021;15(11):1245–1251. doi:10.1080/17474124.2021.1973431
6. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. doi:10.1056/NEJMoa0708857
7. Hyun MH, Lee YS, Kim JH, et al. Hepatic resection compared to chemoembolization in intermediate- to advanced-stage hepatocellular carcinoma: a meta-analysis of high-quality studies. Hepatology. 2018;68(3):977–993. doi:10.1002/hep.29883
8. Hsieh PM, Hsiao P, Chen YS, et al. Clinical prognosis of surgical resection versus transarterial chemoembolization for single large hepatocellular carcinoma (>/=5 cm): a propensity score matching analysis. Kaohsiung J Med Sci. 2023;39(3):302–310. doi:10.1002/kjm2.12640
9. Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a Phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25–34. doi:10.1016/S1470-2045(08)70285-7
10. Wang S, Wang Y, Yu J, Wu H, Zhou Y. Lenvatinib as first-line treatment for unresectable hepatocellular carcinoma: a systematic review and meta-analysis. Cancers. 2022;14(22):5525. doi:10.3390/cancers14225525
11. Lyu N, Lin Y, Kong Y, et al. FOXAI: a Phase II trial evaluating the efficacy and safety of hepatic arterial infusion of oxaliplatin plus fluorouracil/leucovorin for advanced hepatocellular carcinoma. Gut. 2018;67(2):395–396. doi:10.1136/gutjnl-2017-314138
12. Obi S, Sato S, Kawai T. Current Status of Hepatic Arterial Infusion Chemotherapy. Liver Cancer. 2015;4(3):188–199. doi:10.1159/000367746
13. Tsai WL, Lai KH, Liang HL, et al. Hepatic arterial infusion chemotherapy for patients with huge unresectable hepatocellular carcinoma. PLoS One. 2014;9(5):e92784. doi:10.1371/journal.pone.0092784
14. Tsai WL, Sun WC, Chen WC, et al. Hepatic arterial infusion chemotherapy vs transcatheter arterial embolization for patients with huge unresectable hepatocellular carcinoma. Medicine. 2020;99(32):e21489. doi:10.1097/MD.0000000000021489
15. He MK, Le Y, Li QJ, et al. Hepatic artery infusion chemotherapy using mFOLFOX versus transarterial chemoembolization for massive unresectable hepatocellular carcinoma: a prospective non-randomized study. Chin J Cancer. 2017;36(1):83. doi:10.1186/s40880-017-0251-2
16. Li QJ, He MK, Chen HW, et al. Hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin versus transarterial chemoembolization for large hepatocellular carcinoma: a randomized Phase III trial. J Clin Oncol. 2022;40(2):150–160. doi:10.1200/JCO.21.00608
17. Yau T, Hsu C, Kim TY, et al. Nivolumab in advanced hepatocellular carcinoma: sorafenib-experienced Asian cohort analysis. J Hepatol. 2019;71(3):543–552. doi:10.1016/j.jhep.2019.05.014
18. Xiang YJ, Wang K, Yu HM, et al. Transarterial chemoembolization plus a PD-1 inhibitor with or without lenvatinib for intermediate-stage hepatocellular carcinoma. Hepatol Res. 2022;52(8):721–729. doi:10.1111/hepr.13773
19. Qu S, Zhang X, Wu Y, et al. Efficacy and safety of TACE combined with lenvatinib plus PD-1 inhibitors compared with TACE alone for unresectable hepatocellular carcinoma patients: a prospective cohort study. Front Oncol. 2022;12:874473. doi:10.3389/fonc.2022.874473
20. He MK, Liang RB, Zhao Y, et al. Lenvatinib, toripalimab, plus hepatic arterial infusion chemotherapy versus lenvatinib alone for advanced hepatocellular carcinoma. Ther Adv Med Oncol. 2021;13:17588359211002720. doi:10.1177/17588359211002720
21. Han Z, Yang F, Zhang Y, et al. Prognostic efficacy and prognostic factors of TACE plus TKI with ICIs for the treatment of unresectable hepatocellular carcinoma: a retrospective study. Front Oncol. 2022;12:1029951. doi:10.3389/fonc.2022.1029951
22. Guo Z, Zhu H, Zhang X, et al. The efficacy and safety of conventional transcatheter arterial chemoembolization combined with PD-1 inhibitor and anti-angiogenesis tyrosine kinase inhibitor treatment for patients with unresectable hepatocellular carcinoma: a real-world comparative study. Front Oncol. 2022;12:941068. doi:10.3389/fonc.2022.941068
23. Mei J, Tang YH, Wei W, et al. Hepatic arterial infusion chemotherapy combined with PD-1 inhibitors plus lenvatinib versus PD-1 inhibitors plus lenvatinib for advanced hepatocellular carcinoma. Front Oncol. 2021;11:618206. doi:10.3389/fonc.2021.618206
24. Bruix J, Reig M, Sherman M. Evidence-based diagnosis, staging, and treatment of patients with hepatocellular carcinoma. Gastroenterology. 2016;150(4):835–853. doi:10.1053/j.gastro.2015.12.041
25. Shi M, Lu LG, Fang WQ, et al. Roles played by chemolipiodolization and embolization in chemoembolization for hepatocellular carcinoma: single-blind, randomized trial. J Natl Cancer Inst. 2013;105(1):59–68. doi:10.1093/jnci/djs464
26. Llovet JM, Lencioni R. mRECIST for HCC: performance and novel refinements. J Hepatol. 2020;72(2):288–306. doi:10.1016/j.jhep.2019.09.026
27. Rubin DB, Thomas N. Matching using estimated propensity scores: relating theory to practice. Biometrics. 1996;52(1):249–264. doi:10.2307/2533160
28. Abou-Alfa GK. TACE and sorafenib: a good marriage? J Clin Oncol. 2011;29(30):3949–3952. doi:10.1200/JCO.2011.37.9651
29. Llovet JM, De Baere T, Kulik L, et al. Locoregional therapies in the era of molecular and immune treatments for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2021;18(5):293–313. doi:10.1038/s41575-020-00395-0
30. Chung AS, Lee J, Ferrara N. Targeting the tumour vasculature: insights from physiological angiogenesis. Nat Rev Cancer. 2010;10(7):505–514. doi:10.1038/nrc2868
31. Ringelhan M, Pfister D, O’Connor T, Pikarsky E, Heikenwalder M. The immunology of hepatocellular carcinoma. Nat Immunol. 2018;19(3):222–232. doi:10.1038/s41590-018-0044-z
32. Chang X, Lu X, Guo J, Teng GJ. Interventional therapy combined with immune checkpoint inhibitors: emerging opportunities for cancer treatment in the era of immunotherapy. Cancer Treat Rev. 2019;74:49–60. doi:10.1016/j.ctrv.2018.08.006
33. Pinato DJ, Murray SM, Forner A, et al. Trans-arterial chemoembolization as a loco-regional inducer of immunogenic cell death in hepatocellular carcinoma: implications for immunotherapy. J Immunother Cancer. 2021;9(9):e003311. doi:10.1136/jitc-2021-003311
34. Ochoa de Olza M, Navarro Rodrigo B, Zimmermann S, Coukos G. Turning up the heat on non-immunoreactive tumours: opportunities for clinical development. Lancet Oncol. 2020;21(9):e419–e430. doi:10.1016/S1470-2045(20)30234-5
35. Tischfield DJ, Gurevich A, Johnson O, et al. Transarterial embolization modulates the immune response within target and nontarget hepatocellular carcinomas in a rat model. Radiology. 2022;303(1):215–225. doi:10.1148/radiol.211028
36. Liu WM, Fowler DW, Smith P, Dalgleish AG. Pre-treatment with chemotherapy can enhance the antigenicity and immunogenicity of tumours by promoting adaptive immune responses. Br J Cancer. 2010;102(1):115–123. doi:10.1038/sj.bjc.6605465
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


