Back to Journals » Infection and Drug Resistance » Volume 17
Clinical Significance of Preoperative Pyrazinamide-Containing Therapy in Tuberculous Constrictive Pericarditis
Authors Fang L, Yu W , Yu G , Chen G, Ye B
Received 16 October 2023
Accepted for publication 6 January 2024
Published 12 January 2024 Volume 2024:17 Pages 131—139
DOI https://doi.org/10.2147/IDR.S445025
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Likui Fang, Wenfeng Yu, Guocan Yu, Gang Chen, Bo Ye
Department of Thoracic Surgery, Hangzhou Red Cross Hospital, Hangzhou, 310003, People’s Republic of China
Correspondence: Likui Fang, Department of Thoracic Surgery, Hangzhou Red Cross Hospital, Hangzhou, 310003, People’s Republic of China, Email [email protected]
Background: Tuberculous constrictive pericarditis (TCP) is recommended to be treated with anti-tuberculosis (TB) therapy before pericardiectomy. Whether different preoperative anti-TB regimens may lead to different outcomes is unclear.
Methods: We retrospectively collected patients diagnosed as TCP and received pericardiectomy from April 2016 to June 2023. The study patients were assigned into the active TCP (A-TCP) group and the inactive TCP (IA-TCP) group according to the results of Mycobacterium tuberculosis (MTB) culture and MTB RNA assay. Baseline characteristics including anti-TB regimens and surgical outcomes were compared between the two groups. Logistic regression analysis and subgroup analysis were conducted to identify the protective factors of A-TCP.
Results: Of the 102 study patients, 24 was in the A-TCP group and 78 was in the IA-TCP group. The rate of preoperative anti-TB regimen containing pyrazinamide was 37.5% in the A-TCP group, as compared with 74.4% in the IA-TCP group (P = 0.001). Multivariate analysis showed that preoperative use of pyrazinamide was the protective factor of A-TCP (OR 0.194, 95% CI 0.053– 0.703, P = 0.013). Subgroup analysis based on age also showed consistent findings. In the analyses of surgical outcomes, A-TCP was the independent risk factor of postoperative cardiac complications (OR 4.231, 95% CI 1.317– 13.593, P = 0.015) and associated with longer hospital stay (P = 0.004) and higher hospitalization cost (P = 0.001).
Conclusion: A strategy involving anti-TB regimen containing pyrazinamide before pericardiectomy was superior to that without pyrazinamide in the patients with TCP. The strategy was associated with lower risk of A-TCP and might lead to better postoperative recovery and cost-effectiveness.
Keywords: tuberculous constrictive pericarditis, pericardiectomy, pyrazinamide, outcomes
Introduction
Constrictive pericarditis is a form of diastolic heart failure characterized by fibrotic and inelastic pericardium.1 Although the prevalence or overall incidence of constrictive pericarditis has been poorly established, it appears to be rare and life-threatening. The etiology of constrictive pericarditis varies according to countries, but tuberculosis (TB) remains a major cause worldwide, especially in developing countries.2–5 Because constrictive pericarditis is chronic and progressive in most cases, conservative treatment such as diuretic therapy is only palliative.6 Pericardiectomy is indicated to remove the diseased pericardium but accompanied with high perioperative mortality rates.7–9
It is worth noting that tuberculous pericarditis, accounting for 1% of all forms of tuberculosis, may be the only presentation of Mycobacterium tuberculosis (MTB) infection, and up to 50% of cases will develop constrictive pericarditis if without effective pharmacotherapy for tuberculosis.10 Initial anti-TB chemotherapy is recommended in tuberculous constrictive pericarditis (TCP) before surgery.11 Previous studies suggested that combination therapy strategy should be administered for 1 to 2 months before pericardiectomy, but whether different preoperative anti-TB regimens may lead to different outcomes is unclear.12,13 This study aims to explore the effect of different anti-TB regimens on pericardial TB activity and prognosis in patients with TCP.
Materials and Methods
Study Population
Patients were eligible for inclusion in the study if they were diagnosed as TCP and received pericardiectomy in our department from April 2016 to June 2023. Patients with drug resistant TCP, liver cirrhosis, and cancer were not eligible, and those without records of detailed anti-TB regimens were also excluded. Eventually, 102 out of a total of 108 patients were included in this study. Their clinical characteristics were collected from the hospital electronic medical records system. The study protocol was approved by the Institutional Review Board of Hangzhou Red Cross Hospital (No. 2023104). Because of the retrospective nature of the study and without any specific intervention, the informed consent has been agreed to be waived. The data were maintained with confidentiality. The present study complied with the Declaration of Helsinki.
Diagnosis
The preoperative diagnosis of constrictive pericarditis mainly depended on clinical symptoms, echocardiography, chest enhanced computed tomography and central venous pressure (CVP), which was measured via a central venous catheter. The preoperative diagnosis of pericardial TB relied on interferon-gamma release assay, purified protein derivative test, chest imaging and laboratory tests for pericardial effusion including acid-fast bacilli (AFB) smear, Mycobacterium tuberculosis (MTB) culture, GeneXpert MTB/RIF, CapitalBio Mycobacterium real-time polymerase chain reaction (RT-PCR) detection assay and MTB-RNA assay.
The diagnosis of pericardial TB was verified postoperatively by pathologic examination and laboratory tests on resected pericardial tissue including AFB smear, MTB culture, GeneXpert MTB/RIF, RT-PCR detection assay and MTB-RNA assay. Resected diseased pericardial tissue was mixed and aliquoted in a homogeneous sampling and then sent for the above tests. Active tuberculous constrictive pericarditis (A-TCP) was defined as positive results of MTB culture or MTB RNA assay on resected pericardial tissue.14 If AFB smear, GeneXpert MTB/RIF, RT-PCR detection assay and pathologic examination were positive but MTB culture and MTB RNA assay were negative, these patients were classified into inactive tuberculous constrictive pericarditis (IA-TCP).
Chemical Reagents
Smear microscopy was performed by Ziehl-Neelsen acid-fast staining. MTB culture was performed by Lowenstein–Jensen solid medium and liquid culture medium (BACTEC MGIT 960 Mycobacteria Culture System, BD Diagnostic Systems, Sparks, MD) according to the manufacturer’s instructions. The first-generation Xpert MTB/RIF (Cepheid, Sunnyvale, CA, USA) reaction cassette was used for GeneXpert MTB/RIF. The CapitalBio Mycobacterium RT-PCR detection assay (CapitalBio Technology Inc., Beijing, China) was performed according to the manufacturer’s instructions. An RT-fluorescence quantification PCR instrument (SLAN-96S Real-Time PCR System ZEESAN Xiamen, CN) was used for nucleic acid amplification to detect IS6110 and HSP65 multicopy elements for MTB and NTM, respectively. The MTB-RNA assay (Shanghai Rendu Biotechnology, China) was performed according to the manufacturer’s instructions, with the use of Moloney murine leukemia virus reverse transcriptase and T7 RNA polymerase (RD Bioscience, Inc., San Diego, CA, USA).
Treatment
All patients were routinely recommended to receive the standard first-line anti-TB regimen (isoniazid, rifampin, pyrazinamide, and ethambutol), but the regimen was adjusted according to individual physical condition and tolerance ability. Monotherapy was unacceptable. Preoperative anti-TB therapy was usually continued for at least 1 month unless the patient strongly requested surgical treatment. A patient was regarded as receiving the anti-TB drugs if he or she could tolerate them.
All included patients received general anesthesia and single-lumen endotracheal tube assisted ventilation. Median sternotomy was performed in all cases without the use of cardiopulmonary bypass. The extent of pericardiectomy included the anterolateral pericardium between the two phrenic nerves, the basal pericardium over the diaphragmatic surface, the pericardium on the great arteries and the pericardium from superior vena cava-right atrium junction to inferior vena cava-right atrium junction.15
Statistical Analysis
The studied participants were divided into the A-TCP group and the IA-TCP group according to the results of pathologic examination and laboratory tests on resected pericardial tissue. The comparison of categorical data was done with the chi-square test, the corrected chi-square test or the Fisher's exact test when appropriate. T-test was used for the comparison of continuous data. Odds ratios (ORs) for A-TCP and postoperative cardiac complications were estimated by the binary logistic regression model, including two-sided 95% CIs. Confounders were included, based on univariate analysis. Subgroup analysis was conducted to assess the consistency of the effect of anti-TB regimens on A-TCP if necessary. These analyses were conducted using SPSS software (version 24.0, IBM SPSS Inc., United States). All analyses were two-sided, and statistical significance was set at P value <0.05.
Results
Baseline Characteristics
From April 2016 to June 2023, a total of 108 patients were retrospectively screened and 102 were included in this study, with 24 (23.5%) in the A-TCP group and 78 (76.5%) in the IA-TCP group. Four patients were excluded because of drug resistant TCP, and two patients were excluded because of incomplete records. The characteristics of the patients at baseline were almost similar in the two groups except age, preoperative use of pyrazinamide and fluoroquinolones, serum total protein and albumin (Table 1).
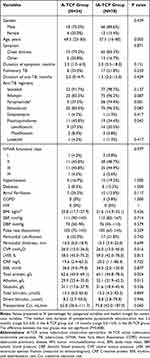 |
Table 1 Baseline Characteristics of the Patients |
In the A-TCP group, age at diagnosis of TCP ranged from 23 to 83 years, with a median of 69.5 years, which was older than that in the IA-TCP group (P = 0.005). In the IA-TCP group, 74.4% (58/78) of patients were treated with the anti-TB regimen containing pyrazinamide, but the rate was only 37.5% (9/24) in the A-TCP group (P = 0.001). However, the proportion of fluoroquinolones use was higher in the A-TCP group than in the IA-TCP group (45.8% vs 24.4%, P = 0.043). In addition to these, the serum total protein level and albumin level of the A-TCP group were significantly lower than those of the IA-TCP group (P = 0.024 and 0.013, respectively).
Protective Factors of A-TCP
In order to detect protective factors of A-TCP, multivariate analysis was performed by logistic regression model. We included factors that were statistically different in the univariate analysis in the multivariate analysis model (Table 2). Demographic characteristics generally did not have a direct effect on pericardial TB activity but were indirectly associated with it through other clinical factors such as nutrition-related factors. Therefore, age was not included in the multivariate analysis. The results showed that preoperative use of pyrazinamide was the independent protective factors of A-TCP (OR 0.194, 95% CI 0.053–0.703, P = 0.013), while the effects of fluoroquinolones, total protein and albumin on A-TCP were not significant.
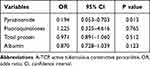 |
Table 2 Protective Factors of A-TCP |
Subgroup analysis was also conducted to eliminate the potential interference of age. Age of the overall study population ranged from 16 to 83 years, with a median of 61 years. The patients were divided into two subgroups, with 49 cases in the age <61 subgroup and 53 in the age ≥61 subgroup. The results were consistent across prespecified subgroups (Figure 1).
 |
Figure 1 Subgroup analysis of the effect of pyrazinamide on A-TCP according to age. Abbreviations: A-TCP, active tuberculous constrictive pericarditis; OR, odds ratio; CI, confidence interval. |
Effect of A-TCP on Outcomes
Surgical outcomes were compared between the two groups (Table 3). No in-hospital death was observed in both groups, but a total of 21 cases occurred cardiac complications after pericardiectomy (Supplemental Table 1). The incidence of cardiac complications was significantly higher in the A-TCP group than in the IA-TCP group (41.7% vs 14.1%, P = 0.008). The patients in the A-TCP group were noted to have longer durations of postoperative chest drainage and hospital stay than those in the IA-TCP group (P = 0.014 and 0.004, respectively). The hospitalization cost was also higher in the A-TCP group (P = 0.001). Other surgical outcomes such as postoperative duration of vasoactive agent use, intubation and ICU stay were similar in the two groups.
 |
Table 3 Comparison of Surgical Outcomes |
In order to determine the degree of contribution of A-TCP on postoperative cardiac complications, we performed univariate analysis at first and then included the statistically significant factors in multivariate logistic regression analysis (Supplemental Table 2). Univariate analysis revealed that risk factors of postoperative cardiac complications were age, A-TCP, preoperative NYHA functional class and preoperative CVP. Multivariate analysis that included these covariates revealed that A-TCP was a significant predictor of postoperative cardiac complications (OR 4.231, 95% CI 1.317–13.593, P = 0.015) (Table 4).
 |
Table 4 Effect of A-TCP on Postoperative Cardiac Complications |
Discussion
The results of this study showed that a strategy involving preoperative anti-TB treatment containing pyrazinamide was superior to that without pyrazinamide in relation to the risk of A-TCP during pericardiectomy. The efficacy of pyrazinamide was consistent across subgroups that were defined according to the median age of overall study population. Patients with A-TCP were at increased risk of postoperative cardiac complications in the analysis of surgical outcomes. This study could provide some reference value for clinicians in the selection of preoperative anti-TB regimens in TCP.
Pyrazinamide is a nicotinamide analogue that can be activated by human and mycobacterial amidases and converted to pyrazinoic acid (POA) which accumulates within MTB and interferes with various important cellular processes, such as the maintenance of metabolic homeostasis and adaptation to stress conditions.16–19 Therefore, pyrazinamide is considered a key sterilizing drug included in the first-line anti-TB regimen and plays a critical role in shortening treatment duration. Previous studies have reported that pyrazinamide could target a subpopulation of MTB within acidified portions of the lesion and distribute rapidly and homogenously into all lesion types, both cellular and caseous lesions.20,21 Recently, it was shown that pyrazinamide could be a PARP1 inhibitor and that PARP1 inhibition might play a mechanistic role in pyrazinamide’s host-directed activity, but its mechanism of action remained incompletely understood.22
The anti-TB effect of pyrazinamide on the TCP has been unclear. This study retrospectively analyzed the eligible patients with TCP in our department. We observed that patients whose preoperative anti-TB regimen did not contain pyrazinamide were more likely to have positive MTB culture and MTB RNA assay in pericardial tissue, which was defined as A-TCP. This implied a greater bacterial burden of pericardial tissue in these patients. Pasipanodya et al have suggested that mortality in proven TB pericarditis was high and the bacillary load was a significant risk factor of poor prognosis.23 Our study validated this finding to some extent. The analysis of surgical outcomes in this study revealed that patients with A-TCP were more vulnerable to postoperative cardiac complications, and multivariate analysis indicated that A-TCP was the independent risk factor of cardiac complications. On top of that, this group of patients had longer hospital stay and higher hospitalization cost, which meant slower postoperative recovery and heavier financial burden for these patients.
It was worth mentioning that patients in the A-TCP group had lower levels of total protein and albumin than those in the IA-TCP. Previous studies have indicated that older patients and those at risk of malnutrition have impaired immune function which could have a negative impact on TB activity.24,25 However, we performed multivariate analysis and found that serum total protein and albumin were not the significant risk factors of A-TCP.
The proportion of preoperative use of pyrazinamide was higher in patients diagnosed with IA-TCP, which was broadly in line with the findings of previous studies. Preclinical disease model that recapitulated the major lung lesions observed in human TB was used to confirm that pyrazinamide could not only penetrate all lesion types but also reduce bacterial burden and sterilize both cellular and necrotic lesions.26–28 However, given the relatively common adverse effects of pyrazinamide, some frail patients may be unable to tolerate standard first-line regimen containing pyrazinamide. Drug-induced liver injury is the main concern, while increased plasma uric acid and gastrointestinal intolerance may also occur.21 Fluoroquinolones are regarded as core agents in case of resistance or intolerance against first-line anti-TB drugs, but the results of trials investigating the outcome of fluoroquinolone-containing regimens are not in favor of fluoroquinolones for TB.29 In this study, a high percentage of patients used an alternative regimen containing fluoroquinolones, with 45.8% in the A-TCP group and 24.4% in the IA-TCP. Univariate analysis suggested that the difference was significant, but fluoroquinolones were not an independent risk factor of A-TCP in multivariate analysis.
This study has some limitations. First, unavoidable bias existed due to the nature of single-center retrospective design. Multivariate logistic regression analysis was conducted to control the effect of confounders to some degree. Moreover, subgroup analysis was performed according to age, eliminating the potential influence of demographic characteristic. Second, because of the low incidence of TCP, the study sample was small, especially the group of A-TCP. Therefore, a multi-institutional, large-scale trial is required to confirm our results. Moreover, this study focused on drug-sensitized TB. Regarding drug-resistant TCP, we would further collect cases for analysis and discussion. Finally, the drug concentration of pyrazinamide in pericardial tissues, as well as the bacterial load in pericardial tissues, remains to be further explored.
Conclusion
Our study showed that preoperative use of pyrazinamide could significantly reduce the risk of A-TCP which was associated with poor surgical outcomes. The results suggested that there was value in considering anti-TB regimen containing pyrazinamide for the patients with TCP to maximize cost-effectiveness and outcome benefit.
Abbreviations
TB, tuberculosis; A-TCP, active tuberculous constrictive pericarditis; IA-TCP, active tuberculous constrictive pericarditis; AFB, acid-fast bacilli; MTB, Mycobacterium tuberculosis; RT-PCR, real-time polymerase chain reaction; NYHA, New York Heart Association; CVP, central venous pressure; OR, odds ratio; CI, confidence interval.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
The study protocol was approved by the Institutional Review Board of Hangzhou Red Cross Hospital (No. 2023104). Because of the retrospective nature of the study and without any specific intervention, the informed consent has been agreed to be waived. The data were maintained with confidentiality. The present study complied with the Declaration of Helsinki.
Funding
There is no funding to report.
Disclosure
The authors declare that they have no competing interests
References
1. Welch TD, Oh JK. Constrictive pericarditis. Cardiol Clin. 2017;35(4):539–549. doi:10.1016/j.ccl.2017.07.007
2. George TJ, Arnaoutakis GJ, Beaty CA, et al. Contemporary etiologies, risk factors, and outcomes after pericardiectomy. Ann Thorac Surg. 2012;94(2):445–451. doi:10.1016/j.athoracsur.2012.03.079
3. Noubiap JJ, Agbor VN, Ndoadoumgue AL, et al. Epidemiology of pericardial diseases in Africa: a systematic scoping review. Heart. 2019;105(3):180–188. doi:10.1136/heartjnl-2018-313922
4. Karima T, Nesrine BZ, Hatem L, et al. Constrictive pericarditis: 21 years’ experience and review of literature. Pan Afr Med J. 2021;38:141. doi:10.11604/pamj.2021.38.141.22884
5. Fang LK, Yu GC, Huang JP, et al. Predictors of postoperative complication and prolonged intensive care unit stay after complete pericardiectomy in tuberculous constrictive pericarditis. J Cardioth Surg. 2020;15(1):148–155. doi:10.1186/s13019-020-01198-9
6. Welch TD. Constrictive pericarditis: diagnosis, management and clinical outcomes. Heart. 2018;104(9):725–731. doi:10.1136/heartjnl-2017-311683
7. Inamdar KY, Aikebaier M, Mulati A. Pericardiectomy: prompt surgical management of constrictive pericarditis. Heart Surg Forum. 2014;17(6):E319–22. doi:10.1532/HSF98.2013183
8. Adler Y, Charron P, Imazio M, et al. 2015 ESC guidelines for the diagnosis and management of pericardial diseases: the task force for the diagnosis and management of pericardial diseases of the European society of cardiology (ESC) Endorsed by: the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2015;36(42):2921–2964. doi:10.1093/eurheartj/ehv318
9. Depboylu BC, Mootoosamy P, Vistarini N, et al. Surgical treatment of constrictive pericarditis. Texas Heart Inst J. 2017;44(2):101–106. doi:10.14503/THIJ-16-5772
10. Dybowska M, Blasinska K, Gatarek J, et al. Tuberculous pericarditis-own experiences and recent recommendations. Diagnostics. 2022;12(3):619. doi:10.3390/diagnostics12030619
11. Syed FF, Schaff HV, Oh JK. Constrictive pericarditis--A curable diastolic heart failure. Nat Rev Cardiol. 2014;11(9):530–544. doi:10.1038/nrcardio.2014.100
12. Mayosi BM, Burgess LJ, Doubell AF. Tuberculous pericarditis. Circulation. 2005;112(23):3608–3616. doi:10.1161/CIRCULATIONAHA.105.543066
13. Fang L, Yu G, Ye B, et al. The optimal duration of anti-tuberculous therapy before pericardiectomy in constrictive tuberculous pericarditis. J Cardiothorac Surg. 2021;16(1):313. doi:10.1186/s13019-021-01691-9
14. Zumla A, Raviglione M, Hafner R, et al. Tuberculosis. New Engl J Med. 2013;368(8):745–755. doi:10.1056/NEJMra1200894
15. Cho YH, Schaff HV. Extent of pericardial resection for constrictive pericardiectomy. Ann Thorac Surg. 2012;94(6):2180. doi:10.1016/j.athoracsur.2012.04.116
16. Via LE, Savic R, Weiner DM, et al. Host-mediated bioactivation of pyrazinamide: implications for efficacy, resistance, and therapeutic alternatives. ACS Infect Dis. 2015;1(5):203–214. doi:10.1021/id500028m
17. Yadon AN, Maharaj K, Adamson JH, et al. A comprehensive characterization of PncA polymorphisms that confer resistance to pyrazinamide. Nat Commun. 2017;8(1):588. doi:10.1038/s41467-017-00721-2
18. Stehr M, Elamin AA, Singh M. Pyrazinamide: the importance of uncovering the mechanisms of action in mycobacteria. Expert Rev Anti Infect Ther. 2015;13(5):593–603. doi:10.1586/14787210.2015.1021784
19. Wahan SK, Sharma S, Chawla PA. Anti-tubercular activity of pyrazinamide conjugates: synthesis and structure-activity relationship studies. Mini Rev Med Chem. 2023;23(6):700–718. doi:10.2174/1389557522666220819092431
20. Prideaux B, Via LE, Zimmerman MD, et al. The association between sterilizing activity and drug distribution into tuberculosis lesions. Nature Med. 2015;21(10):1223–1227. doi:10.1038/nm.3937
21. Peloquin CA, Davies GR. The Treatment of Tuberculosis. Clin Pharmacol Ther. 2021;110(6):1455–1466. doi:10.1002/cpt.2261
22. Krug S, Gupta M, Kumar P, et al. Inhibition of host PARP1 contributes to the anti-inflammatory and antitubercular activity of pyrazinamide. Nat Commun. 2023;14(1):8161. doi:10.1038/s41467-023-43937-1
23. Pasipanodya JG, Mubanga M, Ntsekhe M, et al. Tuberculous pericarditis is multibacillary and bacterial burden drives high mortality. EBioMedicine. 2015;2(11):1634–1639. doi:10.1016/j.ebiom.2015.09.034
24. Liu QX, Tang DY, Xiang X, et al. Associations between nutritional and immune status and clinicopathologic factors in patients with tuberculosis: a comprehensive analysis. Front Cell Infect Microbiol. 2022;12:1013751. doi:10.3389/fcimb.2022.1013751
25. Sinha P, Davis J, Saag L, et al. Undernutrition and tuberculosis: public health implications. J Infect Dis. 2019;219(9):1356–1363. doi:10.1093/infdis/jiy675
26. Subbian S, Tsenova L, Yang G, et al. Chronic pulmonary cavitary tuberculosis in rabbits: a failed host immune response. Open Biol. 2011;1(4):110016. doi:10.1098/rsob.110016
27. Blanc L, Sarathy JP, Alvarez Cabrera N, et al. Impact of immunopathology on the antituberculous activity of pyrazinamide. J Exp Med. 2018;215(8):1975–1986. doi:10.1084/jem.20180518
28. Gopal P, Gruber G, Dartois V, et al. Pharmacological and molecular mechanisms behind the sterilizing activity of pyrazinamide. Trends Pharmacol Sci. 2019;40(12):930–940. doi:10.1016/j.tips.2019.10.005
29. Pranger AD, van der Werf TS, Kosterink JGW, et al. The role of fluoroquinolones in the treatment of tuberculosis in 2019. Drugs. 2019;79(2):161–171. doi:10.1007/s40265-018-1043-y
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
