Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 16
Clopidogrel as a Distinctive Cause of Insulin Autoimmune Syndrome: A Systematic Case Review
Authors Chen S, Qiang J, Zhao B, Tian R, Yuan T, Li M, Li M, Gu Z, Yang L, Zhu H, Pan H , Tang Y, Li Y
Received 1 May 2023
Accepted for publication 16 August 2023
Published 25 August 2023 Volume 2023:16 Pages 2583—2592
DOI https://doi.org/10.2147/DMSO.S418845
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Juei-Tang Cheng
Shi Chen,1,* Jiaqi Qiang,1,2,* Bin Zhao,3 Ran Tian,4 Tao Yuan,1 Ming Li,1 Mei Li,1 Zhaoqi Gu,5 Liping Yang,6 Huijuan Zhu,1 Hui Pan,1,7 Yan Tang,3 Yuxiu Li1
1Key Laboratory of Endocrinology of National Health Commission, Department of Endocrinology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 100730, People’s Republic of China; 2Eight-Year Program of Clinical Medicine, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 100730, People’s Republic of China; 3Department of Pharmacy, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 100730, People’s Republic of China; 4Department of Cardiology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 100730, People’s Republic of China; 5Department of Radiotherapy, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 100730, People’s Republic of China; 6Department of Pharmacy, Handan First Hospital, Handan, Hebei Province, People’s Republic of China; 7State Key Laboratory of Complex Severe and Rare Diseases, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 100730, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yuxiu Li, Key Laboratory of Endocrinology of National Health Commission, Department of Endocrinology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, No. 1 Shuaifuyuan, Wangfujing, Dongcheng District, Beijing, 100730, People’s Republic of China, Email [email protected] Yan Tang, Department of Pharmacy, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, No. 1 Shuaifuyuan, Wangfujing, Dongcheng District, Beijing, 100730, People’s Republic of China, Email [email protected]
Abstract: The sulfhydryl group of clopidogrel metabolite could induce insulin autoimmune syndrome (IAS) with hypoglycemia as the major symptom. For patients with cardiovascular disease taking clopidogrel for vascular protection, this adverse event hypoglycemia increases the risk of cardiovascular events. However, discontinuing clopidogrel leaves patients without appropriate antiplatelet therapy. Treating IAS with glucocorticoids is also risky for these patients’ primary cardiovascular diseases. Early recognition and appropriate treatment of clopidogrel-induced IAS (CIAS) would be beneficial for patients. This research aimed to discover the clinical features and investigate optimal therapeutic management of CIAS. We systematically searched for cases of CIAS in PubMed and Embase and performed data mining in Food and Drug Administration Adverse Event Reporting System (FAERS). In the CIAS series, clinical features were summarized and compared to 287 IAS cases, including demographic information, HLA alleles, onset, and symptoms. The therapeutic effect of glucocorticoids was compared between the receiving group and the not-receiving group. The possibilities of common antiplatelet drugs to induce hypoglycemia/IAS were investigated with chemical structure and FAERS reports. A CIAS series of 51 patients was established. CIAS had an onset age of 74.8± 8.6 years old, 92.2% male, and a balanced proportion of East Asians and non-East Asians. Confusion occurred more frequently in CIAS than in IAS from various causes, while the other symptoms and hypoglycemia types were similar. The recovery time was approximately the same whether using glucocorticoids/immunotherapy in CIAS or not. Among common antiplatelet drugs, ticagrelor and rivaroxaban were unlikely to induce hypoglycemia/IAS. Clopidogrel is a distinctive cause of IAS featuring an elderly male presenting confusion as the symptom of hypoglycemia. Glucocorticoids/immunotherapy might not be necessary for the long-term recovery of CIAS. To balance the risks of hypoglycemia and cardiovascular events, substituting clopidogrel with ticagrelor and rivaroxaban might be considered.
Keywords: autoimmune hypoglycemia, antiplatelet therapy, glucocorticoids, cardiovascular diseases, ticagrelor
Introduction
Insulin autoimmune syndrome (IAS) is a relatively rare cause of spontaneous hypoglycemia featured with hyperinsulinism and an increase in insulin autoantibody (IAA). The incidence is higher in populations with genetic backgrounds of susceptible human leukocyte antigen (HLA) alleles.1 One of the major triggers of IAS was the medication containing the sulfhydryl group or generating the sulfhydryl group during metabolism. The underlying mechanism has been proposed that the sulfhydryl group disrupts disulfide bonds of the endogenous insulin molecule. As the conformation changes, the insulin is recognized and presented by the major histocompatibility complex (MHC). Subsequently, IAA is produced and captures insulin. Since the binding of IAA and insulin is unstable, hypoglycemia occurs when insulin is released in large quantities into the blood at once.2,3
Multiple drugs with the sulfhydryl group have been discovered to be associated with this autoimmune process, including methimazole, captopril, diltiazem, hydralazine, isoniazid, penicillamine, alpha lipoic acid, and the active metabolite of clopidogrel.4 Clopidogrel is a cornerstone antiplatelet medication frequently recommended in clinical guidelines, commonly prescribed in a massive number of cardiovascular, cerebral vascular, and peripheral artery disease patients. Hypoglycemia manifesting in IAS could induce severe outcomes in the cardiovascular and neural systems. Since this harm could exacerbate the state of primary illness and even threaten the patient’s life,5,6 it is important to improve early recognition by summarizing clinical characteristics of clopidogrel-induced IAS (CIAS). For appropriate treatment, it is urgent to maintain control over primary diseases while treating IAS by exploring possible antiplatelet alternatives and the necessity of glucocorticoids.
In this study, we aimed to establish a CIAS case series by a systematic case review, including an index patient diagnosed and treated in our hospital, reports from literature and real-world data from Food and Drug Administration Adverse Event Reporting System (FAERS). To discover the distinctiveness of CIAS, clinical characteristics would be summarized and compared to that of IAS from various causes. Since we substituted clopidogrel with alternative antiplatelet drugs without using glucocorticoids in the index case, we also aimed to explore more optimal therapeutic management in this CIAS series. Given that antiplatelet therapy is essential in the secondary prevention of cardiovascular and cerebrovascular diseases, we would like to investigate the possibilities of other antiplatelet drugs to induce IAS and find possible choices to substitute clopidogrel.
Materials and Methods
Case Presentation
We first reported an index case diagnosed with CIAS and treated in our hospital. An 82-year-old Chinese male was admitted to our hospital on November 17th, 2020. He had experienced episodic hypoglycemia for about a month. During hypoglycemia onset, he had blood glucose <2.8 mmol/L (50.4 mg/dL), insulin >25 μIU/mL, and IAA > 400 RU/mL. He was diagnosed with coronary heart disease in April 2019, and non-ST-elevation myocardial infarction (NSTEMI) in June 2020. Considering the symptom of hypoglycemia, high level of insulin and IAA, we considered him as hyperinsulinism hypoglycemia. After he was hospitalized and fully examined, he was diagnosed with IAS. Among his regular medications, clopidogrel was the only one that could produce sulfhydryl group, a trigger of IAS. His HLA typing was HLA-DRB1*0403/1147 and DQB1*0301/0302. He discontinued clopidogrel on November 20th, 2020. Since then, IAA titer fell from 7290.4 RU/mL to 326.03 RU/mL in 12 days and total insulin fell from 831 μIU/mL to 322 μIU/mL in two weeks. His hypoglycemia never recurred, with blood glucose maintaining in normal range. According to the European Society of Cardiology (ESC) Guidelines,7 rivaroxaban was prescribed as alternative to provide “dual pathway” protection with aspirin. Antiplatelet drugs were not selected as we were uncertain of IAS impact on platelet aggregation. He was discharged on December 17th, 2020 and monitored in the outpatient service. He had planned to have coronary stenting, so ticagrelor was prescribed to substitute rivaroxaban on April 8th, 2021 to restart his dual antiplatelet therapy (DAPT). On June 2nd, 2021, he was implanted with two stents in the right coronary artery. Up to the latest follow-up on July 9th, 2021, he had no signs of angina pectoris or hypoglycemia, and his IAA maintained in the normal range.
Literature Searching Strategy
Further CIAS cases were searched in PubMed and Embase from inception to September 4th, 2022. To avoid missing, we searched for both IAS and autoimmune hypoglycemia related to clopidogrel in the title, abstract, keywords, and full text. The searching strategy was (Clopidogrel OR Duoplavin OR Plavix OR Zyllt) AND (“insulin autoimmune syndrome” OR “Hirata disease” OR ((hypoglycemia OR hypoglycaemia) AND (autoimmune OR autoimmunity))). No restriction was set on language or publication time. Full texts of all the searching results were retrieved.
FAERS Data Mining Strategy
FAERS is a FDA post-marketing safety surveillance program for approved drugs and biologics. It provides adverse events and medication error reports at the patient level.8 “Insulin autoimmune syndrome” (code: 10022472) and “hypoglycemia” (code: 10020993) were selected as the preferred term (PT) in the adverse event (REAC) file. The brand name (“CLOPIDOGREL”) and generic names (“PLAVIX”, “DUOPLAVIN”, “ZYLLT”) of clopidogrel were selected in the drug information (DRUG) file. “PS” (primary suspected) was chosen as the role_cod. The MedDRA (Version 23.1) and the MICROMEDEX® were used for data mapping. The MICROMEDEX® was utilized as a drug dictionary.
Selection Criteria
For literature searching results, all were screened according to the following predefined criteria. Inclusion criteria: (1) The patient had a definitive diagnosis of IAS and other causes of hypoglycemia and autoimmune diseases were excluded; (2) The patient took clopidogrel before the onset of hypoglycemia; (3) The patient took clopidogrel at an indication dose; (4) possible adverse events of hypoglycemia and IAS caused by concomitant drugs were excluded. Exclusion criteria: (1) The case was published without peer review; (2) The clinical records were not clearly reported, eg, the patient was not clearly diagnosed of CIAS; (3) Clopidogrel was not the only IAS-inducible medication taken prior to onset. For FAERS data mining results, data deduplication was conducted according to the FDA’s recommendations. We removed data from the deleted file and kept the latest FDA_DT when the CASEIDs were the same and the higher PRIMARYID when the CASEID and FDA_DT were the same.
Data Extraction and Combination
For each included case from the literature, we extracted the following information with a standard collection form: report time, age, sex, race, HLA genotype, clinical symptoms, hypoglycemia types (postprandial, fasting, exercise‐induced), concomitant drugs, treatment, and outcomes were extracted. For each included case from FAERS, we extracted records of demography, indication, drug administrative information, onset time, and outcome. Cases from literature and FAERS were combined into one CIAS series. After combination, cases with the same age, sex, race, and report time were removed to avoid possible duplication.
Quality Assessment
The literature searching, FAERS data mining, data selection, and data extraction were performed with critical appraisal according to a methodological guide for case reports and case series.9 Double-blind searching and selection were conducted by JQ and BZ. Disagreements were solved by consulting a third investigator SC.
Statistical Analyses
Clinical characteristics of the CIAS case series were summarized and compared to features of 287 IAS cases from a systematic case review.10 The authors searched with the terms “insulin autoimmune syndrome” and “Hirata disease” in the time range of 1960 to August 2021. Given that the authors performed statistics on East Asian and non-East Asian patients separately, we manually calculated the clinical data of all patients. In the comparative analysis of CIAS versus all IAS, continuous data were analyzed with the t-test, while categorical data were analyzed with the χ2 test and two‐sided Fisher’s exact test (when sample sizes were small). P-value <0.05 was considered statistically significant. All statistical analyses were conducted with the R studio (version 4.0.0).
Investigation of the Possibility of IAS/Hypoglycemia Induced by Other Antiplatelet Drugs
We further investigated the possibilities of IAS/hypoglycemia induced by other antiplatelet drugs (aspirin, ticagrelor, prasugrel, dipyridamole, tirofiban) and rivaroxaban. Rivaroxaban was included because it is recommended as an alternative to clopidogrel or ticagrelor to combine with aspirin in the ESC Guidelines of non-ST-segment elevation acute coronary syndrome.7 Criteria of IAS/hypoglycemia-inducible were defined as (1) The chemical structure of the drug contains the sulfhydryl group (–SH); (2) The drug has been reported to induce IAS/hypoglycemia at therapeutic dose in clinical practice.
Chemical structures of these drugs were achieved from the DrugBank Online, Database for Drug and Drug Target Info (https://go.drugbank.com/, accessed on February 13th, 2022). Reports of IAS/hypoglycemia in clinical practice were investigated with a similar strategy to clopidogrel (literature searching strategy, selection criteria, data extraction and combination, quality assessment). We searched with the strategy (generic name OR brand name) AND (“insulin autoimmune syndrome” OR ((hypoglycemia OR hypoglycaemia) AND (autoimmune OR autoimmunity))) from inception to September 10th, 2022 (brand names and generic names were shown in Table S1).
Results
Searching Results
The flowchart of the identification, screening, and inclusion process is shown in Figure 1 according to the PRISMA guide.11 We obtained 12 CIAS cases from electronic literature search4,12–21 and 45 cases from FAERS. We combined records of these cases with the index case diagnosed with CIAS and treated in our hospital. Seven were considered possible duplications and were removed. At last, 51 patients were included in the case series. Individual clinical data of these cases were extracted and shown in Tables S2–S4.
Demographic Information
In the CIAS case series, the onset age was 74.8±8.6 years old, which was significantly greater than 53.0±20.0 years old of IAS from various causes (p < 0.001). The CIAS patients were almost all males while there was no obvious sex difference in IAS (p < 0.001). There were 25 East Asian and 26 non-East Asian patients in CIAS, and 145 East Asian and 142 non-East Asian patients in IAS, both showing a balanced race distribution (p = 0.946) (Table 1). In the CIAS series, the East Asian group had an onset age of 76.2±8.4 years old and 88% of males while the non-East Asian group had an onset age of 73.4±8.7 years old and 96% of males. No significant age or sex differences were observed between these two races (Table S5).
 |
Table 1 Reported Demography of CIAS and Comparison to All IAS |
HLA Alleles
HLA genotyping results were reported in nine CIAS cases and 98 IAS cases. In the CIAS series, DRB1*0403, DRB1*0404, DRB1*0406, and DQB1*0302 were the most frequent HLA alleles, similar to that of IAS. In the East Asian race group, DRB1*0403 was the most common type in CIAS (p = 0.0463), while DRB1*0406 was the most in all IAS (p = 0.0315). In the non-East Asian race group, there was no HLA distribution difference between CIAS and IAS (all p > 0.05) (Table 2). In the CIAS series, though East Asians tended to carry more DRB1*0403, DRB1*0406, and DQB1*0302 than non-East Asians, the difference was not statistically significant (all p > 0.05). In the IAS series, DRB1*0406 was more frequent in East-Asians (p < 0.001), while DRB1*0403 (p < 0.001) and DRB1*0404 were more frequent in non-East Asians (p=0.027) (Table 2).
 |
Table 2 Reported HLA Alleles of CIAS and Comparison to IAS from Various Causes |
Clinical Onset and Symptoms
In CIAS, indications of clopidogrel were mainly cardiovascular or cerebrovascular diseases, including coronary artery disease, stroke, and peripheral vascular disease. The onset time was reported in 11 cases from literature, varying as 1 week, 1 week, 10 days, 3 weeks, 1 months, 4 months, 9 months, “months”, 2 years, and 2 years (Table S3). The main symptom was spontaneous recurrent hypoglycemia in all cases. Laboratory tests showed high titer of IAA and increase in total insulin. In comparison of CIAS to IAS from various causes, hypoglycemia types and symptoms were similar except that CIAS patients were more likely to be presented with confusion in hypoglycemia (p = 0.013) (Table 3).
 |
Table 3 Reported Clinical Onset and Symptoms of CIAS and Comparison to All IAS |
Treatment and Outcome
In both CIAS and IAS series, most patients were treated with traditional IAS therapy, including drug discontinuation, diet modification, and glucocorticoids. Plasmapheresis, immunoadsorption or rituximab were also administrated in few patients with severe symptoms. There was no treatment strategy difference between CIAS and IAS (all p > 0.05) (Table S6). In cases using alternative antiplatelet drugs after discontinuing clopidogrel, ticagrelor and rivaroxaban were mentioned as possible options (Table S4). In the CIAS series, patients receiving glucocorticoids/immunotherapy or not showed no difference in age, sex, race, and the recovery time (all p > 0.05) (Table 4).
 |
Table 4 Reported GC/Immunotherapy Use in the Treatment of Clopidogrel-Induced IAS |
IAS and Autoimmune Hypoglycemia Induced by Other Antiplatelet Drugs
Chemical structures of the active metabolite of clopidogrel, aspirin, ticagrelor, prasugrel, dipyridamole, tirofiban, and rivaroxaban were obtained. Only the active metabolite of clopidogrel and prasugrel carry sulfhydryl groups (Figure S1). In literature search, we screened all the search results and no antiplatelet drug was found related to IAS or autoimmune hypoglycemia except for clopidogrel (Table 5).
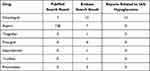 |
Table 5 Reports Related to IAS or Autoimmune Hypoglycemia of Antiplatelet Drugs |
Discussion
This study focused on IAS and autoimmune hypoglycemia as adverse events of clopidogrel. We generated findings as follows: (1) A CIAS series of 51 patients was established, which was the CIAS case series with the largest sample number to our knowledge; (2) CIAS featured an elderly male patient with confusion as the symptom of hypoglycemia, indicating clopidogrel might be a distinctive cause of IAS; (3) Glucocorticoids and immune therapy might not be necessarily determining for recovery of CIAS; (4) Drug substitution after clopidogrel discontinuation should be considered to protect over cardiovascular risk. Among common antiplatelet medications, only clopidogrel was associated with IAS and autoimmune hypoglycemia. Ticagrelor and rivaroxaban might be possible alternatives to clopidogrel based on current clinical experience.
In previous reviews of CIAS, the maximum number of cases was at most four.4 None of these reviews included reports from real-world data as FAERS. In our study, we first identified 12 cases in literature based on systematic literature research. Combined with real-world data from FAERS, we established a CIAS series of 51 patients in total, far more than the prior series. Typically, IAS was observed occurring in patients over 40 years old, with a peak at 60–69 years of age.3,22 In our case review, the average age of onset was around 75 years old, indicating the vulnerable population was older than the general IAS patients. IAS was reported to affect both sexes equally.2,3 However, 92.2% of patients were males in our case series. A possible explanation was males were more likely to develop cardiovascular, cerebrovascular, and peripheral artery diseases, thus taking clopidogrel as a regular medication. In a word, a patient susceptible to IAS when taking clopidogrel was characterized as a male in the seventies.
One more to be noticed was that approximately the same numbers of East Asian and non-East Asian patients were found in this case review. This result was inconsistent with the geographic distribution of IAS that more Asian patients were found than Caucasians.23 It is reported that patients carrying specific HLA alleles, which encode the major histocompatibility complex (MHC) molecules, are more vulnerable. HLA-DRB1*0403 and HLA-DRB1*0406 were reported to be such susceptible alleles.3,24–27 Asians, especially Japanese, carry these alleles at a higher frequency, thus more vulnerable to developing IAS.2 Our case review demonstrated that susceptible HLA alleles of CIAS were DRB1*0406, DRB1*0403, DRB1*0404, and DQB1*0302. All these four alleles have been previously reported to be associated with IAS.3,24–27 Given that age, sex, race, and HLA-genotype are essential diagnostic clues of IAS, our summary provided specific points for CIAS early recognition.
The duration from the medication initiation of clopidogrel to the onset of CIAS varied among patients. As this research was the first attempt to uncover the onset time of IAS, there were no previous reports for reference. The result ranged from one week to 2 years, which probably originated from the complex mechanism of clopidogrel to induce IAS. This multi-step autoimmune process involved the interaction of clopidogrel metabolite with insulin and endogenous antigen with innate immune response. As a result, the onset time heavily depended on the heterogeneity of the immune system. More cases might be needed for a more statistically convincing conclusion. Confusion was a distinctive feature more common in CIAS than in IAS. Considering confusion was a relatively worrying event for an elderly male patient, it is important to recognize and prevent CIAS.
The index patient in our hospital got recovered by diet modification and clopidogrel substitution with rivaroxaban and then ticagrelor without taking glucocorticoids. To previous knowledge, IAS is generally self-limiting after discontinuation of the triggering drug. Tradition treatment also includes frequent, low-carbon diets and glucocorticoids. Medications such as diazoxide, octreotide, and acarbose have also been applied to regulate insulin secretion and glucose metabolism.2,28 For cases with refractory hypoglycemia and severe symptoms, plasmapheresis and rituximab would be beneficial.2,4,29 In this CIAS series, all patients were treated consistently with the traditional strategy. To alleviate symptoms, glucocorticoid is the first choice to downregulate antibody levels. And in the point of etiology, drug discontinuation is also of importance. However, none of these cases emphasized drug substitution after clopidogrel discontinuation.
In fact, the use of glucocorticoids is a balance of risks and benefits. On one hand, glucocorticoid alleviates hypoglycemia and cures IAS. On the other hand, it also exposes the patient to risks of refractory angina and even aggravation of the primary disease. Glucocorticoid was reported to increase cardiovascular risk even at low doses in patients with immune-mediated diseases.30 It influences vascular function, arteriosclerosis, and vascular remodeling after intravascular injury or ischemia.31 In this CIAS case series, most patients recovered in several months except for those treated with plasma exchange. The risk of glucocorticoids could not be ignored during this long recovery period. Four CIAS patients recovered without glucocorticoids or other immunotherapy in the same time period as those with immunotherapy, indicating drug cessation alone was effective enough for patients with mild symptoms. As discontinuing the medication is not a decision of tradeoff, it would be more beneficial if considered a priority. To be noticed, the IAA titer of the index patient plummeted in the first 10 days after drug cessation, which is consistent with the pharmacodynamics and pharmacokinetics. Therefore, the first 1~2 weeks might be a key monitoring time to evaluate the effect of drug cessation and decision on glucocorticoid use.
Considering that IAS and the primary disease should be balanced in treatment, we proposed that it might be beneficial to incorporate drug substitution into the treatment of CIAS. In the index patient, clopidogrel was a crucial element of dual antiplatelet therapy (DAPT) for his acute coronary syndrome. In searching for non-hypoglycemia inducible alternatives, we investigated both chemical structures and related literature reports. Chemicals either containing a sulfhydryl group or with IAS/autoimmune hypoglycemia adverse events were excluded. Given that prasugrel carries a sulfhydryl group, ticagrelor, and rivaroxaban were selected in the treatment of the index patient according to ESC guidelines for non-ST-segment elevation acute coronary syndrome.7 Ticagrelor has also been presented as an alternative to clopidogrel after hypoglycemia attacks in a pharmacovigilance study.32
There were some limitations in this study. First, though we reached all available resources including real-world data to consummate the case series, there might still be unreported cases. Also, evidence based on chemical structures and literature reports seemed limited to prove the association between IAS and other antiplatelet medications. However, more evidence seemed limited because hypoglycemia is a relatively rare endpoint in clinical trials or observational studies.
Conclusion
In summary, based on a systematic case review with most cases, a CIAS patient is characterized as a male in the seventies carrying susceptible HLA alleles when compared to IAS patients from various causes. Antiplatelet substitution after clopidogrel discontinuation is essential in therapy to ensure control over cardiovascular risks. Ticagrelor and rivaroxaban were optional alternatives according to current clinical experience. Considering the balance between the harm to primary diseases and the treatment effect of IAS, glucocorticoids might not be the ideal first-line therapy. We believe that the clinical characteristics and therapeutic management of CIAS proposed in this study would help clinicians to early recognize such patients and provide practical implications in treating for better prognosis.
Abbreviations
IAS, insulin autoimmune syndrome; IAA, insulin autoantibody; HLA, human leukocyte antigen; MHC, major histocompatibility complex; CIAS, clopidogrel-induced IAS; FAERS, Food and Drug Administration Adverse Event Reporting System; PT, preferred term; ESC, European Society of Cardiology; DAPT, dual antiplatelet therapy; MHC, major histocompatibility complex.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for Publication
We have obtained written consents from the index patient to present his clinical data and genomic sequencing data in this article.
Acknowledgments
We extend thanks to the index patient for their cooperation in diagnosis, treatment, and their contribution to science.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
National High Level Hospital Clinical Research Funding (2022-PUMCH-A-015).
Disclosure
The author reports no conflicts of interest in this work.
References
1. Kittah NE, Vella A. Management of endocrine disease: pathogenesis and management of hypoglycemia. Eur J Endocrinol. 2017;177(1):R37–R47. doi:10.1530/EJE-16-1062
2. Censi S, Mian C, Betterle C. Insulin autoimmune syndrome: from diagnosis to clinical management. Ann Transl Med. 2018;6(17):335. doi:10.21037/atm.2018.07.32
3. Eisenbarth GS. Medical Intelligence Unit 13: Molecular Mechanisms of Endocrine and Organ Specific Autoimmunity. R.G. Landes Company; 1999.
4. Calder GL, Ward GM, Sachithanandan N, MacIsaac RJ. Insulin autoimmune syndrome: a case of clopidogrel-induced autoimmune hypoglycemia. J Clin Endocrinol Metab. 2020;105(4):dgz301. doi:10.1210/clinem/dgz301
5. Hanefeld M, Frier BM, Pistrosch F. Hypoglycemia and cardiovascular risk: is there a major link? Diabetes Care. 2016;39(Suppl 2):S205–S209. doi:10.2337/dcS15-3014
6. Amiel SA, Aschner P, Childs B; International Hypoglycaemia Study Group. Hypoglycaemia, cardiovascular disease, and mortality in diabetes: epidemiology, pathogenesis, and management. Lancet Diabetes Endocrinol. 2019;7(5):385–396. doi:10.1016/S2213-8587(18)30315-2
7. Collet JP, Thiele H, Barbato E, et al. 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2021;42(14):1289–1367. doi:10.1093/eurheartj/ehaa575
8. Ji HH, Tang XW, Dong Z, Song L, Jia YT. Adverse event profiles of anti-CTLA-4 and anti-PD-1 monoclonal antibodies alone or in combination: analysis of spontaneous reports submitted to FAERS. Clin Drug Investig. 2019;39(3):319–330. doi:10.1007/s40261-018-0735-0
9. Murad MH, Sultan S, Haffar S, Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med. 2018;23(2):60–63. doi:10.1136/bmjebm-2017-110853
10. Oest L, Roden M, Müssig K. Comparison of patient characteristics between East Asian and non-East Asian patients with insulin autoimmune syndrome. Clin Endocrinol. 2022;96(3):328–338. doi:10.1111/cen.14634
11. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372. doi:10.1136/bmj.n71
12. Bortolotti D, Mothe-Satney I, Ferrari P, et al. Spontaneous hypoglycaemia in the presence of both anti-insulin antibody and anti-insulin receptor antibody. Diabetes Metab. 2006;32(6):598–603. doi:10.1016/S1262-3636(07)70314-2
13. Yamada E, Okada S, Saito T, Osaki A, Ozawa A, Yamada M. Insulin autoimmune syndrome during the administration of clopidogrel. J Diabetes. 2016;8(4):588–589. doi:10.1111/1753-0407.12385
14. Rajpal A, Kassem LS, Moscoso-Cordero M, Arafah BM. Clopidogrel-induced insulin autoimmune syndrome: a newly recognized cause of hypoglycemia in a patient without diabetes. J Endocr Soc. 2017;1(9):1217–1223. doi:10.1210/js.2017-00316
15. Hunter A, Graham U, Lindsay JR. Insulin autoimmune syndrome: a rare case of hypoglycaemia resolving with immunosuppression. Ulster Med J. 2018;87(1):34–36.
16. Jiang Y, Wang L, Shi F, et al. Insulin autoimmune syndrome after exposure to clopidogrel: a case report. Endocr Metab Immune Disord Drug Targets. 2020;20(8):1355–1362. doi:10.2174/1871530320666191220111615
17. Yoshino H, Kawakami K, Kohriyama K, Yoshino G, Matsunaga S, Takechi H. Long-term follow-up of insulin autoimmune syndrome in an elderly patient. Clin Case Rep. 2020;8(12):2941–2944. doi:10.1002/ccr3.3150
18. Yang CC, Gu WJ, Lyu ZH, et al. 氯吡格雷致胰岛素自身免疫综合征一例 [One case report of insulin autoimmune syndrome induced by clopidogrel]. Zhonghua Nei Ke Za Zhi. 2021;60(1):55–57. Chinese. doi:10.3760/cma.j.cn112138-20200221-00109
19. Rajan J, Cobos –salinas Leopoldo M, Cigarroa C. Insulin autoimmune syndrome after coronary stent placement. J Am Coll Cardiol. 2021;77(18_Supplement_1):1895. doi:10.1016/S0735-1097(21)03251-4
20. de Castro T, Beier C, Terkamp C, et al. Insulin autoimmune syndrome: a rare, but important differential diagnosis of hypoglycemia. Internist. 2022;63(2):217–220. doi:10.1007/s00108-021-01180-0
21. Zhu Q, Zhao H, Qiu W, et al. Case report: recurrent autoimmune hypoglycemia induced by non-hypoglycemic medications. Front Immunol. 2022;13:855350. doi:10.3389/fimmu.2022.855350
22. Lupsa BC, Chong AY, Cochran EK, Soos MA, Semple RK, Gorden P. Autoimmune forms of hypoglycemia. Medicine. 2009;88(3):141–153. doi:10.1097/MD.0b013e3181a5b42e
23. Uchigata Y, Hirata Y, Omori Y, Iwamoto Y, Tokunaga K. Worldwide differences in the incidence of insulin autoimmune syndrome (Hirata disease) with respect to the evolution of HLA-DR4 alleles. Hum Immunol. 2000;61(2):154–157. doi:10.1016/s0198-8859(99)00144-5
24. Uchigata Y, Kuwata S, Tokunaga K, et al. Strong association of insulin autoimmune syndrome with HLA-DR4. Lancet. 1992;339(8790):393–394. doi:10.1016/0140-6736(92)90080-m
25. Uchigata Y, Hirata Y, Iwamoto Y. Drug-induced insulin autoimmune syndrome. Diabetes Res Clin Pract. 2009;83(1):e19–e20. doi:10.1016/j.diabres.2008.10.015
26. Uchigata Y, Omori Y, Nieda M, Kuwata S, Tokunaga K, Juji T. HLA-DR4 genotype and insulin-processing in insulin autoimmune syndrome. Lancet. 1992;340(8833):1467. doi:10.1016/0140-6736(92)92654-x
27. Uchigata Y, Tokunaga K, Nepom G, et al. Differential immunogenetic determinants of polyclonal insulin autoimmune syndrome (Hirata’s disease) and monoclonal insulin autoimmune syndrome. Diabetes. 1995;44(10):1227–1232. doi:10.2337/diab.44.10.1227
28. Yuan T, Li J, Li M, et al. Insulin autoimmune syndrome diagnosis and therapy in a single Chinese center. Clin Ther. 2019;41(5):920–928. doi:10.1016/j.clinthera.2019.03.009
29. Yaturu S, DePrisco C, Lurie A. Severe autoimmune hypoglycemia with insulin antibodies necessitating plasmapheresis. Endocr Pract. 2004;10(1):49–54. doi:10.4158/EP.10.1.49
30. Pujades-Rodriguez M, Morgan AW, Cubbon RM, Wu J, Rahimi K. Dose-dependent oral glucocorticoid cardiovascular risks in people with immune-mediated inflammatory diseases: a population-based cohort study. PLoS Med. 2020;17(12):e1003432. doi:10.1371/journal.pmed.1003432
31. Walker BR. Glucocorticoids and cardiovascular disease. Eur J Endocrinol. 2007;157(5):545–559. doi:10.1530/EJE-07-0455
32. Chen S, Qiang J, Tian R, et al. Clopidogrel-associated hypoglycemia and alternative antiplatelet therapy: a Real-World, Pharmacovigilance Study. Eur Heart J Cardiovasc Pharmacother. 2022:pvac050. doi:10.1093/ehjcvp/pvac050
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

