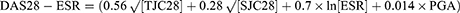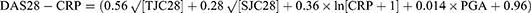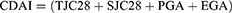Back to Journals » International Journal of General Medicine » Volume 17
Combining Traditional Chinese Herbs and csDMARDs for the Treatment of Rheumatoid Arthritis Involves Tapering and Discontinuing Glucocorticoids: Protocol for a Two-Stage Non-Randomized Controlled Trial
Authors Wang X , Yang X, Wang S, Tian X, Yin J, Liu N, Di P, Qi J, Li Y, Chen J, Wu Y, Wu J, Zhao W , Peng J, Zhang L, Gu L
Received 10 October 2023
Accepted for publication 10 February 2024
Published 5 March 2024 Volume 2024:17 Pages 827—839
DOI https://doi.org/10.2147/IJGM.S444056
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Woon-Man Kung
Xingqiang Wang,1,2,* Xianna Yang,3,* Shiqi Wang,3,* Xiaofang Tian,1 Jianmei Yin,3 Nian Liu,3 Pengtao Di,1 Jipeng Qi,3 Ya Li,3 Jimin Chen,3,4 Yang Wu,1 Jingjin Wu,1,2 Weiqing Zhao,5 Jiangyun Peng,1,2 Ling Zhang,1,2 Lingli Gu1
1Department of Rheumatology, The No. 1 Affiliated Hospital of Yunnan University of Traditional Chinese Medicine, Kunming, Yunnan, 650021, People’s Republic of China; 2Yunnan Provincial Clinical Medicine Research Center of Rheumatism in TCM, Yunnan Provincial Hospital of Traditional Chinese Medicine, Kunming, Yunnan, 650021, People’s Republic of China; 3The First School of Clinical Medicine, Yunnan University of Chinese Medicine, Kunming, Yunnan, 650021, People’s Republic of China; 4Department of Integrative Internal Medicine, The Lijiang Hospital of Traditional Chinese Medicine, Lijiang, Yunnan, 674100, People’s Republic of China; 5Department of Rheumatology and Immunology, The First People’s Hospital of Yunnan Province and The Affiliated Hospital of Kunming University of Science and Technology, Kunming, Yunnan, 650034, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Ling Zhang; Lingli Gu, Email [email protected]; [email protected]
Abstract: Glucocorticoids (GC) are crucial in the treatment of rheumatoid arthritis (RA), but discontinuing GC effectively in RA patients poses a significant challenge for rheumatologists. In this two-stage, single-center, non-randomized controlled trial, we investigated the benefits of combining Chinese traditional herbal treatment with csDMARDs to aid GC discontinuation in terms of GC tapering, disease control, and safety. A total of 231 participants were enrolled, of which 150 eligible subjects were included in the first phase and allocated to three groups (control group, treatment group 1, and treatment group 2) based on their willingness to take traditional Chinese medicine and syndrome differentiation, in a 1:1:1 ratio. All groups received basic treatment consisting of methotrexate tablets (10 mg, qw), leflunomide (10 mg, qd), and stratified GC bridging therapy and tapering regimen (The intervention regimen was developed based on rigorous adherence to available evidence). Treatment group 1 received basic treatment combined with Juanbi Granule, while treatment group 2 received basic treatment combined with Yupingfeng Guizhi Decoction Granule. Efficacy was evaluated after a 12-week follow-up, with slightly adjustments to the treatment group based on efficacy and change of syndrome, followed by continued observation until 24 weeks to complete the study. The efficacy evaluation and data analysis were conducted in a blinded manner, including group label concealment, data cleaning, confounder and control regimen analysis, and outcome analysis. This project has received ethical approval from the Ethics Committee of Yunnan Provincial Hospital of Traditional Chinese Medicine (YLZ [2022] Ethical Review No. (006)-01) and has been registered with the China Clinical Trials Registry (Registration number: ChiCTR2300067676, Registered 17 January 2023, https://www.chictr.org.cn/showproj.html?proj=184908). This trial was the first to evaluate the clinical efficacy of combining Chinese herbal medicines with standard Western medicines to facilitate the discontinuation of glucocorticoid (GC) therapy in patients with rheumatoid arthritis (RA).
Keywords: rheumatoid arthritis, glucocorticoids tapering, clinical trial, traditional Chinese medicine, Yupingfeng Guizhi Decoction, Juanbi Decoction
Introduction
Glucocorticoids (GCs) are commonly used in the treatment of systemic autoimmune diseases because of their powerful anti-inflammatory and immunosuppressive effects, and their role in the treatment of rheumatoid arthritis (RA) is beyond doubt.1 The use of GCs in RA can rapidly control joint symptoms, reduce disease activity and prevent radiological damage to the bone, and many guidelines recommend the combination of conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) with GCs for rapid disease control or as bridging therapy during the initial treatment with csDMARDs and when switching from csDMARDs.2,3 However, the long-term use of various doses of GC increases the risk of dosage-related and duration-related side effects such as infection, peptic ulcer, cardiovascular events, osteoporosis, fracture, osteonecrosis, diabetes, cataract, etc., especially in long-term maintenance treatment with GC doses greater than 10 mg/d. Therefore, guidelines from different countries all emphasize that GC should be discontinued for RA patients as soon as their disease reaches a low disease activity state or remission (preferably within 3 months).2–7
Since 2017, rheumatologists in various countries have prescribed oral prednisone for RA at initial doses ranging from 5 mg to 30 mg in daily clinical practice, with varying discontinuation regimens.8,9 A real-world study in China showed that the starting dose of prednisone in RA patients was 48.3% at 10 mg, 24.6% at 5 mg, and 27.1% at other doses, and the median time to GC discontinuation was 27 months (IQR: 19.2–34.8), with a 6-month cumulative discontinuation rate of only 9.7%.9 A French early RA cohort (ESPOIR cohort) also showed that 55% of patients were on GC for more than two years, and even 11.8% of patients were on GC for more than nine years.6 These data suggest that there is a large gap between the reality of GC use and guideline recommendations. At present, the discontinuation of GC in RA treatment has attracted much attention, and the main issues are the initial dose of the GC, the duration of maintenance use and the discontinuation regimen, and the lack of detailed guidelines for physicians to perform tapering and discontinuation after GC bridging therapy.
With the development of traditional Chinese medicine (TCM) modernization and the accumulation of evidence-based medical evidence, the advantages of TCM in controlling the progression of RA patients and assisting in GC discontinuation are gradually emerging.10,11 Preclinical experimental studies have shown that (1) some chinese herbs have steroid hormone effects and can be used as supplemental replacement drugs for adrenal cortical hormones; (2) the combination of Western medicine with TCM treatment can also improve the efficacy of RA, indirectly reducing the cumulative dosage and the exposure time of GC.12 Yupingfeng Guizhi Decoction (YG) Granule and Juanbi (JB) Granule, which have the efficacy of warming yang for dispelling cold, and dispelling wind and eliminating dampness, are two commonly used formulas in TCM for the treatment of RA and have been used clinically for decades. Animal experiments have shown that the anti-rheumatic herbs contained in these two formulas, such as aconitum carmichaelii (fuzi), Asarum heterotropoides (xixin), Gentiana macrophylla (qinjiao), Sabia japonica (qingfengteng), have steroids-like effects; the warming yang drugs such as aconitum carmichaelii (fuzi) and Epimedium sagittatum (yinyanghuo), reduce the effects of exogenous GCs by promoting the release of adrenocorticotropic hormones or by counteracting the hypothalamus-pituitary-adrenal axis (HPA) to reduce side effects caused by exogenous GCs.13 In conclusion, we hypothesized that YG Granule and JB Granule have similar pharmacological effects with GCs and can be used as ancillary drugs for GC discontinuation in RA. Therefore, we attempted to develop an active stratified glucocorticoids bridging therapy and tapering (sGCBT) regimen and prepared to implement a non-randomized controlled trial with a two-stage design to observe the efficacy, safety, and potential efficacy mechanisms of TCM combined with csDMARDs in assisting GC discontinuation.
Trial Objectives
Objectives of the study: The primary objective of the study is to observe and compare the differences in safety, disease control, and GC discontinuation of TCM combined with csDMARDs-assisted GC bridging therapy and discontinuation regimen to explore the advantages of this combined therapy in the treatment of RA in terms of GC discontinuation or GC dependence. The secondary objectives are (1) to explore and analyze the risk factors (eg, sex, age, disease duration, disease condition, adrenocortical function, etc.) for GC discontinuation failure and relapse in RA. (2) to collect biological samples (peripheral blood and feces) to explore potential mechanisms of GC discontinuation and the effect of drugs on the gut microbiome. (3) to pilot a staged-GC bridging therapy and discontinuation regimen for RA to gain experience for future clinical validation of this regimen.
Methods and Analyses
Study Design
This study is designed to explore the effect of TCM-assisted discontinuation in RA patients after using GC and belongs to a two-stage, single-center, non-randomized controlled trial. In addition to animal experiments, the TCM method has decades of prior experience in clinical use, but there are only a few unpublished clinical cases and no systematic clinical trial data yet. Hence this is the first prospective clinical observation of TCM combined with csDMARDs to assist with GC discontinuation; secondly, the sGCBT regimen developed according to the guideline-recommended GC discontinuation principles may appear slightly aggressive, with risks of an increased rate of disease relapse, decreased disease remission rate, and induced adrenal insufficiency. Hence only pre-clinical exploration studies were performed and confirmatory studies were not conducted. Among them, the two-stage design is a clinical trial divided into two stages, and at the end of the first stage, an interim analysis is performed to determine whether a second stage should be conducted to save manpower, material resources, and financial resources and measures to protect the rights of the patients.14
All eligible participants were divided into three groups according to their willingness to take TCM and TCM-combined therapy: control group, treatment group 1, and treatment group 2, in a ratio of 1:1:1. All participants were treated with western medicine of methotrexate tablets (10 mg, qw), leflunomide (10 mg, qd) and sGCBT regimen throughout the study period, while the control group was treated with standard western medicine only. Treatment group 1 was treated with JB granule based on western medicine treatment, and treatment group 2 was treated with YG granule based on the western medicine treatment regimen, and the GC discontinuation status was evaluated in 12 weeks of follow-up. For patients with complete GC discontinuation, the original regimen was continued until 24 weeks. If GC was still not withdrawal, the control group was given oral TCM treatment after the combined treatment. The treatment group can continue the treatment after adding or subtracting the original TCM formula. The schematic diagram of the study design is shown in Figure 1.
Study Setting and Participants
The study was conducted at the Department of Rheumatology, Yunnan Provincial Hospital of Traditional Chinese Medicine, a three-level first-class academic general hospital and a clinical medical research center in Yunnan Province. In addition to the Project Director, the study team also has Project Quality Controllers, Evaluator Team, Investigator Team, and Facilitators. There are two Project Quality Controllers, who are responsible for the training of investigators, quality control of project implementation, and supervision and verification; Evaluator Team is composed of at least three TCM rheumatologists above the attending level, who are responsible for performing physical examinations, disease assessment, GC dosage collection, TCM syndrome identification and symptom efficacy scoring, without knowing the patient group; Investigator Team is responsible for patient information collection, case report form (CRF) filling, study interpretation, drug distribution, and patient follow-up. Facilitators were responsible for guiding patients through the follow-up process and volunteer recruitment, and qualified facilitators would not disclose subject grouping information to any evaluator.
At the beginning of the study, all volunteers participating in the clinical trial were first guided to complete and sign the final informed consent form by the facilitator, then guided to Evaluator Team for assessment, and later handed over to Investigator Team for informed consent confirmation, issuance of a screening checklist, and selection of eligible subjects regarding inclusion and exclusion criteria. Eligible subjects were divided into groups according to the TCM syndrome types and the willingness to TCM treatment: those who did not wish to take TCM treatment were in the control group; those who wish to take TCM and with cold dampness obstruction syndrome were in the treatment group 1; those who wished to take TCM and with wind dampness obstruction syndrome were in the treatment group 2. Finally, Investigator Team distributed the medications, explained the precautions (eg, nonsteroidal anti-inflammatory drugs are not allowed during study) and scheduled follow-up visits. At the follow-up visit, subjects were first assessed by Evaluator Team, and then Investigator Team collected follow-up information, issued a review list, distributed medications, and scheduled follow-up visits.
The Project Quality Controllers conducted two rounds of investigator training before the start of the study, and performed CRF spot checks, completed quality check documentation, investigator study performance summary reports, and follow-up on-site supervision, etc. every 2–3 months after the start of the study.
Eligibility Criteria
Inclusion Criteria
- Meet the western medical criteria for RA classification: the 1987 American College of Rheumatology (ACR) Rheumatoid Arthritis Classification for adults or ACR/EULAR 2010 rheumatoid arthritis classification criteria;5
- Meet the TCM diagnostic criteria for RA with wind dampness obstruction syndrome or with cold dampness obstruction syndrome (Additional File 1);
- Patients with active RA (DAS28 > 2.6);5
- Patients who have been/are taking GCs or have indications for oral GC therapy;
- Aged 18–65 years, male or female;
- Those who voluntarily participate in this trial, agree to cooperate with the clinical study, and sign the informed consent form.
Exclusion Criteria
- Patients with a history of severe respiratory (including pulmonary interstitial fibrosis), blood circulation, gastrointestinal, endocrine, or urinary system diseases, and malignancies prior to participation in the study;
- Patients with active gastrointestinal disease with bleeding tendency and patients with a recent history of surgery;
- Patients with active hepatitis or tuberculosis;
- The presence of infections that cannot be controlled by antimicrobial drugs, serious infections, or opportunistic infections in the two months prior to participation in the trial;
- Patients having used biological agents in the past month;
- The patient was participating in other drug clinical trials, or was out of the group of drug clinical trials for less than 1 week;
- Patients whose blood ALT/AST is elevated more than three times the normal value (ie, > 120 U/L), whose serum creatinine exceeds 177 μmol/L and whose urea nitrogen exceeds 7.0 mmol/L;
- Patients with allergic constitutions (allergic to herbal formulas of TCM and experimental drugs such as leflunomide);
- Pregnant and lactating women;
- Patients with a history of neuropsychiatric abnormalities (epilepsy, depression, etc.);
- Patients with a suspected or confirmed history of alcohol or drug abuse, or other circumstances that increase the likelihood of missed visits, or that complicate the study management, such as frequent changes in the workplace, and other circumstances that predispose to missed visits.
Trial Interventions
A real-world study in China showed that 81.1% of RA patients were treated with a double combination of csDMARDs, of which the most common combination regimens were methotrexate combined with leflunomide and methotrexate combined with hydroxychloroquine.9 However, the use of hydroxychloroquine requires fundus screening, so the base regimen of methotrexate tablets (10 mg, qw) combined with leflunomide (10 mg, qd) was used in this study.
The dose of oral GC is positively correlated with disease improvement in patients with RA, however, the higher the GC dose, the greater the drug-related adverse effects and the earlier they appear, and the longer the time to discontinue the GC. Therefore, it is more reasonable to choose the initial dose of GC bridging therapy based on RA disease activity.15 In this study, the initial dose was 20 mg of prednisone acetate tablets (or equivalent dose of the methylprednisolone tablets) orally if the DAS28 score was ≥ 5.1, and 10 mg if the DAS28 score was between 2.6 and 5.1 (Figure 1).
GC in patients with rheumatic diseases should be tapered gradually, and the rate of reduction should be adjusted according to the improvement of the disease. In the study, if the dose of prednisone acetate was greater than 10 mg, it was reduced by 5 mg per week; if it was less than or equal to 10 mg, a modified alternate-day dosing method was used, reducing the dose by 2.5 mg every two weeks. The glucocorticoid reduction rate was adjusted according to the DAS28 score and the patient’s response to treatment, as detailed in Table 1.
 |
Table 1 The Adjustment Schedule of Glucocorticoids Doses |
At follow-up, when patients responded to current therapy (moderate or good response), GC continued to be reduced according to the original schedule (prednisone tapering schedule in Additional File 2); Investigator Team administered GC dose adjustments when:
- The patient did not respond to treatment and had a current DAS28 score > 5.1 and ΔDAS28 ≤ 0.6. The dose was increased to the prior step GC dose for 2 weeks therapy before reassessment.
- The patient did not respond to treatment and has a current DAS28 score ≤ 5.1 or 0.6 < ΔDAS28 ≤ 1.2. The current GC dosage was maintained for 2 weeks before reassessment; see Table 1 for details.
It is well known that the use of GCs requires consideration of the circadian rhythm of endogenous GC secretion, the suppressive effect of exogenous GCs on the HPA, and the need for disease treatment.16 Oral GC for RA patients are recommended to be taken in the morning, and short-acting GCs such as prednisone and methylprednisolone should be chosen whenever possible, and alternate-day administration has less inhibition of the HPA axis than daily administration, facilitating GC discontinuation.17 The standard alternate-day dosing requires patients to take the total dose of glucocorticoids for two days in the morning every other day. However, it has been found that the relapse rate of rheumatoid arthritis increases in practice. This may be due to the fact that RA patients often have relative adrenal insufficiency, and the cortisol fluctuation caused by this method cannot be compensated for by the adrenal function of the patients. The modified alternate-day dosing method still requires daily use of GC, but the dosage on alternate days is reduced. For example, if a patient’s glucocorticoid dose is 7.5 mg per day, the standard every-other-day dosing method is to take 15 mg in the morning today, skip the next day, take 15 mg on the third day morning, and so on (15, 0, 15, 0, 15..). The improved every-other-day dosing method is to take 10 mg in the morning today, 5 mg in the morning the next day, 10 mg on the third day morning, 5 mg, and so on (10, 5, 10, 5, 10..). For details see Prednisone tapering schedule in Additional File 2.
The experimental drugs in the study, JB Granule and YG Granule were both manufactured by Tianjiang Pharmaceutical Co., Ltd Jiangsu province. JB Granule is mainly composed of more than ten herbs such as aconitum carmichaelii (fuzi), Cinnamomum cassia (guizhi), Asarum heterotropoides (xixin), Ligusticum chuanxiong (chuanxiong), and Paeonia lactiflora (baishao), which have the functions of warming yang, dispelling cold, and dispelling wind to eliminate dampness and relieving pain; YG Granule is mainly composed of more than ten herbs including Astragalus membranaceus (huangqi), Saposhnikovia divaricata (fangfeng), Cinnamomum cassia (guizhi), and Paeonia lactiflora (baishao), and has the effect of dispersing cold and removing dampness, invigorating qi for consolidating superficies, and relieving the muscles and opening the channels of qi. Due to the applied patent, it is not convenient to publish a detailed composition. The granules were taken with warm water, twice a day, one sachet each time.
Considering that the dialectical theory of governance is the core idea of TCM and the evolution of transformation of individual syndromes similar to precision medicine, a comprehensive assessment was conducted after 12 weeks of intervention, and those with complete reduction of GC will continue the original regimen until 24 weeks. If GC is still not discontinuation, the control group will be given TCM orally after symptom-based treatment, and the treatment group can continue the treatment after adding or subtracting formula herbs based on the original TCM formula, to follow the basic principles of experimental design under the premise of maximizing the advantages of TCM.
Blinding
The study primarily used the assessor-blinded and analysts-blinded approach. To achieve this, Evaluator Team and Investigator Team were in two separate rooms, and Evaluator Team performed the efficacy evaluation of all subjects without knowing the group assignment (physical examination, condition assessment, GC dosage collection, TCM syndrome identification and symptom efficacy scoring). At the same time, objective indicators were tested by the hospital laboratory and imaging department without knowing the subject grouping. Furthermore, the group allocation of data was masked via code (ie, A, B or C) by the investigator team, and then the statistical analysis was performed by a third party. These blinding procedures ensured the reliability of the study results.
Sample Size
This study is still in the exploratory stage and no relevant studies are currently available. A TARRA registry cohort in China observed the GC discontinuation in patients with RA. And the results showed that at 6 months, the GC discontinuation rate was 12.7% in naive patients and 6.2% in patients who had experienced with DMARDs, and the overall GC discontinuation rate was 9.7%.9 In this study, Simon’s minimax and optimal two-stage designs were used for sample size estimation.14 The parameters were set as the 6-month GC discontinuation rate p0 at 15% for the control group and the 6-month GC discontinuation rate p1 at 30% for the experimental group 1 and 2, with α = 0.05 and power = 0.9. Considering the 20% loss rate, 50 cases in each group in the first stage and 27 cases in the second stage were enrolled if the first stage was effective, making a total of 77 cases in each group and 231 cases in the three groups.
Recruitment
Subject recruitment information was released from the following channels: The Rheumatology Clinic and ward of Yunnan Provincial Hospital of Traditional Chinese Medicine, the hospital bulletin board, the hospital’s official website, and the patient’s WeChat group. Volunteers who wish to participate in the study can contact the facilitators or the rheumatologist to obtain information about participating in the study.
Visits
As this is an exploratory study, in view of patient safety and efficacy, the study arranged monthly face-to-face follow-up visits at the study center for a total of 7 follow-up visits. The visit timing and contents are listed in Table 2 according to SPIRIT statement. Subjects in the follow-up visits are left with the contact information of the investigator to facilitate timely communication. In addition, the facilitator would follow up by telephone every 2–3 weeks to ask about the recent status of the disease and medication taking and to remind the patient of follow-up visits. When the patient is found to have poor disease control or adverse reactions, the facilitator, Investigator Team members, and the patient would communicate promptly to resolve the situation and increase follow-up visits if necessary.
 |
Table 2 The Timing and Contents of Follow-Up in the Study |
Additional face-to-face follow-up is required when the following conditions are present during follow-ups:
- Patients with progressively increasing fatigue, edema, rash, nausea, melena, abdominal pain, and other significant discomforts within 7 days require an immediate visit of the subject to the research center.
- When the patient’s condition is poorly controlled and does not respond adequately to treatment (Table 1).
Subjects may be discontinued from the study if any of the following occurs during follow-ups:
- For patients with serious adverse events, the clinical trial should be stopped according to the physician’s judgment.
- If the condition worsens during the course of the disease, or other conditions occur during the trial and affect the observation of the trial (such as acute cardiovascular or cerebrovascular events), the clinical trial should be stopped according to the judgment of the investigator and treated as an invalid case.
- Serious deviations occurred in the implementation of the clinical trials protocol, such as poor adherence and difficulty in evaluating drug effects.
Of course, Subjects can request to withdraw from this study without any reason during the course of this trial, which will not affect the health services of subsequent patients.
Harms
At each visit, clinical assessments and laboratory tests were used to evaluate adverse events or side effects. Evaluators are responsible for determining the link between the trial medications and the aforementioned occurrences. Any symptom, physical sign, disease, or injury that occurs after taking trial medications may be considered an adverse event. Serious adverse events (SAEs) are defined as events which can cause hospitalization, loss of ability to work, disability, congenital deformity or death. Any SAEs must be informed to the study leader, and participants with SAEs must be in emergency treatment. Besides, a SAEs report form should be submitted to the Ethics Committee and the Data and Safety Monitoring Committee within 24 hours.
Data Collection
The investigator followed the investigator’s manual to complete data collection and preservation. In addition, participants who had withdrawn from our project ought to be contacted for follow-up data. And in our follow-up, we need to collect the following data:
General information: patient’s name and their phonetic initials, outpatient or hospitalization status, date of visit, telephone number, and home address.
Demographic characteristics: gender, age, marriage, ethnicity, education level, living environment, allergy history, tobacco and alcohol use history, medical history and family history, disease duration, body measurements (height, weight, waist circumference, and vital signs), and some laboratory testing (rheumatoid factor and anti-cyclic citrullinated peptide antibodies (CCP)).
Safety indicators: (1) Blood count tests (white blood cells, hemoglobin, platelets), liver function (alanine aminotransferase, aspartate aminotransferase), kidney function (blood urea nitrogen, serum creatinine), blood sugar, serum lipid profiles (total cholesterol, phospholipids, total lipid), serum electrolytes (sodium, potassium, chloride), electrocardiogram, urine test, stool routine, fecal occult blood test (OB), etc. (2) Any symptoms or physical signs related to the trial drug during follow-up should be recorded.
Outcome measures: glucocorticoid usage status (yes/no still suing), glucocorticoid dosage, recurrence of disease flare, TCM syndrome improvement score (Additional File 2), swollen joint counts (SJC, 28 joint), tender joint counts (TJC, 28 joint), the 10 cm pain visual analogue scale (VAS), patient’s global assessment (PGA), evaluator’s global assessment (EGA), erythrocyte sedimentation rate (ESR), C reactive protein (CRP), and the morning cortisol and adrenocorticotropic hormone (ACTH). Here, recurrence of disease flare was defined as those who experienced disease flares during glucocorticoid tapering or within the observation period (24 weeks), and the judgment criteria were clear: (1) increase of DAS28 beyond 0.60; (2) GC reinitiation; (3) adding or increment of any csDMARDs, biological DMARDs or targeted synthetic DMARDs; (4) rheumatologist’s judgement.
Other indicators: The Health Assessment Questionnaire (HAQ), lymphocyte subsets, cytokines (IL17, IL-1β, IL-4, IL-6, TNF-A), and intestinal flora profile.
Outcomes
Primary Outcome Measures
The primary outcome measures for the study were cumulative GC discontinuation rate, cumulative GC dose, and cumulative GC dose duration, obtained by collecting GC doses at baseline and throughout the follow-up period.
Secondary Outcome Measures
Secondary outcome measures for the study included DAS28, ACR20, SDAI, CDAI, relapse rate, and incidence of adverse events. Among them, DAS28, SDAI, and CDAI were defined as:18
ACR20 is defined as ≥ 20% improvement in tender AND swollen joint count AND ≥ 3 of the following parameters:
- Patient assessment of pain.
- Patient assessment of global disease activity.
- Physician assessment of global disease activity.
- Patient assessment of physical function, eg, PGA, VAS.
- Acute stage reactants (ESR or CRP).
New indicators explored are lymphocyte subsets, cytokines, and gut microbiome profiles (alpha diversity, beta diversity, species variation, etc.).
Human Specimen’s Procedure
In this study, the subjects came to the study center in the morning (7:30–10:30 BST) on an empty stomach for venous blood collection and were immediately sent to the Clinical Laboratory of Yunnan Provincial Hospital of Traditional Chinese Medicine for a blood routine test, liver function, renal function, blood glucose, blood lipids, electrolytes, cortisol and ACTH tests. The remaining blood was separated into serum, plasma and mononuclear cells and then frozen at −80°C for subsequent scientific studies. Urine was collected for routine urinalysis. Fecal samples were collected regarding the Human Microbiome Project (HMP) fecal collection protocol19 and were usually collected from subjects before and after early morning blood collections. If the subject was having an enema, taking an oil-based laxative, or taking antibiotics, the stool sample collection was deferred to the next follow-up visit. The collected samples were stored immediately (within half an hour) at −80°C for gut microbiome studies (collected samples can be transferred to a −80°C freezer with an ice box within 8 hours for short-distance transport, or on dry ice for long-distance transport).
Data Monitoring and Management
Data Quality Control
The clinical trial is overseen by the project leader, while the project quality controller ensures the quality of the whole trial, adhering to the standards of Good Clinical Practice (GCP). Before the initiation, comprehensive investigator training was conducted, encompassing a range of topics including the study process, protocol implementation, informed consent procedures, specific operations of clinical observation indices, standardized methods for clinical sign examinations, filling out study medical records and CRF forms, observation and reporting of adverse events, identification of TCM syndromes, and consistency of symptom efficacy, etc. This training was meticulously conducted in accordance with the standard operating procedure (SOP) for investigator training, ensuring the accuracy of data sources. Throughout the trial, the controller conducted a minimum of three on-site spot checks of the CRFs and related data.
The source documents of this clinical study include study medical records, informed consent forms, physical and chemical examination reports, drug management documents, and quality inspection documents. Any observation and examination results in the study should be recorded in the source documents timely, accurately, completely, standardized, and truthfully. Modification of source documents and source data must be well documented, otherwise, the first judgment (documentation) prevails. All case report forms have appropriate signatures and need to be properly stored for traceability after completion of entry and verification.
Data Storage and Processing
During the follow-up, data were entered into the TCM evidence-based project management system (http://oa.yn-tcm-hospital.com:19100/cd/h5weblogin.jsp), which is connected to the medical hospital information system (HIS) of the sponsor of the project and can effectively extract the physical and chemical examination data of the subjects. The investigator only needs to enter general information, physical examination data, GC dosage, recurrence, TCM syndrome score, and other efficacy evaluation data and adverse events. The data should be entered and verified within one month after the follow-up, and the verification should be done by two persons to ensure the data are correct and complete. The database will be locked after the last CRF entry is completed and reviewed for errors.
Statistical Analysis Plan
Statistical analysis was preceded by data cleaning and discussion between the statistician and the principal investigator to determine if the case was valid and to exclude the case if the following criteria were met:
- Cases found after inclusion that did not meet the inclusion criteria.
- Cases that have not been medicated since inclusion.
- Cases without any data before and after the trial.
The baseline comparability of cases between groups was then analyzed to compare sex, age, marriage, disease duration, GC medication history, cortisol, ACTH, and CCP to explore the confounding factors affecting the results in this study. Finally, the effect of confounding factors on the study results was adjusted by multiple regression analysis, propensity score or stratified analysis. The analysis and comparison between study groups were made on the full analysis set and per-protocol set, respectively. GC discontinuation and safety were analyzed at the end of the first stage of the study to determine whether to proceed to the second stage of the study.
Survival analysis was used to compare the differences between groups and time points in cumulative GC discontinuation rate, cumulative GC dose, and cumulative GC dose duration; and the Cox proportional hazards regression was used to correct for confounders in it. Furthermore, sensitivity analyses were also performed using stratified analyses, generalized estimating equations, and corrected mean difference methods to test the robustness of the results. The safety analysis was performed by the chi-square test. Paired t-test, paired rank sum test, repeated measures ANOVA, and multiple regression models were used for comparisons of between-group efficacy (DAS28). Logistic regression models, nomograms, receiver operator characteristic (ROC) curves, calibrate analysis, net benefit and decision curve analysis (DCA), etc. were used for exploratory analysis of risk factors for RA GC discontinuation failure and relapse.
According to whether the measurement data are normally distributed or not, the statistical description method: the mean ± standard deviation is used for normal distribution, otherwise, it is expressed as median and quartile. The counting data are expressed as frequencies and percentages. All statistical tests were performed using two-sided tests, and P < 0.05 would be considered statistically significant. Statistical analysis was performed using SPSS 26.0, R4.2.2. Graphing was drawn using GraphPad prism v.8.0 and R4.2.2.
Ethics Approval and Consent to Participate
Although the study is exploratory, no serious adverse reactions have been observed in the clinical use of the test drug, and the two-stage design can greatly reduce the risk of ineffective drug exposure. To ensure that this clinical study followed the Declaration of Helsinki and the relevant Chinese regulations on clinical research studies, the ethical review and filing of this research project were conducted by the Ethics Committee of Yunnan Provincial Hospital of Traditional Chinese Medicine (National Drug Clinical Trial Body Certification in China) before the start of the study (YLZ [2022] Ethical Review No. (006)-01). Before the start of the study, the investigator fully explained the study objectives, observation indicators, intervention methods, tests and assessments performed, possible risks, disposal of biological samples, publication of study results to the subjects, and participation in the clinical study was permitted only after written signed consent was obtained from each subject/legal representative to ensure the patient’s right to informed consent. In the event of a serious adverse event during the study, the investigator will provide reasonable emergency treatment, and the hospital ethics committee will convene a timely meeting to review and inform the project leader of the review findings. There will be no additional financial burden on the patients in the study and no financial compensation for the subjects will be involved. Subjects may request discontinuation at any time without any reason and this will not affect subsequent treatments and services for the subject.
Consent for Publication
The consent form signed by participants includes their consent for publication. In addition, the data collected will be used for academic research only. In general, personal information and raw data will be kept strictly confidential, and the subjects’ privacy will not be disclosed in the publication of research results or in the reporting of academic conferences. If raw data must be shared, the subjects’ personal information will be anonymized before dissemination.
Discussion
Evidence-based clinical practice is a major advancement in the history of medical development and is also receiving increasing attention and recognition in today’s health services. The innovative development of TCM heritage requires us to translate from experience to evidence, thus improving clinical care. GC are a double-edged sword that plays an irreplaceable role in the treatment of rheumatologic diseases, but the effective discontinuation of GC is also a great challenge for all rheumatologists. Therefore, it is a pressing issue to find an effective GC discontinuation regimen.
This study is the first to develop an aggressive GC bridging therapy and discontinuation regimen for RA based on the accumulated evidence and an exploratory study using a two-stage non-randomized controlled trial. The protocol may have important reference value for TCM modernization research and clinical practice of GC discontinuation in RA, and the innovative and practical significance of the study cannot be overstated. However, this study also has certain limitations: (1) the study is a non-randomized controlled trial, not a confirmatory trial design, and may produce selection bias. Therefore, a multi-center large-sample randomized controlled clinical trial or a real-world study is required to validate the results in the future. (2) although the study results were corrected for confounding factors using statistical methods, potential unknown factors may still exist.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work is supported by grants from National Natural Science Foundation of China (81960863, 82160901, 82360924), the Construction Project of Yunnan Provincial Fund for Medical Research Center (202102AA310006), the Construction Project of National Traditional Chinese Medicine Clinical Research Base (2018 No. 131), the Construction Project for the focal branch of National Traditional Chinese Medicine (2023 No. 85), the Expert Workstation of zhangxuan in Yunnan Province (202305AF150175), the Yunnan Applied Basic Research Projects (202101AT070230), the Funding of Yunnan Applied Basic Research Projects-Union Foundation (2019FF002(−082), 2019FF002(−031), 202101AZ070001-072), Construction Project Funding of University Key Laboratories in Yunnan Province (2018TGZ01), and the Funding of Yunnan Provincial Health Science and Technology Plan (2018NS0045, 2018NS0046).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Hardy R, Cooper MS. Unravelling how glucocorticoids work in rheumatoid arthritis. Nat Rev Rheumatol. 2018;14(10):566–567. doi:10.1038/s41584-018-0079-4
2. Chinese Rheumatology Association. 2018 Chinese guideline for the diagnosis and treatment of rheumatoid arthritis. Zhonghua Nei Ke Za Zhi. 2018;57(4):242–251. doi:10.3760/cma.j.issn.0578-1426.2018.04.004
3. Smolen JS, Landewe RBM, Bijlsma JWJ, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheumatic Dis. 2020;79(6):685–699. doi:10.1136/annrheumdis-2019-216655
4. Burmester GR, Buttgereit F, Bernasconi C, et al. Continuing versus tapering glucocorticoids after achievement of low disease activity or remission in rheumatoid arthritis (SEMIRA): a double-blind, multicentre, randomised controlled trial. Lancet. 2020;396(10246):267–276. doi:10.1016/S0140-6736(20)30636-X
5. Singh JA, Saag KG, Bridges SL, et al. 2015 American college of rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2016;68(1):1–26. doi:10.1002/art.39480
6. Roubille C, Coffy A, Rincheval N, et al. Ten-year analysis of the risk of severe outcomes related to low-dose glucocorticoids in early rheumatoid arthritis. Rheumatology. 2021;60(8):3738–3746. doi:10.1093/rheumatology/keaa850
7. Fraenkel L, Bathon JM, England BR, et al. 2021 American college of rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2021;73(7):1108–1123. doi:10.1002/art.41752
8. van Ouwerkerk L, Palmowski A, Nevins IS, et al. Systematic literature review of observational cohorts and clinical trials into the success rate of glucocorticoid discontinuation after their use as bridging therapy in patients with rheumatoid arthritis. Ann Rheumatic Dis. 2022;81(7):937–943. doi:10.1136/annrheumdis-2022-222338
9. Xie W, Huang H, Li G, et al. Dynamical trajectory of glucocorticoids tapering and discontinuation in patients with rheumatoid arthritis commencing glucocorticoids with csDMARDs: a real-world data from 2009 to 2020. Ann Rheumatic Dis. 2021;80(8):997–1003. doi:10.1136/annrheumdis-2021-220112
10. Zhang D, Lyu JT, Zhang B, Zhang XM, Jiang H, Lin ZJ. Comparative efficacy, safety and cost of oral Chinese patent medicines for rheumatoid arthritis: a Bayesian network meta-analysis. BMC Complement Med Therap. 2020;20(1):210. doi:10.1186/s12906-020-03004-4
11. Wang Y, Han M, Pedigo CE, Xie ZM, Wang WJ, Liu JP. Chinese herbal medicine for systemic lupus erythematosus: a systematic review and meta-analysis of randomized, placebo-controlled trials. Chin J Integr Med. 2021;27(10):778–787. doi:10.1007/s11655-021-3497-0
12. Zhu H, Zhang L, Qian M, et al. Microgravity versus microgravity and irradiation: investigating the change of neuroendocrine-immune system and the antagonistic effect of traditional Chinese medicine formula. Biomed Res Int. 2020;2020:2641324. doi:10.1155/2020/2641324
13. Huang J, Li J, Zheng S, et al. Epimedium flavonoids counteract the side effects of glucocorticoids on hypothalamic-pituitary-adrenal axis. Evid Based Complement Alternat Med. 2013;2013:938425. doi:10.1155/2013/938425
14. Qin F, Wu J, Chen F, et al. Optimal, minimax and admissible two-stage design for phase II oncology clinical trials. BMC Med. Res. Method. 2020;20(1):126. doi:10.1186/s12874-020-01017-8
15. van Ouwerkerk L, Boers M, Emery P, et al. Individual patient data meta-analysis on continued use of glucocorticoids after their initiation as bridging therapy in patients with rheumatoid arthritis. Ann Rheumatic Dis. 2022;82(4):468–475. doi:10.1136/ard-2022-223443
16. Cutolo M, Sulli A, Pincus T. Circadian use of glucocorticoids in rheumatoid arthritis. Neuroimmunomodulation. 2015;22(1–2):33–39. doi:10.1159/000362733
17. Alves C, Robazzi TC, Mendonca M. Withdrawal from glucocorticosteroid therapy: clinical practice recommendations. J Paediatr. 2008;84(3):192–202. doi:10.2223/JPED.1773
18. Smolen JS, Breedveld FC, Eberl G, et al. Validity and reliability of the twenty-eight-joint count for the assessment of rheumatoid arthritis activity. Arthritis Rheum. 1995;38(1):38–43. doi:10.1002/art.1780380106
19. Qian XB, Chen T, Xu, YP et al. A guide to human microbiome research: study design, sample collection, and bioinformatics analysis. Chin Med J. 2020;133:1844–1855— doi:10.1097/CM9.0000000000000871.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.