Back to Journals » Infection and Drug Resistance » Volume 16
Clostridium perfringens Liver Abscess Disguised as Biliary Disease: A Report of Two Cases and a Review of the Literature
Authors Peng J, Zhai Q, Li J , Chen X, Wu H, Zhong T, Tang G, Yu D, He L, Li J
Received 18 April 2023
Accepted for publication 26 July 2023
Published 11 August 2023 Volume 2023:16 Pages 5209—5222
DOI https://doi.org/10.2147/IDR.S415347
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Jialun Peng,1,* Qilong Zhai,1,* Jinzheng Li,1 Xingyu Chen,1 Hongyu Wu,1 Tao Zhong,1 Gangyi Tang,2 Dajun Yu,2 Lixian He,2 Jinxu Li2
1Department of Hepatobiliary Surgery, The Second Affiliated Hospital of Chongqing Medical University, Chongqing, 400010, People’s Republic of China; 2Department of General Surgery, Wushan County People’s Hospital of Chongqing, Chongqing, 404700, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jinzheng Li; Gangyi Tang, Tel +86 138 9600 6584 ; +86 159 2387 3984, Fax +86 023 6369 3000, Email [email protected]; [email protected]
Abstract: Liver abscesses caused by Clostridium perfringens are rare but rapidly fatal. In only a few days, patients progress from liver abscess to sepsis, intravascular hemolysis, multiple organ failure, and even death. These abscesses often occur in patients after trauma or surgery or in those with immunodeficiency. Because patients only show non-specific symptoms such as fever and abdominal pain in the early stage, they can easily be misdiagnosed and miss the therapeutic window, resulting in a poor prognosis. The diagnosis of Clostridium perfringens liver abscess mainly depends on computed tomography (CT), needle aspiration, and/or blood culture. After diagnosis, treatments such as antibiotic therapy, surgical abscess drainage, blood transfusion as needed, and correction of metabolic disturbances must be immediately administered to prevent severe complications. Here, we present two cases of liver abscess due to Clostridium perfringens infection. Both patients initially presented only with fever, abdominal pain, and jaundice, symptoms that were easily confused with cholangitis caused by cholelithiasis. The patients then progressed rapidly and, despite receiving antimicrobial and multimodal sepsis treatment, both eventually died of multiple organ dysfunction syndrome. Clinicians should be on high alert for Clostridium perfringens liver abscesses disguised as biliary disease. Early diagnosis and treatment with the appropriate antibiotics and surgery are fundamental for the survival of the affected patients.
Keywords: intravascular hemolysis, biliary diseases, diagnosis, treatment
Introduction
Clostridium perfringens is an anaerobic, Gram-positive bacterium normally found in the gastrointestinal and genital tracts of humans.1,2 This bacterium grows rapidly, showing a doubling time of approximately 7 min, and can tolerate up to 3% oxygen.3 Its virulence mainly depends on the production of more than 20 types of potent toxins, including α-toxin, β-toxin, and Clostridium perfringens enterotoxin (CPE). High levels of these toxins in the blood circulation result in the rapid deterioration of the condition of affected patients, including progression to organ and circulatory failure, and even death.4 Clostridium perfringens is classified into seven categories (A–G) according to the types of toxins produced.5 It is an opportunistic pathogen that can cause a variety of diseases, including food poisoning, emphysematous cholecystitis, and liver abscess.6 Infection with Clostridium perfringens has a wide range of clinical manifestations, from asymptomatic bacteremia, to shock, and death.6–8 However, liver abscesses caused by Clostridium perfringens are very rare, especially as a result of biliary tract infection, but are rapidly fatal and are associated with a high mortality rate. It often takes only a few days for patients to progress from liver abscess to sepsis, intravascular hemolysis, Multiple Organ Dysfunction Syndrome (MODS), and death. Patients with Clostridium perfringens liver abscess need to be promptly identified, given that early and accurate diagnosis, followed by specific treatment, can prevent death. Here, we present two cases of Clostridium perfringens liver abscess. Both patients initially presented only with fever, abdominal pain, and jaundice, symptoms that were easily confused with cholangitis caused by cholelithiasis.
Case Report
Case 1
Case 1 was a 59-year-old male presenting with right upper abdominal pain, chills, diarrhea, and jaundice who was referred to our hospital. Right upper abdominal pain started four days before admission. The pain was sharp, repeated, non-radiating, unrelated to food, and resolved on its own. He denied nausea and vomiting. Gradually, the patient developed chills, jaundice, and diarrhea, and the abdominal pain was relieved after defecation. He was subsequently treated at a local hospital and underwent a computed tomography (CT) scan of the abdomen. CT showed a hypodense shadow consistent with entrapped air in the right liver lobe and an inflamed gallbladder with gallbladder stones. The patient denied diabetes mellitus, coronary heart disease, and hypertension. On physical examination, his heart rate was 109 beats per min, his blood pressure was 80/69 mmHg, and he had scleral and cutaneous icterus. Abdominal examination showed upper abdominal pressure, rebound pain, and muscle tension. Initial laboratory data showed hemoglobin 97 g/L (normal range: 130–175 g/L), a white blood cell count of 41.17×109/L (3.5–9.5×109/L), total bilirubin 529 µmol/L (5.1–28.0 µmol/L), direct bilirubin 271.5 µmol/L (0.0–10.0 µmol/L), aspartate transaminase 1869 U/L (15–40 U/L), alanine transaminase 813 U/L (9–50 U/L), γ-GGT 395 U/L (10–60 U/L), alkaline phosphatase 244 U/L (45–125 U/L), and a glomerular filtration rate of 19.6 mL/min (80.0–300.0 mL/min). We performed an esophagogastroduodenoscopy, which included an endoscopic retrograde cholangiography, and observed purulent secretion from the common bile duct (Figure 1). Secretion culture was positive for Clostridium perfringens. The patient was admitted to the intensive care unit for further treatment after surgery and gradually developed sepsis with hemolysis, hepatic insufficiency, and renal failure. The relevant clinical indicators are listed in Figure 2. Abdominal ultrasound and CT detected a 9×3.5 cm abscess with entrapped air in liver segment VI and a 4×3.5 cm abscess with entrapped air in liver segment VII (Figure 3). CT was repeated after puncture and drainage of the patient’s two liver abscesses (Figure 4). The patient received antibiotics (mainly penicillin), blood product transfusion, plasma exchange, an artificial liver, and hemodialysis but eventually died 15 days after admission to the hospital.
 |
Figure 1 Endoscopic retrograde cholangio-pancreatography (ERCP) image. The common bile duct shows purulent secretions (marked by red arrow). |
 |
Figure 3 Computer tomography of the abdomen. Two areas of the right hepatic lobe have liver abscesses and both contain air (marked by red arrows). |
 |
Figure 4 Computed tomography of the abdomen after drainage of liver abscess. Liver abscesses in both areas of the right hepatic lobe have shrunk (marked by red arrows). |
Case 2
Case 2 was a 62-year-old male admitted to the hospital with abdominal pain, fever, chills, vomiting, and diarrhea after eating, all of which occurred acutely within half a day. The patient presented with dull, recurrent epigastralgia and vomiting after eating. At that time, he was febrile (axillary temperature: 39.8 °C) but had a normal level of consciousness. The vomit was stomach contents, and the stool was yellow watery material. The patient had no relief of symptoms after taking non-steroidal anti-inflammatory drugs. His medical history included diabetes mellitus, hypertension, and hyperlipidemia. The initial vital signs were axillary temperature 38.6 °C, pulse 82 beats per min, blood pressure 119/70 mmHg, and oxygen saturation 95% in ambient air. Physical examination revealed epigastric pain, rebound pain, and muscle tension in the right upper abdomen. Laboratory findings showed hemoglobin 94 g/L (normal range: 130–175 g/L), a white blood cell count of 10.6×109/L (3.5–9.5×109/L), total bilirubin 281.6 µmol/L (3.42–20.5 µmol/L), direct bilirubin 233.9 µmol/L (0.0–6.84 µmol/L), aspartate transaminase 782.2 U/L (15–40 U/L), alanine transaminase 324.7 U/L (9–50 U/L), γ-GGT 453.7 U/L (10–60 U/L), alkaline phosphatase 124.2 U/L (45–125 U/L), and a glomerular filtration rate of 84 mL/min (>90 mL/min). CT of the abdomen revealed a gas-filled area in the seventh liver segment with perihepatic fluid exudate (Figure 5). Abdominal X-ray showed subdiaphragmatic free gas (Figure 6). We performed emergency surgery, which showed an enlarged gallbladder with edema, a thickened common bile duct (1.2 cm), and hemorrhagic exudation around the pancreas. We then removed the gallbladder and incised the common bile duct for drainage. After the incision of the gallbladder and bile duct, we observed sediment-like stones and placed a drainage tube around the pancreas for drainage. The patient was admitted to the intensive care unit for further treatment after surgery. A few days after undergoing the procedure, he appeared severely jaundiced and presented with deterioration in hemoglobin levels and renal function as well as anemia. The relevant clinical indicators are listed in Figure 7. Blood cultures grew Clostridium perfringens and Klebsiella. A repeat CT scan of the abdomen after the procedure showed a gas-forming abscess in segment VII of the liver and subdiaphragmatic free gas. We performed a combination of anti-infective treatments including penicillin, metronidazole, and other antibiotics, plasma exchange, CRRT, and blood transfusion. The patient eventually died of sepsis combined with MODS.
 |
Figure 5 Computer tomography of the abdomen. The right lobe of the liver shows an inflated area (marked by a red arrow) and is accompanied by perihepatic fluid exudate. |
 |
Figure 6 Plain film of the abdomen. There is a crescent-shaped free gas formation under the diaphragm (marked with a red arrow). |
Discussion
Clostridium perfringens was first discovered in 1891 by William H. Welch following an autopsy on a male.9 This bacterium is ubiquitous and is also normally found in the gastrointestinal and genital tracts of humans. Clostridium perfringens can produce more than 20 toxins,4 and is categorized into seven toxinotypes (A–G) according to which combinations of 6 of these toxins (α-toxin, β-toxin, ε-toxin, ι-toxin, CPE, and necrotic enteritis B-like toxin) is produced.5 However, to date, only toxinotype A has been associated with human cases of hemolysis.10 These toxins are usually disease-specific. For example, α-toxin is involved in human clostridial myonecrosis or gas gangrene,11 CPE causes food poisoning in humans,12 and β-toxin, ε-toxin, and Clostridium perfringens necrotic enteritis B-like toxin primarily mediate infection in animals.5 In humans, these toxins, especially α-toxin, can enter the blood circulation and cause toxemia, resulting in circulatory failure, organ failure, and death.13
Clostridium perfringens infections are common in immunocompromised patients and after trauma or surgery. Indeed, invasive hepatobiliary and gastrointestinal surgery, gynecological surgery, catheter placement, and other traumatic operations can easily lead to Clostridium perfringens infection. Advanced age, type II diabetes, and malignant tumors are also risk factors for infection.2 However, it has been reported that Clostridium perfringens can cause septicemia through bacterial translocation even in the absence of any clear potential risk factors.14 Meanwhile, Clostridium perfringens-induced sepsis is associated with a high mortality rate, ranging from 70% to 100%, and patients usually experience rapid deterioration.15 This happened in Case 1 in our report, where the patient was infected with Clostridium perfringens in the absence of chronic disease, and eventually died of sepsis.
Liver abscesses can be categorized into different types depending on the etiology, with amebic liver abscesses (ALAs) and pyogenic liver abscesses (PLAs) representing the major types.16 ALAs are more common in developing countries and are caused by Entamoeba.17 In contrast, PLAs constitute the bulk of liver abscesses in developed countries and are usually caused by Escherichia coli, Klebsiella, and Streptococcus,16 with the bacteria mainly spreading through the portal vein or hepatic artery.1 In most of Asia, the pathogens most commonly associated with PLA are Klebsiella pneumoniae serotypes K1 and K2.18 Cases of PLA due to Clostridium perfringens infection are very rare, albeit rapidly fatal.15 At first, patients only show non-specific symptoms such as fever and abdominal pain, or jaundice and right upper abdominal pain, which can be easily misdiagnosed as biliary diseases. The PLA subsequently develops into a gas-forming pyogenic liver abscess (GPLA) within a few days, and the condition of patients rapidly deteriorates, often complicated with septicemia, massive intravascular hemolysis, and renal failure, eventually progressing to MODS or even death.1
The total mortality rate for liver abscesses ranges from 6% to 14%.19 However, liver abscesses caused by Clostridium perfringens infection are markedly more deadly than other types of liver abscesses due to their early and rapid undetectable progression. We searched PubMed and identified 60 cases of Clostridium perfringens liver abscess (Table 1). A detailed search strategy is provided in the Supplementary Material. Among the 60 cases reviewed in the literature, the mortality rate was 60% (36/60), with 65% of cases (39/60) experiencing intravascular hemolysis and 21.7% (13/60) MODS. Among the 36 patients who died, only one patient survived for 48 days after early surgery and multidisciplinary support treatment, while the average survival time for the remaining 35 patients was only 27.6 h (0.8–168 h). Notably, of the 35 patients who died, 71.4% (25/35) received no treatment or received only a single treatment (antibiotics or abscess drainage), and the average survival time for these patients was only 12.6 h (1.5–48 h). The remaining 10 patients received two or more treatments, including antibiotics, abscess drainage, and surgery, and the average survival time was 64.9 h (2.5–168 h), substantially longer than that for patients who received only a single treatment. This observation emphasizes the importance of combined treatment, especially the combined use of antibiotics and abscess drainage, which can significantly improve the prognosis of patients and increase their average survival time. The most common underlying disease was diabetes (23/60), followed by an underlying malignancy (17/60), and both were seen in elderly patients. This suggests that immunocompromised populations are susceptible to infection by Clostridium perfringens, particularly so elderly patients with underlying disease, and reminds clinicians that they should be highly vigilant for Clostridium perfringens infection in such populations. Interestingly, 12 patients were found to have no clear underlying medical disease, like the patient in Case 1 in this report.
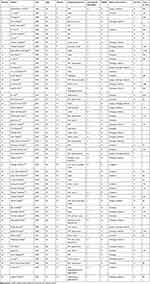 |
Table 1 Literature Overview of Human Cases with Clostridium perfringens Liver Abscess |
In the early stage, it is critical to accurately diagnose patients with Clostridium perfringens liver abscess, thereby significantly improving their prognosis. First, infection by Clostridium perfringens necessitates high vigilance for all types of trauma, epigastric pain, vomiting, nausea, and disturbance of consciousness after surgery,73 especially biliary and gastrointestinal surgery. If a patient’s condition progresses, GPLA can be diagnosed by abdominal X-ray, ultrasound, and CT. After diagnosis, needle aspiration and/or blood culture of the liver abscess should be performed immediately to determine the types of bacteria causing the infection14 and provide patients with the correct treatment. In the laboratory, the identification of Clostridium perfringens is divided into two steps. Purified isolates are preliminary identified based on their culture and morphological features, motility testing, double hemolysis on blood agar, and the reverse Christie–Atkins–Munch–Petersen test.74 Subsequently, the isolates are confirmed using biochemical tests, such as nitrite reduction, lactose fermentation, and lecithinase production assays.75 In addition, the disk diffusion method should be used to determine the sensitivity of Clostridium perfringens isolates to a variety of antibiotics, which is important for guiding the use of appropriate antibiotics in subsequent treatment.76 In the future, greater availability of rapid polymerase chain reaction-based testing should assist with diagnosis.77
If there is sufficient clinical evidence to suspect/diagnose a liver abscess caused by Clostridium perfringens, early and specific treatment must be administered immediately to prevent the death of patients. Specific therapy includes antibiotic treatment and surgical abscess drainage, while other treatments involve correcting metabolic disorders and performing blood transfusion as needed.57 First, the immediate use of antibiotics can significantly improve patient survival.34,49 Simon et al78 reported that the combination of penicillin and clindamycin was the most effective regimen for the treatment of Clostridium perfringens infection. Clindamycin can inhibit the activity of α-toxin secreted by Clostridium perfringens in the early stage, which greatly reduces the risk of fatal toxemia in patients.79 Secondly, timely drainage is essential for suspected Clostridium perfringens liver abscesses. Patients who underwent surgical excision and drainage of the infected lesion not only had a significantly higher survival rate but also had a significantly longer mean time to death compared with those who received conservative treatment.78 This indicates that puncture and drainage, continuous irrigation, and anti-infective treatment must be performed immediately after abscess formation. When surgical debridement is difficult, hyperbaric oxygen therapy (HBOT) may be a viable adjunct therapeutic option because it can reduce toxin production and disrupt the anaerobic environment required for bacterial growth.2 The use of HBOT in acute necrotizing infections may save the lives of patients. In addition, adjuvant hemodialysis therapy, which is effective against infectious shock, may be used to treat patients with Clostridium perfringens liver abscesses in the future.80,81
Here, we report two cases of Clostridium perfringens liver abscess, one with postprandial infection and many underlying diseases, and the other with unknown etiology and no underlying diseases. However, although the basic conditions of the patients were different, the initial clinical manifestations were similar to biliary diseases, and rapidly progressed to GPLA. Both patients were diagnosed with Clostridium perfringens infection and received multidisciplinary treatment; however, neither of them survived. This highlights the importance of early identification of Clostridium perfringens infection by clinicians, especially in patients with atypical clinical manifestations and without any underlying diseases, given that the infection progresses rapidly and is highly fatal. Once patients progress to GPLA or sepsis before treatment, the best window for treatment has often already been missed, resulting in a poor prognosis. Therefore, it is strongly suggested that clinicians should be more alert to Clostridium perfringens infection and that the strains infecting suspected cases are identified as soon as possible, so that specific treatment can be administered promptly, thereby improving patient prognosis.
This study had several limitations. The 60 cases reviewed were all from PubMed and were all open access. Accordingly, studies with non-open access were not included, which may have resulted in the exclusion of cases similar to those of the two patients presented here. In addition, although all 60 cases reviewed in this study referred to patients presenting with Clostridium perfringens liver abscess, the specific etiology differed among patients, and was mainly seen in postoperative infection, such as pancreatic resection,29 pancreaticoduodenectomy,68 and cholecystectomy.36 At the same time, there were also cases of post-eating infection that initially presented as biliary disease, similar to that seen in Case 2 in this study, but these cases were less common.51 In the future, we encourage the publication of cases of Clostridium perfringens liver abscess, as well as their collection and summation according to etiology, to propose better diagnostic and therapeutic protocols, and thus improve patient survival.
Conclusion
Liver abscesses caused by Clostridium perfringens infection can rapidly progress to sepsis, diffuse intravascular coagulation, MODS, and even death. The two cases presented here initially presented only with abdominal pain, fever, and jaundice, symptoms that were easily confused with cholangitis caused by cholelithiasis. Owing to the rapid deterioration of the condition of the patients, the window for antibiotic therapy and/or surgical treatment was minimal.15 Clinicians should be alert to patients with initial clinical manifestations similar to biliary diseases. In particular, when a patient’s condition rapidly progresses to GPLA, Clostridium perfringens infection should be highly suspected. Early accurate diagnosis and treatment with the appropriate antibiotics and surgery are fundamental for the survival of affected patients.
Ethics Statement
The study involving a human participant was reviewed and approved by the Ethics committee of Chongqing Medical University. The families of both patients provided written informed consent to participate in this study. Written informed consent was obtained from the families of both patients for the publication of any potentially identifiable images or data included herein.
Acknowledgments
We would like to thank patients of this case report. They gave us permission to write down their disease course and they were very helpful in sharing their patient history.
Funding
The work was supported by the Joint project of Chongqing Health Commission and Science and Technology Bureau (NO:2022GDRC004), Kuanren Talents Program of the second affiliated hospital of Chongqing Medical University.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Guo J, Li C, Gao X. Liver abscess after microwave ablation of hepatocellular carcinoma caused by Clostridium perfringens. J Infect Dev Ctries. 2022;16(1):222–225. doi:10.3855/jidc.13756
2. Law ST, Lee MK. A middle-aged lady with a pyogenic liver abscess caused by Clostridium perfringens. World J Hepatol. 2012;4(8):252–255. doi:10.4254/wjh.v4.i8.252
3. Rives C, Chaudhari D, Swenson J, Reddy C, Young M. Clostridium perfringens liver abscess complicated by bacteremia. Endoscopy. 2015;47(1). doi:10.1055/s-0034-1392867
4. Kiu R, Hall LJ. An update on the human and animal enteric pathogen Clostridium perfringens. Emerg Microbes Infect. 2018;7(1):141. doi:10.1038/s41426-018-0144-8
5. Rood JI, Adams V, Lacey J, et al. Expansion of the Clostridium perfringens toxin-based typing scheme. Anaerobe. 2018;53:5–10. doi:10.1016/j.anaerobe.2018.04.011
6. Rechner PM, Agger WA, Mruz K, Cogbill TH. Clinical features of clostridial bacteremia: a review from a rural area. Clin Infect Dis. 2001;33(3):349–353. doi:10.1086/321883
7. Kapoor JR, Monteiro B, Tanoue L, Siegel MD. Massive intravascular hemolysis and a rapidly fatal outcome. Chest. 2007;132(6):2016–2019. doi:10.1378/chest.07-0853
8. Shibazaki S, Yasumoto T, Nakaizumi T. Massive intravascular haemolysis due to Clostridium perfringens. BMJ Case Rep. 2018;2018. doi:10.1136/bcr-2017-223464
9. Liu F, Xue S, Zhang Y, et al. Clostridium perfringens sepsis in three patients with acute leukemia and review of the literature. Int J Hematol. 2021;113(4):508–517. doi:10.1007/s12185-020-03060-z
10. Woittiez NJC, van Prehn J, van Immerseel F, et al. Toxinotype A Clostridium perfringens causing septicaemia with intravascular haemolysis: two cases and review of the literature. Int J Infect Dis. 2022;115:224–228. doi:10.1016/j.ijid.2021.12.331
11. Awad MM, Bryant AE, Stevens DL, Rood JI. Virulence studies on chromosomal alpha-toxin and theta-toxin mutants constructed by allelic exchange provide genetic evidence for the essential role of alpha-toxin in Clostridium perfringens-mediated gas gangrene. Mol Microbiol. 1995;15(2):191–202. doi:10.1111/j.1365-2958.1995.tb02234.x
12. Sarker MR, Carman RJ, McClane BA. Inactivation of the gene (cpe) encoding Clostridium perfringens enterotoxin eliminates the ability of two cpe-positive C. perfringens type A human gastrointestinal disease isolates to affect rabbit ileal loops. Mol Microbiol. 1999;33(5):946–958. doi:10.1046/j.1365-2958.1999.01534.x
13. Stabler S, Titecat M, Duployez C, et al. Clinical relevance of Clostridium bacteremia: an 8-year retrospective study. Anaerobe. 2020;63:102202. doi:10.1016/j.anaerobe.2020.102202
14. Kwon YK, Cheema FA, Maneckshana BT, Rochon C, Sheiner PA. Clostridium paraputrificum septicemia and liver abscess. World J Hepatol. 2018;10(3):388–395. doi:10.4254/wjh.v10.i3.388
15. Ng H, Lam SM, Shum HP, Yan WW. Clostridium perfringens liver abscess with massive haemolysis. Hong Kong Med J. 2010;16(4):310–312.
16. Jha AK, Das A, Chowdhury F, Biswas MR, Prasad SK, Chattopadhyay S. Clinicopathological study and management of liver abscess in a tertiary care center. J Nat Sci Biol Med. 2015;6(1):71–75. doi:10.4103/0976-9668.149091
17. Pérez J. Amoebic Liver Abscess: revisited; 2006.
18. Longworth S, Han J. Pyogenic liver abscess. Clin Liver Dis. 2015;6(2):51–54. doi:10.1002/cld.487
19. Huang CJ, Pitt HA, Lipsett PA, et al. Pyogenic hepatic abscess. Changing trends over 42 years. Ann Surg. 1996;223(5):600–7; discussion 607–9. doi:10.1097/00000658-199605000-00016
20. Kivel RM, Kessler A, Cameron DJ. Liver abscess due to Clostridium perfringens. Ann Intern Med. 1958;49(3):672–679. doi:10.7326/0003-4819-49-3-672
21. Mera CL, Freedman MH. Clostridium liver abscess and massive hemolysis. Unique demise in Fanconi’s aplastic anemia. Clin Pediatr. 1984;23(2):126–127. doi:10.1177/000992288402300215
22. Nakano O, Okita M, Miura M, et al. ク ロ ス ト リジ ウ ム菌 に よ る肝 膿 瘍 の1剖 検 例 [An autopsy case of clostridial liver abscess]. Nihon Naika Gakkai Zasshi. 1988;77(5):690–694. Japanese. doi:10.2169/naika.77.690
23. Eckel F, Lersch C, Huber W, Weiss W, Berger H, Schulte-Frohlinde E. Multimicrobial sepsis including Clostridium perfringens after chemoembolization of a single liver metastasis from common bile duct cancer. Digestion. 2000;62(2–3):208–212. doi:10.1159/000007815
24. Kreidl KO, Green GR, Wren SM. Intravascular hemolysis from a Clostridium perfringens liver abscess. J Am Coll Surg. 2002;194(3):387. doi:10.1016/s1072-7515(01)01169-3
25. Au WY, Lau LS. Massive haemolysis because of Clostridium perfringens [corrected] liver abscess in a patient on peritoneal dialysis. Br J Haematol. 2005;131(1):2. doi:10.1111/j.1365-2141.2005.05634.x
26. Ohtani S, Watanabe N, Kawata M, Harada K, Himei M, Murakami K. Massive intravascular hemolysis in a patient infected by a Clostridium perfringens. Acta Med Okayama. 2006;60(6):357–360. doi:10.18926/amo/30725
27. Daly JJ, Haeusler MN, Hogan CJ, Wood EM. Massive intravascular haemolysis with T-activation and disseminated intravascular coagulation due to clostridial sepsis. Br J Haematol. 2006;134(6):553. doi:10.1111/j.1365-2141.2006.06177.x
28. Umgelter A, Wagner K, Gaa J, Stock K, Huber W, Reindl W. Pneumobilia caused by a clostridial liver abscess: rapid diagnosis by bedside sonography in the intensive care unit. J Ultrasound Med. 2007;26(9):1267–1269. doi:10.7863/jum.2007.26.9.1267
29. Tabarelli W, Bonatti H, Cejna M, Hartmann G, Stelzmueller I, Wenzl E. Clostridium perfringens liver abscess after pancreatic resection. Surg Infect. 2009;10(2):159–162. doi:10.1089/sur.2008.014
30. Alarcón Del Agua I, Flores Cortés M, Pareja Ciuró F, Puppo Moreno A, Jiménez Rodríguez R. [Spontaneous rupture of a clostridium perfringens liver abscess into the abdominal cavity]. Absceso hepático por Clostridium perfringens abierto espontáneamente a la cavidad abdominal. Cir Esp. 2009;85(3):187–189. Spanish. doi:10.1016/j.ciresp.2008.04.003
31. Macías I, Salas de Zayas R, Zoila L, Dólera C. [Intravascular hemolysis due to Clostridium perfringens in an immunocompetent patient]. Hemólisis intravascular masiva por Clostridium perfringens en paciente inmunocompetente. Enferm Infecc Microbiol Clin. 2009;27(9):548–549. Spanish. doi:10.1016/j.eimc.2008.11.008
32. Meyns E, Vermeersch N, Ilsen B, Hoste W, Delooz H, Hubloue I. Spontaneous intrahepatic gas gangrene and fatal septic shock. Acta Chir Belg. 2009;109(3):400–404. doi:10.1080/00015458.2009.11680447
33. Rajendran G, Bothma P, Brodbeck A. Intravascular haemolysis and septicaemia due to Clostridium perfringens liver abscess. Anaesth Intensive Care. 2010;38(5):942–945. doi:10.1177/0310057x1003800522
34. van Bunderen CC, Bomers MK, Wesdorp E, Peerbooms P, Veenstra J. Clostridium perfringens septicaemia with massive intravascular haemolysis: a case report and review of the literature. Neth J Med. 2010;68(9):343–346.
35. Merino A, Pereira A, Castro P. Massive intravascular haemolysis during Clostridium perfrigens sepsis of hepatic origin. Eur J Haematol. 2010;84(3):278–279. doi:10.1111/j.1600-0609.2009.01337.x
36. Qandeel H, Abudeeb H, Hammad A, Ray C, Sajid M, Mahmud S. Clostridium perfringens sepsis and liver abscess following laparoscopic cholecystectomy. J Surg Case Rep. 2012;2012(1):5. doi:10.1093/jscr/2012.1.5
37. Kim JH, Jung ES, Jeong SH, et al. A case of emphysematous hepatitis with spontaneous pneumoperitoneum in a patient with hilar cholangiocarcinoma. Korean J Hepatol. 2012;18(1):94–97. doi:10.3350/kjhep.2012.18.1.94
38. Imai J, Ichikawa H, Tobita K, Watanabe N. Liver abscess caused by Clostridium perfringens. Intern Med. 2014;53(8):917–918. doi:10.2169/internalmedicine.53.2139
39. Kurasawa M, Nishikido T, Koike J, Tominaga S, Tamemoto H. Gas-forming liver abscess associated with rapid hemolysis in a diabetic patient. World J Diabetes. 2014;5(2):224–229. doi:10.4239/wjd.v5.i2.224
40. Kusumoto K, Hamada A, Kusaka T, et al. 持続灌流ドレナージを行い救命可能であった敗血症をともなったClostridium perfringensによるガス産生性肝膿瘍の1例 [A patient with sepsis and a gas-forming liver abscess caused by Clostridium perfringens treated with continuous perfusion drainage]. Nihon Shokakibyo Gakkai zasshi. 2014;111(7):1416–1423. Japanese.
41. Kitterer D, Braun N, Jehs MC, Schulte B, Alscher MD, Latus J. Gas gangrene caused by clostridium perfringens involving the liver, spleen, and heart in a man 20 years after an orthotopic liver transplant: a case report. Exp Clin Transplant. 2014;12(2):165–168. doi:10.6002/ect.2013.0034
42. Eltawansy SA, Merchant C, Atluri P, Dwivedi S. Multi-organ failure secondary to a Clostridium perfringens gaseous liver abscess following a self-limited episode of acute gastroenteritis. Am J Case Rep. 2015;16:182–186. doi:10.12659/ajcr.893046
43. Khan MS, Ishaq MK, Jones KR. Gas-forming pyogenic liver abscess with septic shock. Case Rep Crit. 2015;2015:632873. doi:10.1155/2015/632873
44. Li JH, Yao RR, Shen HJ, et al. Clostridium perfringens infection after transarterial chemoembolization for large hepatocellular carcinoma. World J Gastroenterol. 2015;21(14):4397–4401. doi:10.3748/wjg.v21.i14.4397
45. Yoshida J, Nakamura H, Yamada S, et al. Clostridium perfringensを起因菌とするガス産生性肝膿瘍に乾燥ガス壊疽ウマ抗毒素を投与した1例 [A case of freeze-dried gas gangrene antitoxin for the treatment of Clostridium perfringens sepsis]. Nihon Shokakibyo Gakkai zasshi. 2015;112(2):332–338. Japanese. doi:10.11405/nisshoshi.112.332
46. Meeuwes FO, Hukshorn CJ, Bloembergen P. Severe abdominal pain three weeks after a hemi-hepatectomy. Neth J Med. 2015;73(8):392–393.
47. Kyang LS, Bin Traiki TA, Alzahrani NA, Morris DL. Microwave ablation of liver metastasis complicated by Clostridium perfringens gas-forming pyogenic liver abscess (GPLA) in a patient with past gastrectomy. Int J Surg Case Rep. 2016;27:32–35. doi:10.1016/j.ijscr.2016.08.009
48. García Carretero R, Romero Brugera M, Vazquez-Gomez O, Rebollo-Aparicio N. Massive haemolysis, gas-forming liver abscess and sepsis due to Clostridium perfringens bacteraemia. BMJ Case Rep. 2016;2016. doi:10.1136/bcr-2016-218014
49. Hashiba M, Tomino A, Takenaka N, et al. Clostridium perfringens infection in a febrile patient with severe hemolytic anemia. Am J Case Rep. 2016;17:219–223. doi:10.12659/ajcr.895721
50. Lim AG, Rudd KE, Halliday M, Hess JR. Hepatic abscess-associated Clostridial bacteraemia presenting with intravascular haemolysis and severe hypertension. BMJ Case Rep. 2016;2016. doi:10.1136/bcr-2015-213253
51. Paasch C, Wilczek S, Strik MW. Liver abscess and sepsis caused by Clostridium perfringens and Klebsiella oxytoca. Int J Surg Case Rep. 2017;41:180–183. doi:10.1016/j.ijscr.2017.10.033
52. Takemura K, Sekoguchi S, Yamane S, et al. 肝動脈化学塞栓療法後にClostridium perfringensによるガス産生性肝膿瘍を発症した1例 [A case of a gas-forming liver abscess caused by Clostridium perfringens after transcatheter arterial chemoembolization]. Nihon Shokakibyo Gakkai zasshi. 2018;115(6):554–562. Japanese. doi:10.11405/nisshoshi.115.554
53. Martí Gelonch L, Jiménez Agüero R, Rodríguez Canas N, Enríquez Navascués JM. Massive haemolysis due to sepsis caused by Clostridium perfringens secondary to liver abscess. Presentation of two cases with a similar history. Hemólisis masiva debida a sepsis por Clostridium perfringens secundaria a absceso hepático. Presentación de dos casos con un mismo antecedente. Gastroenterol Hepatol. 2018;41(9):562–563. doi:10.1016/j.gastrohep.2017.11.012
54. Guridi Mugica A, Marti Gelonch L, Jimenez Agüero R. Sepsis fulminante porClostridium perfringens. Med Intensiva. 2018;42(2):137. doi:10.1016/j.medin.2016.08.002
55. Hamada K, Sasaki Y. Gas-filled Cavities in the Liver. Intern Med. 2018;57(15):2277. doi:10.2169/internalmedicine.0172-17
56. Yoshikawa T, Ohana M, Fukuda A. High fever after radiofrequency ablation of hepatocellular carcinoma. Gastroenterology. 2018;155(2):e3–e4. doi:10.1053/j.gastro.2017.12.037
57. Amjad W, Chung S, Mumtaz M, Farooq A, Gondal N. Gaseous liver abscess with Clostridium perfringens sepsis in a patient with neutropaenia. Prz Gastroenterol. 2019;14(2):160–161. doi:10.5114/pg.2019.85902
58. Uojima H, Onoue M, Hidaka H, et al. A suspected case of Clostridium perfringens sepsis with intravascular hemolysis after transhepatic arterial chemoembolization: a case report. J Med Case Rep. 2019;13(1):125. doi:10.1186/s13256-019-2023-x
59. Lindberg Å, Wide D. [Sepsis with intravascular hemolysis caused by Clostridium perfringens]. Bakteriemi med C perfringens – ovanligt men livshotande tillstånd. Lakartidningen. 2019;2019:11. Swedish.
60. Sakaue M, Ota K, Nakamura E, et al. Type A fulminant Clostridium perfringens sepsis indicated RBC/Hb discrepancy; a case report. BMC Infect Dis. 2019;19(1):719. doi:10.1186/s12879-019-4350-3
61. Fujikawa H, Araki M. Clostridium perfringens septicemia with massive intravascular hemolysis. Intern Med. 2020;59(4):591. doi:10.2169/internalmedicine.3793-19
62. Dahl SS, Thorsteinsson M, Lambine TL, Penninga L. Severe sepsis caused by a gas-forming Clostridium perfringens and Klebsiella variicola liver abscess following total pancreatectomy. BMJ Case Rep. 2020;13(10). doi:10.1136/bcr-2020-238896
63. Hamura R, Haruki K, Kumagai Y, Shiba H, Wakiyama S, Yanaga K. Subphrenic abscess due to Clostridium perfringens after hepatic resection for hepatocellular carcinoma following emphysematous cholecystitis: report of a case. Int J Surg Case Rep. 2020;67:86–90. doi:10.1016/j.ijscr.2020.01.029
64. De Zylva J, Padley J, Badbess R, Dedigama M. Multiorgan failure following gastroenteritis: a case report. J Med Case Rep. 2020;14(1):74. doi:10.1186/s13256-020-02402-z
65. Olds KL, Gilbert JD, Byard RW. Unexpected Death Associated With Clostridial Sepsis. Am J Forensic Med Pathol. 2021;42(3):289–291. doi:10.1097/paf.0000000000000640
66. Wang MH, Kuo YH, Yen YH, et al. Hepatic Clostridium Perfringens Abscess Formation after Radiofrequency Ablation Therapy for Hepatocellular Carcinoma: report of a Rare Case. Case Rep Oncol. 2021;14(2):906–911. doi:10.1159/000517024
67. Satoh M, Kogure T, Koiwai A, et al. A gas-forming liver abscess with massive bleeding into the abscess cavity due to a ruptured inferior phrenic artery. Intern Med. 2021;60(24):3913–3919. doi:10.2169/internalmedicine.7746-21
68. Takahashi G, Nakamura Y, Hayakawa T, Ono T, Endo K, Yoshida H. Clostridium perfringens sepsis after pancreatoduodenectomy: a case report. Surg Case Rep. 2022;8(1):48. doi:10.1186/s40792-022-01402-z
69. Lang H, Schmidt JJ, Wedemeyer H, Busch M. [Sepsis with hemolysis due to a liver abscess in a 60-year-old male patient]. Sepsis mit Hämolyse im Rahmen eines Leberabszesses bei einem 60-jährigen Patienten. Internist. 2022;63(3):325–329. German. doi:10.1007/s00108-021-01227-2
70. Wong ACC, Chow EY. A case of severe intravascular haemolysis. EJHaem. 2022;3(3):1036–1037. doi:10.1002/jha2.520
71. Itoh N, Akazawa N, Yanaidani T, Kuwahara T. Clinical and microbiological features of intratumor abscess with bloodstream infection caused by plesiomonas shigelloides, citrobacter freundii, streptococcus mitis/oralis, clostridium perfringens, and Candida albicans in a patient with cholangiocarcinoma: a case report. J Infect Chemother. 2022;28(12):1677–1681. doi:10.1016/j.jiac.2022.08.024
72. Osório C, Silva D, Teles L, Ferreira T, Nora M. An unusual fatal outcome of laparoscopic cholecystectomy: a case report. Cureus. 2023;15(1):e34365. doi:10.7759/cureus.34365
73. Fujita H, Nishimura S, Kurosawa S, Akiya I, Nakamura-Uchiyama F, Ohnishi K. Clinical and epidemiological features of Clostridium perfringens bacteremia: a review of 18 cases over 8 year-period in a tertiary care center in metropolitan Tokyo area in Japan. Intern Med. 2010;49(22):2433–2437. doi:10.2169/internalmedicine.49.4041
74. Bendary MM, Abd El-Hamid MI, El-Tarabili RM, et al. Clostridium perfringens associated with foodborne infections of animal origins: insights into prevalence, antimicrobial resistance, toxin genes profiles, and toxinotypes. Biology. 2022;11(4):551. doi:10.3390/biology11040551
75. Park JY, Kim S, Oh JY, et al. Characterization of Clostridium perfringens isolates obtained from 2010 to 2012 from chickens with necrotic enteritis in Korea. Poult Sci. 2015;94(6):1158–1164. doi:10.3382/ps/pev037
76. Udhayavel S, Thippichettypalayam Ramasamy G, Gowthaman V, Malmarugan S, Senthilvel K. Occurrence of Clostridium perfringens contamination in poultry feed ingredients: isolation, identification and its antibiotic sensitivity pattern. Anim Nutr. 2017;3(3):309–312. doi:10.1016/j.aninu.2017.05.006
77. Shindo Y, Dobashi Y, Sakai T, Monma C, Miyatani H, Yoshida Y. Epidemiological and pathobiological profiles of Clostridium perfringens infections: review of consecutive series of 33 cases over a 13-year period. Int J Clin Exp Pathol. 2015;8(1):569–577.
78. Simon TG, Bradley J, Jones A, Carino G. Massive intravascular hemolysis from Clostridium perfringens septicemia: a review. J Intensive Care Med. 2014;29(6):327–333. doi:10.1177/0885066613498043
79. Stevens DL, Maier KA, Mitten JE. Effect of antibiotics on toxin production and viability of Clostridium perfringens. Antimicrob Agents Chemother. 1987;31(2):213–218. doi:10.1128/AAC.31.2.213
80. Korhonen K. Hyperbaric oxygen therapy in acute necrotizing infections. With a special reference to the effects on tissue gas tensions. Ann Chir Gynaecol. 2000;89(214):7–36.
81. Hirn M, Niinikoski J, Lehtonen OP. Effect of hyperbaric oxygen and surgery on experimental gas gangrene. Eur Surg Res. 1992;24(6):356–362. doi:10.1159/000129228
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


