Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 19
Common Pathogeneses Underlying Asthma and Chronic Obstructive Pulmonary Disease -Insights from Genetic Studies
Authors Hizawa N
Received 27 October 2023
Accepted for publication 21 February 2024
Published 4 March 2024 Volume 2024:19 Pages 633—642
DOI https://doi.org/10.2147/COPD.S441992
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Russell
Nobuyuki Hizawa
Department of Pulmonary Medicine, Institute of Medicine, University of Tsukuba, Ibaraki, Japan
Correspondence: Nobuyuki Hizawa, Email [email protected]
Abstract: Neither asthma nor chronic obstructive pulmonary disease (COPD) is a single disease consisting of a uniform pathogenesis; rather, they are both syndromes that result from a variety of basic distinct pathogeneses. Many of the basic pathogeneses overlap between the two diseases, and multiple basic pathogeneses are simultaneously involved at varying proportions in individual patients. The specific combination of different basic pathogeneses in each patient determines the phenotype of the patient, and it varies widely from patient to patient. For example, type 2 airway inflammation and neutrophilic airway inflammation may coexist in the same patient, and quite a few patients have clinical characteristics of both asthma and COPD. Even in the same patient, the contribution of each pathogenesis is expected to differ at different life stages (eg, childhood, adolescence, middle age, and older), during different seasons (eg, high seasons for hay fever and rhinovirus infection), and depending on the nature of treatments. This review describes several basic pathogeneses commonly involved in both asthma and COPD, including chronic non-type 2 inflammation, type 2 inflammation, viral infections, and lung development. Understanding of the basic molecular pathogeneses in individual patients, rather than the use of clinical diagnosis, such as asthma, COPD, or even asthma COPD overlap, will enable us to better deal with the diversity seen in disease states, and lead to optimal treatment practices tailored for each patient with less disease burden, such as drug-induced side effects, and improved prognosis. Furthermore, we can expect to focus on these molecular pathways as new drug discovery targets.
Keywords: Asthma, chronic obstructive pulmonary disease (COPD), endotype, precision medicine, treatable traits approach
Introduction
Asthma and chronic obstructive pulmonary disease (COPD) are both common diseases diagnosed by the presence of chronic symptoms such as cough, sputum, shortness of breath, and airflow obstruction. Several clinical/inflammatory factors are commonly associated with the risk of developing asthma or COPD or with important clinical outcomes such as reduced lung function, exacerbations, reduced quality of life and mortality.1,2 They are characterized by their complex and heterogeneous nature, both clinically and in their molecular pathogenesis. Endotype is a dynamic molecular network that arises when an individual’s genetic factors interact with various environmental factors, such as infections, air pollution, tobacco smoke, antibiotics, and lung flora, driving the phenotype in a particular patient. Given the clinical and biological complexity and heterogeneity of the diseases, the development of therapeutic strategies targeting individual endotypes could help us enable early identification of disease risk with a high degree of accuracy and implementation of preventive strategies.3
Numerous genetic studies, including genome-wide association studies (GWAS), have found a number of loci that influence the development of asthma and COPD, and several genetic factors are common to both diseases. Genetic contribution of individual common variants to disease susceptibility is very small, especially in isolation, and the small proportion of heritability explained by these variants makes it difficult to predict disease onset in a practical clinical setting. At present, the more important significance of GWAS, however, is not to estimate individual risk, but rather to discover the biological pathways underlying complex diseases.4 The pathophysiological pathways identified by GWAS for a disease have important implications not only for carriers of a particular genetic polymorphism, but also in the origins of the disease itself.
This review describes representative endotypes indicated by genetic and molecular data to be commonly involved in both asthma and COPD, including chronic non-type 2 inflammation, type 2 inflammation, increased susceptibility to viral infections, and impaired lung development and repair/remodeling. Advances in genomic medicine in asthma and COPD are critically important for achieving precision medicine, allowing a departure from the current one-size-fits-all medicine according to disease labels or clinical symptoms, and population approach to disease incidence prevention that does not consider individual disease susceptibility.5
Overlap Between Asthma and COPD
Dutch hypothesis was proposed more than 50 years ago.6 In this hypothesis, asthma and COPD are two phenotypes of a syndrome called chronic nonspecific lung disease (CNSLD), where CNSLD is defined as the result of an interaction between intrinsic genetic factors and extrinsic factors such as viral infection, air pollution, tobacco smoke exposure, and allergen exposure. The timing of this interaction during an individual patient’s life stage determines which clinical syndrome develops (ie, asthma or COPD) or whether characteristics of both asthma and COPD appear. Thus, a particular genetic factor may combine with a particular environmental factor to cause asthma, or the same genetic factor may combine with another genetic or environmental factor to cause COPD.7 Several genes and loci have been reported as common factors in susceptibility to asthma and COPD.7,8 We performed a PubMed database search published through September 2012 for asthma, COPD, tuberculosis, and essential hypertension, respectively. For each disease, pathway-based analysis was performed to determine how the identified genes interacted with each other.8 In at least two independent reports, a total of 108 genes were found to be associated with asthma and 58 with COPD. These genes were grouped into multiple networks according to functional annotation. Twelve networks were found in asthma and 11 in COPD, and the overlapping network between the two diseases formed one complex network consisting of 229 common molecules (Figure 1). These overlapping molecules were significantly associated with aryl hydrocarbon receptor (AhR) signaling, the role of cytokines in mediating information transfer between immune cells, glucocorticoid receptor signaling, and pathways involved in IL-12 signaling and production in macrophages. At the network level, the Jaccard similarity index for asthma and COPD was 0.81, with an odds ratio of 3.62 for asthma/COPD pair in comparison to tuberculosis/essential hypertension pair. The overlap in the asthma and COPD gene networks indicated a high degree of pathobiological similarity between these two diseases.
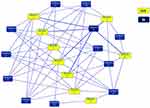 |
Figure 1 Overlapping networks between asthma and COPD. The Ingenuity Pathway Analysis software program identified 229 overlapping molecules between 12 asthma networks and 11 COPD networks, and merged them into a single larger network. In total, 229 genes were common to both diseases, and 190 and 91 genes were unique to asthma and COPD, respectively. Each network is represented by a colored rectangle, and is labeled with its corresponding network number. Adapted with permission from Dove Medical Press. Kaneko Y, Yatagai Y, Yamada H, et al. The search for common pathways underlying asthma and COPD. Int J Chron Obstruct Pulmon Dis. 2013;8:65–78.8 |
Common Pathogenesis Characterized by Chronic Non-Type 2 Airway Inflammation
As discussed in the section above, AhR signaling is implicated in common pathologies of asthma and COPD; AhR acts as a regulator of mucosal barrier function and affects lung immunity by inducing changes in gene expression, intercellular adhesion, mucin production, and cytokine expression.9 Although the binding of this receptor to different ligands leads to what seems to be variable responses, AhR-regulated neutrophils and Th17 cells are involved in the responses to pro-inflammatory stimuli, including tobacco smoke and air pollutants.10 The AhR-ROS-NLRP3 inflammasome functional axis, which regulates Muc5ac expression and airway inflammation, may also be involved in airway inflammation in asthma and COPD.11
We performed a GWAS of adult-onset asthma that developed over the age of 40 and identified the HCG22 gene as a susceptibility gene.12 This gene was also associated with diffuse panbronchiolitis (DPB) and COPD. DPB is a chronic neutrophilic bronchiolitis with chronic cough, sputum, and shortness of breath on exertion as the main symptoms, and its prevalence increases after the age of 40 years. HCG22 is a novel mucin-like gene13 located at 6p21.3, the DPB susceptibility gene region. Furthermore, HCG22 has been reported to be associated with tree-in-bud pattern identified on chest computed tomography in asthmatic patients and with steroid refractoriness requiring high doses of corticosteroids.14 Based on its amino acid sequence, HCG22 has a chitin-binding protein-like structure.15 YKL-40, a chitinase-like protein similar to HCG22, has been reported to be associated with phenotypes characterized by neutrophilic inflammation in asthma and COPD.16,17 Chitin is a pathogen-associated molecular pattern found in mites and fungi, and it is of interest due to its involvement in infection immunity in the airway mucosa, and its association with the pathogenesis of middle-age-onset asthma, COPD, and DPB. Recently, the new disease category of muco-obstructive lung disease has been proposed, and it includes COPD, primary ciliary dyskinesia, and bronchiectasis.18 Mucus-derived obstruction is characterized by altered airway microbiota, mucociliary dysfunction, neutrophilic inflammation, and airway destruction, which are also important features in DPB and a subgroup of patients with non-type 2 asthma.
We have found a gene encoding HA synthase 2 (HAS2) as associated with asthma.19 HAS2 is a glycosaminoglycan found in the extracellular matrix and is highly expressed in the lung. Asthma-associated single nucleotide polymorphisms (SNPs) affected the expression levels of HAS2 mRNA. Hyaluronic acid (HA) is involved in many physiological and pathological processes, including cell migration, morphogenesis, tissue regeneration, wound repair, and tumor cell proliferation and invasion, and increased levels of HA in sputum have been reported in COPD patients.20 Patients with higher levels of hyaluronan had impaired lung function than patients with patients with normal hyaluronan levels. In addition, influx of neutrophil and levels of interleukin-8 and soluble tumor necrosis factor (TNF) receptors were higher in COPD patients with elevated HA levels. Decreased Has2 expression in mice enhanced ovalbumin (OVA)-induced airway inflammation, including increased neutrophils and eosinophils, airway hyperresponsiveness, and attenuated CD44 and transforming growth factor (TGF)-β signaling.21 CD44 is an HA binding protein and decreased CD44 downregulates TGF-β. In addition, lung mRNA sequencing and pathway analysis identified enriched terms “IL-17A signaling in fibroblasts”, “NRF2-mediated oxidative stress response”, and “glucocorticoid receptor signaling”. These terms were thought to be associated with severe asthma and COPD. Furthermore, in a chronic OVA sensitization and challenge-induced asthma model,22 IL-17A levels in lung homogenates were higher in Has2 heteroknockout OVA mice than in wild-type mice, and Has2 heteroknockout OVA mice showed goblet cell hyperplasia and excessive mucus production. Thus, chronic OVA stimulation induced a characteristic phenotype of airway remodeling through Has2-mediated attenuation of IL-17 and TGF-β signaling.
Taken together, neutrophil inflammation is recognized as an important pathogenic factor in asthma as well as COPD.
Common Pathogenesis Characterized by Type 2 Inflammation
Eosinophilic airway inflammation is found in patients with COPD as well as asthma, and the presence of eosinophilic inflammation is associated with exacerbations and responsiveness to inhaled corticosteroids. Overall, 612 (56%) of 1094 Japanese COPD patients had an absolute eosinophil number of 150 cells/mm3 or greater, and 902 (69%) of 1304 Japanese patients had an eosinophil fraction of 2% or greater23 (Figure 2). In a study comparing the comprehensive gene expression in the airway epithelial cells of asthma and COPD patients, the gene expression levels associated with type 2 inflammation were increased not only in asthma patients, but also in COPD patients.24 In particular, the expression of type 2-related genes in COPD patients was associated with stronger airflow limitation, airway eosinophil infiltration, and even the responsiveness to inhaled corticosteroid (ICS).
 |
Figure 2 The distribution of blood eosinophil levels in a Japanese COPD clinical trial database. Distribution of (A) absolute blood eosinophil count and (B) percentage blood eosinophils among Japanese patients with COPD. Reprinted with permission from Dove Medical Press. Barnes N, Ishii T, Hizawa N, et al. The distribution of blood eosinophil levels in a Japanese COPD clinical trial database and in the rest of the world. Int J Chron Obstruct Pulmon Dis. 2018;13:433–440.23 |
In a large GWAS of 8068 patients with the overlapping asthma and COPD pathology in the UK Biobank and 4301 patients with the overlapping pathology from other cohorts, eight loci were identified,25 including the thymic stromal lymphopoietin (TSLP) gene. These eight loci were not clearly associated with smoking habits, but they were strongly associated with the peripheral blood eosinophil counts, immunoglobulin (Ig) E sensitization and asthma, suggesting the importance of type 2 inflammation in the overlapping pathology. Elevated TSLP protein and TSLP mRNA levels have been reported in bronchial epithelium in COPD patients.26 Multiple factors related to exacerbations of asthma and COPD, including respiratory viruses, cigarette smoke, and inflammatory cytokines, have been associated with increased TSLP production.27–30 TSLP gene was also identified as a potential susceptibility locus for impaired lung function in non-COPD, non-asthmatic healthy subjects, which supports the idea that TSLP is a genetic determinant of lung function that influences the risk of developing asthma and COPD.31
The ORMDL3/GSDMB gene located on chromosome 17q has been consistently associated with childhood-onset asthma, and most asthma patients associated with this gene are atopic. In addition, an association of the region with overlap between COPD and asthma without rhinitis has been reported.32 Susceptibility to rhinovirus (RV) infection is associated with this genetic region that affects transcription and protein expression of intercellular adhesion molecule 1 (ICAM1), a major receptor for human RV (HRV).33 ORMDL3/GSDMB has also been implicated in the development of childhood asthma related to indirect exposure to smoking at home.34 Furthermore, the importance of ORMDL3/GSDMB was indicated in susceptibility to early-onset adult asthma in Japanese. While the region was not associated with allergic sensitization, it was strongly associated with increased serum total IgE levels,35 and therefore, the region appears to act as a stress sensor in the airways caused by viral infections and smoking, and promotes airway inflammation through an enhanced innate type 2 immune response.
Common Pathogenesis Characterized by Increased Susceptibility to Viral Infections
HRV is an important risk factor for exacerbations both in asthma and COPD. RV induces several cytokines including IFNα, IFNγ, TNFα, CXCL10/11, and CC chemokine ligand 5 (CCL5) in airway epithelial cells. The airway epithelial cell responses to RV was overlapped with gene expression signatures reported in patients with asthma or COPD.36
We previously found that the gain-of-function −28G allele of a promoter SNP (rs2280788) in the CCL5 gene was a risk factor for adult-onset asthma who developed the disease at age 40 years or older, and also for COPD who had less emphysema lesions.37,38 Given that the CCL5 gene is a pathway involved in both the pathogenesis of older-onset asthma and COPD with less emphysema, it is interesting that CCL5 is shown to contributes to tissue-resident T cell-associated T1 neutrophilic inflammation in asthma and correlates with T2 inflammation and sputum eosinophilia as well.39
The CDHR3 gene, which was identified in GWAS of childhood asthma with frequent severe exacerbations, was found to encode a receptor for RV type C.40 We confirmed that the functional variant at CDHR3 has a significant genetic influence in Japanese adult asthma patients with onset by age 10 years, and that the association is stronger when restricted to allergen sensitization-positive individuals.41 In addition, a 10-year observational study was conducted to examine the genetic impact of the CDHR3 gene on the newly development of asthma or COPD in 1523 healthy adults with no pulmonary disease who had health checkups in 2008. During the 10-year period, a total of 79 cases and 25 cases newly developed asthma and COPD, respectively. The CDHR3 gene had a genetic influence on the development of asthma or COPD, especially in adults with allergen sensitization in 2008.42 A molecular network (endotypes) derived from the susceptibility to RV infection and allergen sensitization was found to be responsible not only for childhood-onset allergic asthma, but also for adult-onset asthma or COPD.
Common Pathogenesis Characterized by Impaired Lung Development and Repair/Remodeling in Asthma and COPD
The primary risk factor for COPD is smoking. However, there is growing evidence to suggest that lung disease in adults may originate from prenatal or early-life exposures to harmful stimuli.43 A whole genome sequencing study44 that compared 3181 moderate/severe asthmatics with 3590 non-asthmatic controls showed that asthma risk is genetically correlated with lung dysfunction. This genetic factor associated with asthma development was shown to be independent of genetic factors associated with eosinophilic inflammation that also contribute to asthma. The polygenic score for impaired lung function was also associated with early-onset of asthma. Thus, genes that influence lung development in utero and in early childhood, in combination with environmental exposure such as cigarette smoke and viral infections, all contribute to both childhood asthma and future COPD development.
Asthma and COPD are heterogeneous and complex diseases, because they are caused by multiple factors, and the impact of individual risk factors is small. A genetic risk score (GRS) has been applied to address the heterogeneity and complexity of these diseases.45 We developed a quantitative GRS according to genotypes at 16 SNPs implicated in impaired lung function in both Japanese and non-Japanese individuals.46 The modest effects of 16 SNPs were combined into a single variable, which was calculated as the weighted sum of the number of high-risk alleles at each SNP. The GRS with a reduced forced expiratory volume/forced lung capacity ratio was consistently associated with asthma or COPD in two independent Japanese populations. Clustering of patients with asthma according to their lung function GRS indicated that elevated GRS may be associated with the development of distinctive phenotype of asthma (early onset, atopy, and severe airflow obstruction). Analysis of the functional relevance of these 16 genes showed that lung function GRS is associated with molecular pathways involved in tissue repair and remodeling induced by lung injury. In addition, a study using UK Biobank data to examine the association of 391 genes known to regulate lung development and lung function in adults47 found that 55 genes were significantly associated with four biological categories including growth factors, transcriptional regulators, intercellular adhesion, and extracellular matrix. These results together showed the importance of lung growth-related genes in regulating lung function and influencing airflow obstruction in adults. Thus, respiratory function measurements from infancy through adolescence may facilitate early identification of individuals prone to lung growth failure, leading to early intervention and prevention of asthma and COPD development.
Several GWAS have indicated the hedgehog signaling pathway as an important pathway underlying lung function and COPD; hedgehog-interacting protein (HHIP) is a negative regulator of the hedgehog pathway and patched 1 (PCTH1) is a receptor that activates the pathway.48 In older adults with asthma, the PTCHD4 gene has recently been associated with the responsiveness to ICS, as indicated by the presence of oral corticosteroid bursts.49 PTCHD4 encodes patched domain-containing protein 4, which represses hedgehog signaling.50 Increased PTCHD4 mRNA expression was associated with aging, and enrichment of methylated CpG sites in the PTCHD4 gene was associated with COPD.51,52 Furthermore, COPD patients with larger lesion with airway smooth muscle cell of bronchial tissue responded better to ICS than those with smaller lesion with airway smooth muscle cell, suggesting that a detailed histological classification of COPD patients may reflect differences in endotypes and help determine treatment strategy.53 These results suggest that responsiveness to ICS in asthma and COPD patients may be strongly influenced by specific patient endotypes, and that patients with specific endotypes related to lung growth abnormalities or impaired injury repair may be less responsive to ICS.
Treatable Traits Approach in Patients with Asthma and COPD
Given the complexity and heterogeneity of chronic inflammatory pulmonary diseases, including asthma and COPD, their appropriate management requires a new approach that includes multidimensional assessment. Patients with chronic inflammatory pulmonary diseases should not be treated according to disease labels such as asthma, COPD, or asthma COPD overlap, but rather on what endotypes play a critical role in individual patients.54 In 2015, I had proposed a plausible approach for positioning ICSs and long-acting β2-agonists (LABAs)/long-acting muscarinic antagonists (LAMAs) in the treatment of COPD based on both the extent of airflow obstruction and the presence of type 2 airway inflammation55 (Figure 3). Thereafter, a management strategy based on the so-called treatable traits was proposed.56,57
 |
Figure 3 Approach to COPD treatment based on the degree of airflow obstruction and peripheral blood eosinophil counts. This proposal for positioning ICSs and bronchodilators for the treatment of COPD in clinical practice follows a personalized medicine approach that is not based on the stratification of patients into subgroups, but rather is based on individual characteristics that consider the heterogeneity and complexity of the disease in patients. Reprinted with permission from Dove Medical Press. Hizawa N. LAMA/LABA vs ICS/LABA in the treatment of COPD in Japan based on the disease phenotypes. Int J Chron Obstruct Pulmon Dis. 2015;10:1093–1102.55 |
We attempted to identify a group of patients who were more prone to exacerbations beyond the name of the diseases using multiple risk factors common to asthma and COPD exacerbations.58 As a result, we identified five distinct clusters, each characterized by high eosinophil counts, smokers with reduced lung function, gastroesophageal reflux, non-allergic women, or allergic rhinitis with high total IgE levels. Clinical heterogeneity of disease exacerbations was shown to possibly indicate the presence of exacerbation-prone endotypes common to asthma and COPD, supporting the benefit of a trait-based approach for exacerbation prevention in patients with chronic inflammatory pulmonary disease.
Recently, it was reported that in COPD patients with type 2 inflammation, in whom both blood eosinophil counts and FeNO are elevated, dupilumab, an antibody against the IL4 receptor alpha chain, leads to a reduction in exacerbation frequency, improvement in lung function and quality of life, and even improvement in respiratory symptoms compared to placebo.59 Considering that patients with currently diagnosed asthma or a history of asthma were excluded from the study, these results appear to support the usefulness of a treatable trait approach.
Conclusion
Both asthma and COPD are syndromes with highly variable clinical manifestations (phenotypes), including severity and course over time, and are caused by complex interactions between individual genetic factors and various environmental factors such as viral infection, allergen exposure, and tobacco smoke exposure (endotype). In this review, I have described four representative endotypes common to asthma and COPD (Figure 4). These endotypes are involved in patient pathogenesis in varying proportions. Furthermore, while the interactions of individual endotypes shape each patient’s pathology, the relative contribution of each endotype in an individual patient may change over time. Clinical traits or biomarkers could be used to identify the presence of each endotype. We must consider that it is not one endotype per patient, but rather the interaction of multiple endotypes that drives individual patient pathologies. With the advancement of genomic medicine, our understanding of endotypes will advance, new therapeutic agents will be developed, and the diseases will be reclassified according to specific phenotypes and biomarkers that reflect differences in molecular pathobiology, ushering in an era of precision medicine that targets the molecular mechanisms underlying the diseases in individual patients.
Disclosure
The author has received speaker fees and/or research funding from AstraZeneca, Boehringer Ingelheim, Kyorin Pharmaceutical, GlaxoSmithKline, Novartis, and Sanofi.
References
1. Suzuki M, Makita H, Konno S, Nishimura M. Clinical characteristics and natural course of chronic obstructive pulmonary disease and/or asthma in Japanese patients: a summary report of two Hokkaido-based cohort studies. Respir Investig. 2023;61(4):527–539. doi:10.1016/j.resinv.2023.05.002
2. Holtjer JCS, Bloemsma LD, Beijers RJHCG, et al. Identifying risk factors for COPD and adult-onset asthma: an umbrella review. Eur Respir Rev. 2023;32(168):230009. doi:10.1183/16000617.0009-2023
3. Hizawa N. The understanding of asthma pathogenesis in the era of precision medicine. Allergol Int. 2023;72(1):3–10. doi:10.1016/j.alit.2022.09.001
4. Hirschhorn JN. Genomewide association studies—illuminating biologic pathways. N Engl J Med. 2009;360(17):1699–1701. doi:10.1056/NEJMp0808934
5. Agustí A, Celli B, Faner R. What does endotyping mean for treatment in chronic obstructive pulmonary disease? Lancet. 2017;390(10098):980–987. doi:10.1016/S0140-6736(17)32136-0
6. Orie N, Sluiter H, DeVries K, et al. The host factor in bronchitis. In: Bronchitis: An International Symposium, 27–29 April 1960, Groningen. Assen, Royal Van Gorcum; 1961:43–59.
7. Weiss ST. What genes tell us about the pathogenesis of asthma and chronic obstructive pulmonary disease Am. J Respir Crit Care Med. 2010;181(11):1170–1173. doi:10.1164/rccm.201001-0069PP
8. Kaneko Y, Yatagai Y, Yamada H, et al. The search for common pathways underlying asthma and COPD. Int J Chron Obstruct Pulmon Dis. 2013;8:65–78. doi:10.2147/COPD.S39617
9. Beamer CA, Shepherd DM. DM Role of the aryl hydrocarbon receptor (AhR) in lung inflammation. Semin Immunopathol. 2013;35(6):693–704. doi:10.1007/s00281-013-0391-7
10. Veldhoen M, Hirota K, Westendorf AM, et al. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453(7191):106–109. doi:10.1038/nature06881
11. Hu X, Shen Y, Zhao Y, et al. Epithelial aryl hydrocarbon receptor protects from mucus production by inhibiting ROS-triggered NLRP3 Inflammasome in asthma. Front Immunol. 2021;12:767508. doi:10.3389/fimmu.2021.767508
12. Yatagai Y, Hirota T, Sakamoto T, et al. Variants near the HLA complex group 22 gene (HCG22) confer increased susceptibility to late-onset asthma in Japanese populations. J Allergy Clin Immunol. 2016;138(1):281–283. doi:10.1016/j.jaci.2015.11.023
13. Hijikata M, Matsushita I, Tanaka G, et al. Molecular cloning of two novel mucin-like genes in the disease-susceptibility locus for diffuse panbronchiolitis. Hum Genet. 2011;129(2):117–128. doi:10.1007/s00439-010-0906-4
14. Nomura N, Matsumoto H, Sunadome H, et al. Importance of mucus burden and mucociliary impairment in asthma. J Allergy Clin Immunol. 2023;151(5):1410–1411. doi:10.1016/j.jaci.2023.01.024
15. Jeong S, Patel N, Edlund CK, et al. Identification of a novel mucin gene HCG22 associated with steroid-induced ocular hypertension. Invest Ophthalmol Vis Sci. 2015;56(4):2737–2748. doi:10.1167/iovs.14-14803
16. Ober C, Tan Z, Sun Y, et al. Effect of variation in CHI3L1 on serum YKL-40 level, risk of asthma, and lung function. N Engl J Med. 2008;358(16):1682–1691. doi:10.1056/NEJMoa0708801
17. James AJ, Reinius LE, Verhoek M, et al. Increased YKL-40 and chitotriosidase in asthma and chronic obstructive pulmonary disease Am. J Respir Crit Care Med. 2016;193(2):131–142. doi:10.1164/rccm.201504-0760OC
18. Boucher RC, Drazen JM. Muco-obstructive lung diseases. N Engl J Med. 2019;380(20):1941–1953. doi:10.1056/NEJMra1813799
19. Yatagai Y, Sakamoto T, Yamada H, et al. Genomewide association study identifies HAS2 as a novel susceptibility gene for adult asthma in a Japanese population. Clin Exp Allergy. 2014;44(11):1327–1334. doi:10.1111/cea.12415
20. Dentener MA, Vernooy JHJ, Hendriks S, Wouters EFM. Enhanced levels of hyaluronan in lungs of patients with COPD: relationship with lung function and local inflammation. Thorax. 2005;60(2):114–119. doi:10.1136/thx.2003.020842
21. Tsunoda Y, Sherpa MT, Kiwamoto T, et al. Has2 deficiency enhances OVA- induced airway inflammation and hyperresponsiveness in mice. Allergy. 2021;76(7):2214–2218. doi:10.1111/all.14715
22. Sherpa MT, Kiwamoto T, Matsuyama M, et al. Has2 regulates the development of ovalbumin-induced airway remodeling and steroid insensitivity in mice. Front Immunol. 2022;12:770305. doi:10.3389/fimmu.2021.770305
23. Barnes N, Ishii T, Hizawa N, et al. The distribution of blood eosinophil levels in a Japanese COPD clinical trial database and in the rest of the world. Int J Chron Obstruct Pulmon Dis. 2018;13:433–440. doi:10.2147/COPD
24. Christenson SA, Steiling K, van den Berge M, et al. Asthma-COPD overlap Clinical relevance of genomic signatures of type 2 inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;191(7):758–766. doi:10.1164/rccm.201408-1458OC
25. John C, Guyatt AL, Shrine N, et al. Genetic Associations and Architecture of Asthma-COPD Overlap. Chest. 2022;161(5):1155–1166. doi:10.1016/j.chest
26. Ying S, O’Connor B, Ratoff J, et al. Expression and cellular provenance of thymic stromal lymphopoietin and chemokines in patients with severe asthma and chronic obstructive pulmonary disease. J Immunol. 2008;181(4):2790–2798. doi:10.4049/jimmunol.181.4.2790
27. Lee HC, Headley MB, Loo YM, et al. Thymic stromal lymphopoietin is induced by respiratory syncytial virus-infected airway epithelial cells and promotes a type 2 response to infection. J Allergy Clin Immunol. 2012;130(5):1187–1196.e5. doi:10.1016/j.jaci.2012.07.031
28. Perez GF, Pancham K, Huseni S, et al. Rhinovirus infection in young children is associated with elevated airway TSLP levels. Eur Respir J. 2014;44(4):1075–1078. doi:10.1183/09031936.00049214
29. Nakamura Y, Miyata M, Ohba T, et al. Cigarette smoke extract induces thymic stromal lymphopoietin expression, leading to T(H)2-type immune responses and airway inflammation. J Allergy Clin Immunol. 2008;122(6):1208–1214. doi:10.1016/j.jaci.2008.09.022
30. Smelter DF, Sathish V, Thompson MA, Pabelick CM, Vassallo R, Prakash YS. Thymic stromal lymphopoietin in cigarette smoke-exposed human airway smooth muscle. J Immunol. 2010;185(5):3035–3040. doi:10.4049/jimmunol.1000252
31. Masuko H, Sakamoto T, Kaneko Y, et al. Lower FEV1 in non-COPD, nonasthmatic subjects: association with smoking, annual decline in FEV1, total IgE levels, and TSLP genotypes. Int J Chron Obstruct Pulmon Dis. 2011;6:181–189. doi:10.2147/COPD.S16383
32. Balantic M, Rijavec M, Flezar M, et al. A polymorphism in ORMDL3 is associated not only with asthma without rhinitis but also with chronic obstructive pulmonary disease. J Investig Allergol Clin Immunol. 2013;23(4):256–261.
33. Zhang Y, Willis-Owen SAG, Spiegel S, Lloyd CM, Moffatt MF, Cookson WOCM; WOCM. The ORMDL3 Asthma Gene Regulates ICAM1 and Has Multiple Effects on Cellular Inflammation. J Respir Crit Care Med. 2019;199(4):478–488. doi:10.1164/rccm.201803-0438OC
34. Bouzigon E, Corda E, Aschard H, et al. Effect of 17q21 variants and smoking exposure in early-onset asthma. N Engl J Med. 2008;359(19):1985–1994. doi:10.1056/NEJMoa0806604
35. Kitazawa H, Masuko H, Kanazawa J, et al. ORMDL3/GSDMB genotype as a risk factor for early-onset adult asthma is linked to total serum IgE levels but not to allergic sensitization. Allergol Int. 2021;70(1):55–60. doi:10.1016/j.alit.2020.04.009
36. Wronski S, Beinke S, Obernolte H, et al. Rhinovirus-induced human lung tissue responses mimic chronic obstructive pulmonary disease and asthma gene signatures Am. J Respir Cell Mol Biol. 2021;65(5):544–554. doi:10.1165/rcmb.2020-0337OC
37. Hizawa N, Yamaguchi E, Konno S, Tanino Y, Jinushi E, Nishimura M. A functional polymorphism in the RANTES gene promoter is associated with the development of late-onset asthma. Am J Respir Crit Care Med. 2002;166(5):686–690. doi:10.1164/rccm.200202-090OC
38. Hizawa N, Makita H, Nasuhara Y, et al. Functional single nucleotide polymorphisms of the CCL5 gene and nonemphysematous phenotype in COPD patients. Eur Respir J. 2008;32(2):372–378. doi:10.1183/09031936.00115307
39. Gauthier M, Kale SL, Oriss TB, et al. CCL5 is a potential bridge between type 1 and type 2 inflammation in asthma. J Allergy Clin Immunol. 2023;152(1):94–106.e12. doi:10.1016/j.jaci.2023.02.028
40. Bochkov YA, Watters K, Ashraf S, et al. Cadherin-related family member 3, a childhood asthma susceptibility gene product, mediates rhinovirus C binding and replication. Proc Natl Acad Sci U S A. 2015;112(17):5485–5490. doi:10.1073/pnas.1421178112
41. Kanazawa J, Masuko H, Yatagai Y, et al. Genetic association of the functional CDHR3 genotype with early-onset adult asthma in Japanese populations. Allergol Int. 2017;66(4):563–567. doi:10.1016/j.alit.2017.02.012
42. Shigemasa R, Masuko H, Hyodo K, et al. Genetic impact of CDHR3 on the adult onset of asthma and COPD. Clin Exp Allergy. 2020;50(11):1223–1229. doi:10.1111/cea.13699
43. Lange P, Celli B, Agustí A, et al. Lung-function trajectories leading to chronic obstructive pulmonary disease. N Engl J Med. 2015;373(2):111–122. doi:10.1056/NEJMoa1411532
44. Chang D, Hunkapiller J, Bhangale T, et al. A whole genome sequencing study of moderate to severe asthma identifies a lung function locus associated with asthma risk. Sci Rep. 2022;12(1):5574. doi:10.1038/s41598-022-09447-8
45. Moll M, Sordillo JE, Ghosh AJ, et al. Polygenic risk scores identify heterogeneity in asthma and chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2023;152(6):1423–1432. doi:10.1016/j.jaci.2023.08.002
46. Yamada H, Masuko H, Yatagai Y, et al. Role of lung function genes in the development of asthma. PLoS One. 2016;11(1):e0145832. doi:10.1371/journal.pone.0145832
47. Portas L, Pereira M, Shaheen SO, et al. Lung development genes and adult lung function. Am J Respir Crit Care Med. 2020;202(6):853–865. doi:10.1164/rccm.201912-2338OC
48. Paré PD. The smoking gun: genetics and genomics reveal causal pathways for COPD. Can J Respir Crit Care Sleep Med. 2017;1(3):126–132. doi:10.1080/24745332.2017.1361203
49. Wang AL, Lahousse L, Dahlin A, et al. Novel genetic variants associated with inhaled corticosteroid treatment response in older adults with asthma. Thorax. 2023;78(5):432–441. doi:10.1136/thoraxjnl-2021-217674
50. Chung JH, Larsen AR, Chen E, Bunz F. A PTCH1 homolog transcriptionally activated by p53 suppresses Hedgehog signaling. J Biol Chem. 2014;289(47):33020–33031. doi:10.1074/jbc.M114.597203
51. Casella G, Munk R, Kim KM, et al. Transcriptome signature of cellular senescence. Nucleic Acids Res. 2019;47(14):7294–7305. doi:10.1093/nar/gkz555
52. Morrow JD, Cho MH, Hersh CP, et al. DNA methylation profiling in human lung tissue identifies genes associated with COPD. Epigenetics. 2016;11(10):730–739. doi:10.1080/15592294.2016.1226451
53. Stolz D, Papakonstantinou E, Pascarella M, et al. Airway smooth muscle area to predict steroid responsiveness in COPD patients receiving triple therapy (HISTORIC): a randomised, placebo-controlled, double-blind, investigator-initiated trial. Eur Respir J. 2023;62(1):2300218. doi:10.1183/13993003.00218-2023
54. Hizawa N. Clinical approaches towards asthma and chronic obstructive pulmonary disease based on the heterogeneity of disease pathogenesis. Clin Exp Allergy. 2016;46(5):678–687. doi:10.1111/cea.12731
55. Hizawa N. LAMA/LABA vs ICS/LABA in the treatment of COPD in Japan based on the disease phenotypes. Int J Chron Obstruct Pulmon Dis. 2015;10:1093–1102. doi:10.2147/COPD.S72858
56. Agusti A, Bel E, Thomas M, et al. Treatable traits: toward precision medicine of chronic airway diseases. Eur Respir J. 2016;47(2):410–419. doi:10.1183/13993003.01359-2015
57. Pavord ID, Beasley R, Agusti A, et al. After asthma: redefining airways diseases. Lancet. 2018;391(10118):350–400. doi:10.1016/S0140-6736(17)30879-6
58. Hyodo K, Masuko H, Oshima H, et al. Common exacerbation-prone phenotypes across asthma and chronic obstructive pulmonary disease (COPD). PLoS One. 2022;17(3):e0264397. doi:10.1371/journal.pone.0264397
59. Bhatt SP, Rabe KF, Hanania NA, et al. Dupilumab for COPD with type 2 inflammation indicated by eosinophil counts. N Engl J Med. 2023;389(3):205–214. doi:10.1056/NEJMoa2303951
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.