Back to Journals » Cancer Management and Research » Volume 14
Clinical Insights into the Management of Blastic Plasmacytoid Dendritic Cell Neoplasm
Received 18 February 2022
Accepted for publication 1 June 2022
Published 28 June 2022 Volume 2022:14 Pages 2107—2117
DOI https://doi.org/10.2147/CMAR.S330398
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Beicheng Sun
Yumeng Zhang,1,2 Lubomir Sokol2
1Department of Internal Medicine, University of South Florida, Tampa, FL, USA; 2Department of Malignant Hematology, Moffitt Cancer Center, Tampa, FL, USA
Correspondence: Yumeng Zhang; Lubomir Sokol, Email [email protected]; [email protected]
Abstract: Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is aggressive hematologic malignancy derived from plasmacytoid dendritic cell precursors of myeloid cell lineage. Patients frequently present with bruise-like skin lesions, which typically are followed months later by progressive cytopenias. Historically, BPDCN prognosis has been dismal, with median overall survival ranging from 9 to 13 months. In the past 2 decades, our understanding of BPDCN pathogenesis has led to the successful development of novel therapeutics. In December 2018, the FDA approved tagraxofusp-erzs for adults and pediatric patients older than 2 years who have either treatment-naïve or relapsed/refractory BPDCN. Acute lymphoblastic leukemia (ALL)-based chemotherapy regimens also provide comparable outcomes to tagraxofusp. In our practice, for patients with good performance status, we use tagraxofusp, ALL-based chemotherapy regimens, or clinical trials as frontline induction therapy, followed by consolidation with allogeneic stem cell transplant once the first complete response has been achieved. Our induction regimen also includes intrathecal chemotherapy for central nervous system prophylaxis. Patients with poor performance status who are treatment-naïve or patients with relapsed/refractory disease have limited therapeutic options, and we strongly recommend enrollment in clinical trials; several novel agents and combinations are currently under clinical investigation for both treatment-naïve and relapsed/refractory BPDCN.
Keywords: tagraxofusp, chemotherapy, allogeneic, transplantation
Introduction
Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is rare, aggressive hematologic malignancy that frequently involve the skin at presentation. BPDCN is derived from the precursors of plasmacytoid dendritic cells (pDCs) of myeloid cell lineage.1–3 This entity was first described by Adachi et al in 1994 as a blastic CD4-positive lymphoma with high expression of CD56.4 Because of the co-expression of CD56, a natural killer cell origin was proposed, and the disease was also known as blastic natural killer cell lymphoma and agranular CD4+ CD56+ hematodermic neoplasm. It was renamed to BPDCN in the 2008 revision of the WHO Classification of Tumors of the Hematopoietic and Lymphoid Tissues. The 2016 WHO taxonomy classified BPDCN as a separate category from myeloid cell-derived neoplasms, thereby recognizing its unique clinical presentation and genetic features.5
Historically, patients with BPDCN experience poor outcomes, with overall survival (OS) rates ranging from 9 to 13 months.6 On December 21, 2018, the FDA approved tagraxofusp-erzs (SL-401, brand name ELZONRIS; manufactured by Stemline Therapeutics, New York City, NY) for patients with untreated or relapsed/refractory (r/r) BPDCN. Despite high response rate, the duration of response is limited in patients who are ineligible for allogeneic stem cell transplant (AlloHSCT). Since then, increasing efforts have been devoted to understanding the pathophysiology of BPDCN and developing novel therapeutics for this patient population.
This review will discuss the clinical presentation, diagnosis, cytogenetic/molecular features, and management of BPDCN in the modern era.
Clinical Presentation
Though the median age of BPDCN diagnosis is 64 years, it has a bimodal distribution with 2 peaks: one for patients younger than 20 years old and one for patients older than 60 years. The male-to-female ratio is 4:1.7–10 The male predominance has been linked with the loss-of-function mutation in ZRSR2, an X chromosome gene resulting in aberrant RNA splicing.10 For more than 90% of patients, skin lesions are the initial signs of disease, which are typically followed by progressive cytopenias months later.11–13 Skin lesions vary in size, color and shape, and includes violaceous, erythematous, red brown and/or purpuric patches, plagues, nodules or ulcerative lesions. The bruise-like infiltrates lesions, most characteristic for BPDCN, could be an early manifestation of neoplastic cell infiltration with red blood cell extravasation in the skin.14 The Modified Skin Weighted Assessment Tool (mSWAT) can be used to estimate and monitor the extent and severity of cutaneous disease.15
Leukemic blastic cells can rapidly disseminate in the bone marrow, leading to transfusion-dependent anemia, bleeding, and infectious complications.1,2,16 The lymph nodes, liver, and spleen are frequently infiltrated during the disease process.2,12,16 In 30% to 60% of cases, BPDCN exhibits central nervous system (CNS) involvement, with or without neurologic signs;15,17,18 however, this percentage is probably underestimated.19
Diagnosis
Our diagnostic work-up for BPDCN is summarized in Figure 1. Morphologically, leukemic blastic cells are intermediate-sized, with a round peripheral nucleus and nucleolus. The cytoplasm is faintly basophilic without granulation.1–3,12 The immunophenotype of malignant cells lacks myeloid-, T-, or B-cell lineage immunophenotypic markers and often expresses CD4, CD56, and high levels of HLA-DR.
The pDC origin can be confirmed by very high expression of CD123, CD303 (BDCA2), or TLC1.16,20,21 Though CD123 and TLC1 can also be expressed in other lymphoid and myeloid malignancies, their expression levels are much higher in BPDCN.22,23 CD303 expression is very specific to BPDCN, but it is only present in 70% of cases.24 Compared to AML and mature pDC proliferation associated with myeloid neoplasm, BPDCN lacks immature markers, such as CD34 or CD133. A comprehensive flow cytometry panel is required for BPDCN diagnosis and must include pDC markers such as CD2AP, CD4, CD56, CD3, CD20, CD2AP, CD123, CD303, CD304, and TLC1.23
Molecular Landscape
In more than half of BPDCN cases, cytogenetics studies reveal a complex karyotype with chromosomal losses.25 The most frequent abnormalities include those in chromosomes 9 or 9q (61%), 12p13 (59%), 13q (55%), 17p (33%), 5q (28%), and 7p12.2 (20%).26 Some losses involve tumor suppressor genes, such as CDKN2A, CDKN2B, CDKN1B, ETV6, RB1, and LATS2;27,28 chromosomal losses involving regulators of the G1/S cell cycle likely contribute to the development of BPDCN. The 8q24 rearrangement is identified in 38% of BPDCN cases.25,29,30 The most frequent partner locus of 8q24 is SUPT3H (6q21), which overlaps with RUNX2, a highly expressed super-enhancer gene.25,29,30 The 8q24 rearrangement results in MYC overexpression and leads to accelerated cell proliferation, growth, and genome instability.31 MYB rearrangement, seen in 20% of BPDCN cases, deregulates the cell cycle and functions as a proto-oncogene.25,32,33
Like other myeloid malignancies, the mutational landscape of BPDCN is characterized by recurrent mutations in epigenetic modifiers, transcriptional regulators, and splicing factors.34,35 Concurrent manifestation of BPDCN and MDS or AML has been described; this suggests a common pathogenesis, with initiating mutations in pluripotent myeloid stem cells.36 The 2 most frequently mutated epigenetic genes in BPDCN are AXSL1 and TET2, and these mutations are associated with poor OS in BPDCN.34,37–40
Further investigations using RNA sequencing showed deregulation of 2 methylation gene signatures that are responsive to DNA hypomethylating agents (HMAs): KDM5B, a histone demethylase gene, and PRMT5, a methyltransferase-associated gene.39,41,42 Interestingly, TP53 is frequently co-mutated with TET2, suggesting a synergistic effect.38 Other frequently observed mutated genes in BPDCN include ARID1A, IDH1, IDH2, U2AF1, SRSF2, IKZF1-3, IRF8, ABL1, GNA13, and KRAS.34,37,39,43–47
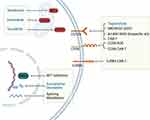
BPDCN also consistently demonstrates overexpression of lncRNA-3q,45 which is thought to activate and change epigenetic programming of leukemia stem cells.48 BRD4, a bromodomain and extraterminal (BET) domain protein, binds to the promoter of lincRNA-3q and regulates lincRNA-3q expression.30 BET inhibitors have been shown to reverse abnormal lincRNA-3q overexpression in animal models and are currently under investigation to treat BPDCN (Figure 2).30 In addition, the overexpression of TCF4, a master transcriptional regulator, has been consistently demonstrated among patients with BPDCN. TCF4 silencing agents are another promising potential epigenetic-based therapy.30
Disease Management
Before December 2018, the treatment paradigm of BPDCN revolved around intensive chemotherapy regimens. However, responses to chemotherapy were typically short-lived; median OS from these regimens range from 5 to 30 months.18,37,49–52 AlloHSCT is the only curative treatment option, but only a small portion of patients are transplant-eligible. Small retrospective case series have reported 3-year OS rates around 60% among patients who underwent transplant after achieving first complete remission (CR1).53–56
Fit Patients
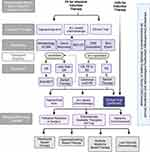
Our preferred frontline agents for fit patients, who have good performance status, include tagraxofusp-erzs; acute lymphoblastic leukemia– (ALL-) based chemotherapy regimens; or clinical trials, if available (Figure 3). There are no prospective randomized studies comparing outcomes from ALL-based chemotherapies and tagraxofusp, but in our previously published real-world experience, first-line ALL-based regimens were associated with a higher response rates than tagraxofusp (95% vs 50%; P =.069) and had comparable progression-free survival and OS rates.36
For fit patients who are candidates for alloHSCT, we aim for them to achieve CR1 prior to alloHSCT. If CR1 is not achieved after induction therapy, we alternate the above frontline regimens until CR is achieved and then proceed with alloHSCT. Among patients with good performance status but not candidates for alloHSCT due to age, we prefer clinical trials or tagraxofusp, as we have observed a small portion of patients experience long-term responses to continuous tagraxofusp therapy.
Frontline Regimens
Tagraxofusp-Erzs
Tagraxofusp is a cytokine-toxin fusion protein connecting truncated diphtheria toxin to recombinant human interleukin 3.57 The IL3 domain binds to CD123, which is strongly expressed on malignant BPDCN cells’ surfaces, and this subsequently leads to the internalization of diphtheria toxin, resulting in inhibition of protein synthesis and cell death.58 Tagraxofusp was first evaluated in a pilot Phase 1/2 trial that included 11 adult patients with BPDCN.6 Seven out of nine evaluable patients experienced either complete remission (CR) or partial remission (PR) after a single course of tagraxofusp.
These promising results led to a subsequent prospective multicenter open-label single-arm study of 47 adult patients with either treatment-naïve or r/r BPDCN who had no clinically evident CNS disease and had a baseline serum albumin level of ≥ 3.2 g/dL.15 The overall response rate was 90%, with a median time-to-response of 43 days. The CR was 72% (95% CI, 53–87%). Among enrolled patients, 45% of treatment-naïve patients underwent consolidation with alloHSCT. The most common adverse events were transaminitis; thrombocytopenia; and capillary leak syndrome (CLS),15 which occurred among 18% of patients and resulted in 2 treatment-related deaths.59
Careful patient selection and supportive measures are essential in reducing toxicity from tagraxofusp-erzs.58 The FDA recommends ensuring patients have a serum albumin level ≥ 3.2 g/dL before initiating each cycle. For otherwise fit patients who have serum albumin levels < 3.2 g/dL, we often prescribe intravenous albumin to increase serum albumin to the target level prior to treatment. The first cycle should be completed in the inpatient setting, with close monitoring for at least 24 hours afterward. Premedication include an H1 histamine antagonist, acetaminophen, and corticosteroids.
Tagraxofusp should be paused if patients develop transaminitis, acute renal injury, hypo- or hypertension, tachy- or bradycardia, fever, or a mild/moderate hypersensitivity reaction. Supportive measures for CLS include intravenous albumin; careful management of volume status; and administration of corticosteroids, diuretics, and vasopressors, if indicated. Intravenous albumin should be administered if serum albumin levels decrease by ≥ 0.5 g/dL from baseline or below 3.5 g/dL or if the patient experiences weight gain of more than 1.5 kg over 24 hours. High-dose methylprednisolone (1 mg/kg or equivalent per day) is recommended until CLS resolves.
For some patients who are transplant-ineligible or underwent previous treatment, the duration of response to tagraxofusp may be limited. The primary resistance mechanism is DPH1 methylation and reduced expression of the diphthamide pathway enzyme.60,61 The addition of an HMA or BCL-2 inhibitor to tagraxofusp is currently under investigation (Figure 2). Notably, CD123 expression is not reduced among patients who developed resistance to tagraxofusp, and so there have been several investigational agents targeting CD123.62 These include IMGN632, which is composed of an anti-CD123 antibody conjugated with a DNA-alkylating payload; MB-102, a CD123 chimeric antigen receptor T-cell therapeutic regimen; and XmAB14045, a bispecific CD3 × CD123 T-cell engager (Figure 2).62,63
ALL-Based Chemotherapy Regimens
Intensive chemotherapy remains an effective option for patients who are fit and eligible for HSCT even in the era of tagraxofusp. Currently, there are no prospective randomized comparison studies between different chemotherapy regimens. Based on the results of multiple retrospective studies, ALL-based regimens conferred better response rates than acute myeloid leukemia– (AML-) based or CHOP- (cyclophosphamide, doxorubicin hydrochloride [hydroxydaunorubicin], vincristine sulfate [Oncovin], and prednisone–) like regimens.36,50–52 The improved survival seen with ALL-based regimens could possibly be explained by the incorporation of CNS prophylaxis in these regimens, which will be further discussed later in the paper. CNS relapse with or without systemic relapse is common in patients with BPDCN and is associated with a poor prognosis.64
Hematopoietic Stem Cell Transplant
Given the aggressive nature of BPDCN, the currently available treatment regimens rarely provide long-term survival despite their high initial response rates. AlloHSCT remains the only potential cure for BPDCN and should be pursued after CR1 for transplant-eligible patients.65 In our practice, we refer patients for bone marrow transplant consultation at the time of diagnosis to initiate a transplant evaluation and donor search. Once CR1 is achieved, eligible patients will proceed with alloHSCT.
The timing of the transplant remains a critical factor effecting post-transplant outcomes. Across studies conducted in Europe, Japan, and North America, transplants performed at the time of CR1 have consistently demonstrated better outcomes than to transplants performed at the second CR (CR2) or later.53,55,56,66 In a meta-analysis of 128 patients with BPDCN who underwent alloHSCT, the pooled OS rate was 50% for all patients.54 Among patients who received alloHSCT during CR1, the pooled OS rate was 67%; among patients who received alloHSCT during CR2 or later, the pooled OS rate was 8%. Because of the small sample size, the study was not powered to compare outcomes between patients who received reduced-intensity conditioning and those receiving myeloablative conditioning; however, the relapse rate was higher among patients receiving reduced-intensity conditioning than those receiving myeloablative conditioning (40% vs 18%).54 The role of post-transplant maintenance therapy is currently under investigation.
The evidence relating to autologous HSCT for BPDCN is limited. A multicenter North American observational study reported a 1-year OS of 11%.53
Unfit Patients or r/r Disease
There is still a significant unmet clinical need for patients with poor performance status and is ineligible for standard first-line regimens or whose diseases have progressed or relapsed.67 Given the poor outcomes seen with current treatment options among these patients, enrollment in clinical trials is strongly recommended (Figure 3).68 Table 1 details the currently active clinical trials for BPDCN.
 |
Table 1 Ongoing Clinical Trials Registered on Clinicaltrials.gov for Blastic Plasmacytoid Dendritic Cell Neoplasm That are Actively Recruiting Patients as of 2/6/2022 |
For patients who cannot participate in clinical trials, preclinical studies and case series have reported results for several commercially available agents: venetoclax, hypomethylating agents, multiple myeloma derived therapies and low intensity chemotherapy regimens.
Venetoclax-Based Regimens
BPDCN highly expresses antiapoptotic protein B cell leukemia/lymphoma-2 (BCL2), and patient-derived mouse models show that malignant BPDCN cells are dependent on BCL2 (Figure 2). Venetoclax, a BCL2 inhibitor, has been used in case reports as a monotherapy for r/r disease.68,69 Most patients achieved either PR or CR; however, responses were short-lived, ranging from 1 to 10 months. The dosing of venetoclax followed that of AML regimens, with a weekly dose escalation. Venetoclax and HMA combinations have also shown promising safety and efficacy profiles against BPDCN.70 In a case series of 10 patients, combined venetoclax and HMA led to a rapid response in the skin, lymph nodes, and bone marrow. Two patients achieved PR and eight achieved CR;70 however, duration of responses varied between 2 to 36 months.
Two triplets of venetoclax combinations—tagraxofusp + 5-azacitidine + venetoclax and tagraxofusp + venetoclax + HCVAD (cyclophosphamide, vincristine sulfate, doxorubicin hydrochloride [Adriamycin], dexamethasone, methotrexate, and cytarabine)/miniCVD (cyclophosphamide, vincristine sulfate and dexamethasone) —are currently being studied in BPDCN clinical trials (Table 1).
Hypomethylating Agents
The primary resistance mechanism to tagraxofusp monotherapy involves hypermethylation of the DPH1 gene and subsequent reduction of the diphthamide pathway enzyme (DPH1). HMAs can potentially reverse such resistance by reversing the hypermethylation.60,61 Standard-dose 5-azacitidine monotherapy (75 mg/m2/day for 7 days) can provide rapid but short-lived responses.71,72 To extend the response, a clinical trial combining 5-azacitidine, venetoclax, and tagraxofusp is currently ongoing.
Multiple Myeloma–Derived Therapies
Several case reports have suggested that myeloma-based therapy has potential role for treating BPDCN. Three patients with BPDCN were treated with bortezomib, lenalidomide, and dexamethasone (VRd) and achieved responses. Dosing consisted of 10 mg of lenalidomide orally on days 1 through 21; 1.3 mg/m2 of bortezomib intravenously on days 1, 8, 15, and 22; and 20 mg of dexamethasone orally on days 1, 8, 15, and 22.73
Daratumumab, an anti-CD38 monoclonal antibody, has been FDA-approved in combination with pomalidomide and dexamethasone for patients with multiple myeloma who have already received at least 1 prior line of therapy. As a monotherapy, daratumumab has been prescribed to elderly patients with BPDCN at a 16 mg/kg weekly dose and elicited rapid responses.74,75
Low-Intensity Chemotherapy Regimens
A low-intensity chemotherapy regimen is a potential option for elderly or unfit patients who cannot participate in clinical trials. However, again, responses are short-lived. In our practice, we prescribe a CHOP-like regimen to patients who are not candidates for induction therapy but present with symptoms from BPDCN. Though the CHOP-like regimen was less effective than ALL-based induction therapy in retrospective studies, it still has an approximately 40% CR rate, with a median duration of response of 7 months.16
Among patients who are not candidates for CHOP-like therapy, case reports have indicated several alternative treatment options, including bendamustine monotherapy (100 mg/m2 on days 1 to 2 every 3 weeks), combined gemcitabine and docetaxel (800 mg/m2 of gemcitabine on day 1 and 8 and 75 mg/m2 of docetaxel on day 8 every 3 weeks), or pralatrexate monotherapy (15–30 mg/m2 weekly); these regimens have varied response rates ranging between 20% and 70%.64,76–80
CNS Prophylaxis
The incidence of CNS involvement in BPDCN is mainly unknown because the previous standard-of-care did not include lumbar puncture (LP) at the time of diagnosis. Martin-Martin et al evaluated 13 consecutive patients with BPDCN and found occult CNS involvement in 60% of cases at diagnosis and 100% at relapse,19 but none of the patients had neurologic symptoms. Similarly, Pemmaraju et al reported CNS involvement in 23 out of 29 (79%) patients who underwent LP analyses;81 of these patients, CNS involvement was detected during routine frontline LP for 57%, and only 43% of 23 patients developed neurologic deficits.19
The role of CNS prophylaxis regimens has not yet been fully explored. However, ALL-based regimens that include CNS prophylaxis demonstrate improved survival outcomes compared to AML-based regimens, which does not routinely include CNS prophylaxis.17,18,82 Pemmaraju et al reported a nonsignificant trend toward improved survival among patients treated for frontline occult CNS disease, supporting the potential benefit of upfront intrathecal therapies.81
In our practice, we perform a diagnostic LP at the time of BPDCN diagnosis, relapse, or any signs of neurologic symptoms. We use both flow cytometry and cytology to test cerebrospinal fluid for the presence of malignant cells. We administer prophylactic intrathecal chemotherapy (alternating cytarabine and methotrexate) during induction treatment regardless of CNS involvement (Figure 3).
Summary
BPDCN is an aggressive hematologic malignancy that affects both young and elderly populations. In the past several decades, our understanding of pathogenesis and molecular targets of disease has successfully led to the development of effective therapeutics, including the first-in-class FDA approval of tagraxofusp. Even though the initial response rates for tagraxofusp or ALL-based regimens are promising for fit patients, these options are not curative without consolidation with alloHSCT. For patients who are unfit or have r/r disease, current alternative treatment options only provide short-lived responses. Fortunately, there are several ongoing clinical trials with novel agents or combinations for both treatment-naïve and r/r BPDCN. We strongly recommend all patient enrollment in clinical trials if possible to treat this aggressive disease.
Abbreviations
ALL, acute lymphoblastic leukemia; alloHSCT, allogeneic stem cell transplant; BET, bromodomain and extraterminal; BPDCN, Blastic plasmacytoid dendritic cell neoplasm; CLS, capillary leak syndrome; CNS, central nervous system; CR, complete response; CR1, first complete remission; CR2, Second complete remission; HMAs, hypomethylating agents; LP, lumbar puncture; MPDCP, mature pDC proliferation associated with myeloid neoplasm; mSWAT, Modified Skin Weighted Assessment Tool; OS, overall survival; pDC, plasmacytoid dendritic cells; PR, partial response; r/r, relapsed/refractory.
Acknowledgments
Editorial assistance was provided by the Moffitt Cancer Center’s Office of Scientific Publishing by Daley Drucker and Gerard Hebert. No compensation was given beyond their regular salaries.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article. This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Disclosure
The authors declare no conflict of interest.
References
1. Tzankov A, Hebeda K, Kremer M, et al. Plasmacytoid dendritic cell proliferations and neoplasms involving the bone marrow: summary of the workshop cases submitted to the 18th Meeting of the European Association for Haematopathology (EAHP) organized by the European Bone Marrow Working Group, Basel 2016. Ann Hematol. 2017;96(5):765–777. doi:10.1007/s00277-017-2947-4
2. Sapienza MR, Pileri A, Derenzini E, et al. Blastic plasmacytoid dendritic cell neoplasm: state of the art and prospects. Cancers. 2019;11(5):595. doi:10.3390/cancers11050595
3. Sabattini E, Bacci F, Sagramoso C, Pileri SA. WHO classification of tumours of haematopoietic and lymphoid tissues in 2008: an overview. Pathologica. 2010;102(3):83–87.
4. Adachi M, Maeda K, Takekawa M, et al. High expression of CD56 (N-CAM) in a patient with cutaneous CD4-positive lymphoma. Am J Hematol. 1994;47(4):278–282. doi:10.1002/ajh.2830470406
5. Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–2405. doi:10.1182/blood-2016-03-643544
6. Frankel AE, Woo JH, Ahn C, et al. Activity of SL-401, a targeted therapy directed to interleukin-3 receptor, in blastic plasmacytoid dendritic cell neoplasm patients. Blood. 2014;124(3):385–392. doi:10.1182/blood-2014-04-566737
7. Venugopal S, Zhou S, El Jamal SM, Lane AA, Mascarenhas J. Blastic plasmacytoid dendritic cell neoplasm-current insights. Clin Lymphoma Myeloma Leuk. 2019;19(9):545–554. doi:10.1016/j.clml.2019.06.002
8. Guru Murthy GS, Pemmaraju N, Atallah E. Epidemiology and survival of blastic plasmacytoid dendritic cell neoplasm. Leuk Res. 2018;73:21–23. doi:10.1016/j.leukres.2018.08.014
9. Sweet K. Blastic plasmacytoid dendritic cell neoplasm: diagnosis, manifestations, and treatment. Curr Opin Hematol. 2020;27(2):103–107. doi:10.1097/MOH.0000000000000569
10. Togami K, Chung SS, Madan V, et al. Sex-biased ZRSR2 mutations in myeloid malignancies impair plasmacytoid dendritic cell activation and apoptosis. Cancer Discov. 2022;12(2):522–541. doi:10.1158/2159-8290.CD-20-1513
11. Amitay-Laish I, Sundram U, Hoppe RT, Hodak E, Medeiros BC, Kim YH. Localized skin-limited blastic plasmacytoid dendritic cell neoplasm: a subset with possible durable remission without transplantation. JAAD Case Rep. 2017;3(4):310–315. doi:10.1016/j.jdcr.2017.03.015
12. Deconinck E, Petrella T, Garnache Ottou F. Blastic plasmacytoid dendritic cell neoplasm: clinical presentation and diagnosis. Hematol Oncol Clin North Am. 2020;34(3):491–500. doi:10.1016/j.hoc.2020.01.010
13. Pileri A, Delfino C, Grandi V, Agostinelli C, Pileri SA, Pimpinelli N. Blastic plasmacytoid dendritic cell neoplasm (BPDCN): the cutaneous sanctuary. G Ital Dermatol Venereol. 2012;147(6):603–608.
14. Hirner JP, O’Malley JT, LeBoeuf NR. Blastic plasmacytoid dendritic cell neoplasm: the dermatologist’s perspective. Hematol Oncol Clin North Am. 2020;34(3):501–509. doi:10.1016/j.hoc.2020.01.001
15. Pemmaraju N, Lane AA, Sweet KL, et al. Tagraxofusp in blastic plasmacytoid dendritic-cell neoplasm. N Engl J Med. 2019;380(17):1628–1637. doi:10.1056/NEJMoa1815105
16. Garnache-Ottou F, Vidal C, Biichle S, et al. How should we diagnose and treat blastic plasmacytoid dendritic cell neoplasm patients? Blood Adv. 2019;3(24):4238–4251. doi:10.1182/bloodadvances.2019000647
17. Pagano L, Valentini CG, Pulsoni A, et al. Blastic plasmacytoid dendritic cell neoplasm with leukemic presentation: an Italian multicenter study. Haematologica. 2013;98(2):239–246. doi:10.3324/haematol.2012.072645
18. Martin-Martin L, Lopez A, Vidriales B, et al. Classification and clinical behavior of blastic plasmacytoid dendritic cell neoplasms according to their maturation-associated immunophenotypic profile. Oncotarget. 2015;6(22):19204–19216. doi:10.18632/oncotarget.4146
19. Martin-Martin L, Almeida J, Pomares H, et al. Blastic plasmacytoid dendritic cell neoplasm frequently shows occult central nervous system involvement at diagnosis and benefits from intrathecal therapy. Oncotarget. 2016;7(9):10174–10181. doi:10.18632/oncotarget.7101
20. Sumarriva Lezama L, Chisholm KM, Carneal E, et al. An analysis of blastic plasmacytoid dendritic cell neoplasm with translocations involving the MYC locus identifies t(6;8)(p2124) as a recurrent cytogenetic abnormality. Histopathology. 2018;73(5):767–776. doi:10.1111/his.13668
21. Angelot-Delettre F, Biichle S, Ferrand C, et al. Intracytoplasmic detection of TCL1–but not ILT7-by flow cytometry is useful for blastic plasmacytoid dendritic cell leukemia diagnosis. Cytometry A. 2012;81(8):718–724. doi:10.1002/cyto.a.22072
22. Petrella T, Meijer CJ, Dalac S, et al. TCL1 and CLA expression in agranular CD4/CD56 hematodermic neoplasms (blastic NK-cell lymphomas) and leukemia cutis. Am J Clin Pathol. 2004;122(2):307–313. doi:10.1309/0QPP-AVTU-PCV9-UCLV
23. Garnache-Ottou F, Feuillard J, Ferrand C, et al. Extended diagnostic criteria for plasmacytoid dendritic cell leukaemia. Br J Haematol. 2009;145(5):624–636. doi:10.1111/j.1365-2141.2009.07679.x
24. Djokic M, Bjorklund E, Blennow E, Mazur J, Soderhall S, Porwit A. Overexpression of CD123 correlates with the hyperdiploid genotype in acute lymphoblastic leukemia. Haematologica. 2009;94(7):1016–1019. doi:10.3324/haematol.2008.000299
25. Sakamoto K, Katayama R, Asaka R, et al. Recurrent 8q24 rearrangement in blastic plasmacytoid dendritic cell neoplasm: association with immunoblastoid cytomorphology, MYC expression, and drug response. Leukemia. 2018;32(12):2590–2603. doi:10.1038/s41375-018-0154-5
26. Lucioni M, Novara F, Fiandrino G, et al. Twenty-one cases of blastic plasmacytoid dendritic cell neoplasm: focus on biallelic locus 9p21.3 deletion. Blood. 2011;118(17):4591–4594. doi:10.1182/blood-2011-03-337501
27. Sakamoto K, Takeuchi K. Cytogenetics of blastic plasmacytoid dendritic cell neoplasm: chromosomal rearrangements and DNA copy-number alterations. Hematol Oncol Clin North Am. 2020;34(3):523–538. doi:10.1016/j.hoc.2020.01.003
28. Kim WY, Sharpless NE. The regulation of INK4/ARF in cancer and aging. Cell. 2006;127(2):265–275. doi:10.1016/j.cell.2006.10.003
29. Nakamura Y, Kayano H, Kakegawa E, et al. Identification of SUPT3H as a novel 8q24/MYC partner in blastic plasmacytoid dendritic cell neoplasm with t(6;8)(p2124) translocation. Blood Cancer J. 2015;5:e301. doi:10.1038/bcj.2015.26
30. Ceribelli M, Hou ZE, Kelly PN, et al. A druggable TCF4- and BRD4-dependent transcriptional network sustains malignancy in blastic plasmacytoid dendritic cell neoplasm. Cancer Cell. 2016;30(5):764–778. doi:10.1016/j.ccell.2016.10.002
31. Lourenco C, Resetca D, Redel C, et al. MYC protein interactors in gene transcription and cancer. Nat Rev Cancer. 2021;21(9):579–591. doi:10.1038/s41568-021-00367-9
32. Suzuki K, Suzuki Y, Hama A, et al. Recurrent MYB rearrangement in blastic plasmacytoid dendritic cell neoplasm. Leukemia. 2017;31(7):1629–1633. doi:10.1038/leu.2017.101
33. George OL, Ness SA. Situational awareness: regulation of the myb transcription factor in differentiation, the cell cycle and oncogenesis. Cancers. 2014;6(4):2049–2071. doi:10.3390/cancers6042049
34. Menezes J, Acquadro F, Wiseman M, et al. Exome sequencing reveals novel and recurrent mutations with clinical impact in blastic plasmacytoid dendritic cell neoplasm. Leukemia. 2014;28(4):823–829. doi:10.1038/leu.2013.283
35. Yin CC, Pemmaraju N, You MJ, et al. Integrated clinical genotype-phenotype characteristics of blastic plasmacytoid dendritic cell neoplasm. Cancers. 2021;13(23):5888. doi:10.3390/cancers13235888
36. Yun S, Chan O, Kerr D, et al. Survival outcomes in blastic plasmacytoid dendritic cell neoplasm by first-line treatment and stem cell transplant. Blood Adv. 2020;4(14):3435–3442. doi:10.1182/bloodadvances.2020001875
37. Alayed K, Patel KP, Konoplev S, et al. TET2 mutations, myelodysplastic features, and a distinct immunoprofile characterize blastic plasmacytoid dendritic cell neoplasm in the bone marrow. Am J Hematol. 2013;88(12):1055–1061. doi:10.1002/ajh.23567
38. Jardin F, Ruminy P, Parmentier F, et al. TET2 and TP53 mutations are frequently observed in blastic plasmacytoid dendritic cell neoplasm. Br J Haematol. 2011;153(3):413–416. doi:10.1111/j.1365-2141.2010.08556.x
39. Sapienza MR, Abate F, Melle F, et al. Blastic plasmacytoid dendritic cell neoplasm: genomics mark epigenetic dysregulation as a primary therapeutic target. Haematologica. 2019;104(4):729–737. doi:10.3324/haematol.2018.202093
40. Nomburg J, Bullman S, Chung SS, et al. Comprehensive metagenomic analysis of blastic plasmacytoid dendritic cell neoplasm. Blood Adv. 2020;4(6):1006–1011. doi:10.1182/bloodadvances.2019001260
41. Rasmussen KD, Jia G, Johansen JV, et al. Loss of TET2 in hematopoietic cells leads to DNA hypermethylation of active enhancers and induction of leukemogenesis. Genes Dev. 2015;29(9):910–922. doi:10.1101/gad.260174.115
42. Li Z, Cai X, Cai CL, et al. Deletion of Tet2 in mice leads to dysregulated hematopoietic stem cells and subsequent development of myeloid malignancies. Blood. 2011;118(17):4509–4518. doi:10.1182/blood-2010-12-325241
43. Sichien D, Scott CL, Martens L, et al. IRF8 transcription factor controls survival and function of terminally differentiated conventional and plasmacytoid dendritic cells, respectively. Immunity. 2016;45(3):626–640. doi:10.1016/j.immuni.2016.08.013
44. Stenzinger A, Endris V, Pfarr N, et al. Targeted ultra-deep sequencing reveals recurrent and mutually exclusive mutations of cancer genes in blastic plasmacytoid dendritic cell neoplasm. Oncotarget. 2014;5(15):6404–6413. doi:10.18632/oncotarget.2223
45. Emadali A, Hoghoughi N, Duley S, et al. Haploinsufficiency for NR3C1, the gene encoding the glucocorticoid receptor, in blastic plasmacytoid dendritic cell neoplasms. Blood. 2016;127(24):3040–3053. doi:10.1182/blood-2015-09-671040
46. Szczepaniak A, Machnicki M, Gniot M, et al. Germline missense NF1 mutation in an elderly patient with a blastic plasmacytoid dendritic cell neoplasm. Int J Hematol. 2019;110(1):102–106. doi:10.1007/s12185-019-02642-w
47. Abdel-Wahab O, Pardanani A, Patel J, et al. Concomitant analysis of EZH2 and ASXL1 mutations in myelofibrosis, chronic myelomonocytic leukemia and blast-phase myeloproliferative neoplasms. Leukemia. 2011;25(7):1200–1202. doi:10.1038/leu.2011.58
48. Jang MK, Mochizuki K, Zhou M, Jeong HS, Brady JN, Ozato K. The bromodomain protein Brd4 is a positive regulatory component of P-TEFb and stimulates RNA polymerase II-dependent transcription. Mol Cell. 2005;19(4):523–534. doi:10.1016/j.molcel.2005.06.027
49. Taylor J, Haddadin M, Upadhyay VA, et al. Multicenter analysis of outcomes in blastic plasmacytoid dendritic cell neoplasm offers a pretargeted therapy benchmark. Blood. 2019;134(8):678–687. doi:10.1182/blood.2019001144
50. Tsagarakis NJ, Kentrou NA, Papadimitriou KA, et al. Acute lymphoplasmacytoid dendritic cell (DC2) leukemia: results from the Hellenic Dendritic Cell Leukemia Study Group. Leuk Res. 2010;34(4):438–446. doi:10.1016/j.leukres.2009.09.006
51. Dalle S, Beylot-Barry M, Bagot M, et al. Blastic plasmacytoid dendritic cell neoplasm: is transplantation the treatment of choice? Br J Dermatol. 2010;162(1):74–79. doi:10.1111/j.1365-2133.2009.09373.x
52. Hashikawa K, Niino D, Yasumoto S, et al. Clinicopathological features and prognostic significance of CXCL12 in blastic plasmacytoid dendritic cell neoplasm. J Am Acad Dermatol. 2012;66(2):278–291. doi:10.1016/j.jaad.2010.12.043
53. Kharfan-Dabaja MA, Al Malki MM, Deotare U, et al. Haematopoietic cell transplantation for blastic plasmacytoid dendritic cell neoplasm: a North American multicentre collaborative study. Br J Haematol. 2017;179(5):781–789. doi:10.1111/bjh.14954
54. Kharfan-Dabaja MA, Reljic T, Murthy HS, Ayala E, Kumar A. Allogeneic hematopoietic cell transplantation is an effective treatment for blastic plasmacytoid dendritic cell neoplasm in first complete remission: systematic review and meta-analysis. Clin Lymphoma Myeloma Leuk. 2018;18(11):703–709 e1. doi:10.1016/j.clml.2018.07.295
55. Roos-Weil D, Dietrich S, Boumendil A, et al. Stem cell transplantation can provide durable disease control in blastic plasmacytoid dendritic cell neoplasm: a retrospective study from the European Group for Blood and Marrow Transplantation. Blood. 2013;121(3):440–446. doi:10.1182/blood-2012-08-448613
56. Aoki T, Suzuki R, Kuwatsuka Y, et al. Long-term survival following autologous and allogeneic stem cell transplantation for blastic plasmacytoid dendritic cell neoplasm. Blood. 2015;125(23):3559–3562. doi:10.1182/blood-2015-01-621268
57. Ray A, Song Y, Das DS, et al. SL-401, a novel IL-3Rα/CD123-directed agent targets stem-like cells in multiple myeloma. Blood. 2016;128(22):4463. doi:10.1182/blood.V128.22.4463.4463
58. Tandon A, Zhang Y, Sokol L. Tagraxofusp, a novel CD123-directed cytotoxin to treat blastic plasmacytoid dendritic cell neoplasm. Drugs Today. 2019;55(12):735–742. doi:10.1358/dot.2019.55.12.3058917
59. Sugita M, Guzman ML. CD123 as a therapeutic target against malignant stem cells. Hematol Oncol Clin North Am. 2020;34(3):553–564. doi:10.1016/j.hoc.2020.01.004
60. Su X, Lin Z, Lin H. The biosynthesis and biological function of diphthamide. Crit Rev Biochem Mol Biol. 2013;48(6):515–521. doi:10.3109/10409238.2013.831023
61. DiNardo CD, Rausch CR, Benton C, et al. Clinical experience with the BCL2-inhibitor venetoclax in combination therapy for relapsed and refractory acute myeloid leukemia and related myeloid malignancies. Am J Hematol. 2018;93(3):401–407. doi:10.1002/ajh.25000
62. Xue T, Budde LE. Immunotherapies targeting CD123 for blastic plasmacytoid dendritic cell neoplasm. Hematol Oncol Clin North Am. 2020;34(3):575–587. doi:10.1016/j.hoc.2020.01.006
63. Kovtun Y, Jones GE, Adams S, et al. A CD123-targeting antibody-drug conjugate, IMGN632, designed to eradicate AML while sparing normal bone marrow cells. Blood Adv. 2018;2(8):848–858. doi:10.1182/bloodadvances.2018017517
64. Kerr D 2nd, Zhang L, Sokol L. Blastic Plasmacytoid Dendritic Cell Neoplasm. Curr Treat Options Oncol. 2019;20(1):9. doi:10.1007/s11864-019-0605-x
65. Kharfan-Dabaja MA, Cherry M. Hematopoietic cell transplant for blastic plasmacytoid dendritic cell neoplasm. Hematol Oncol Clin North Am. 2020;34(3):621–629. doi:10.1016/j.hoc.2020.01.009
66. Bashir Q, Milton DR, Popat UR, et al. Allogeneic hematopoietic cell transplantation for patients with blastic plasmacytoid dendritic cell neoplasm (BPDCN). Bone Marrow Transplant. 2022;57(1):51–56. doi:10.1038/s41409-021-01478-5
67. Kerr D 2nd, Sokol L. The advances in therapy of blastic plasmacytoid dendritic cell neoplasm. Expert Opin Investig Drugs. 2018;27(9):733–739. doi:10.1080/13543784.2018.1512970
68. Lane AA. Novel therapies for blastic plasmacytoid dendritic cell neoplasm. Hematol Oncol Clin North Am. 2020;34(3):589–600. doi:10.1016/j.hoc.2020.01.007
69. Agha ME, Monaghan SA, Swerdlow SH. Venetoclax in a patient with a blastic plasmacytoid dendritic-cell neoplasm. N Engl J Med. 2018;379(15):1479–1481. doi:10.1056/NEJMc1808354
70. Gangat N, Konopleva M, Patnaik MM, et al. Venetoclax and hypomethylating agents in older/unfit patients with blastic plasmacytoid dendritic cell neoplasm. Am J Hematol. 2022;97(2):E62–E67. doi:10.1002/ajh.26417
71. Khwaja R, Daly A, Wong M, Mahe E, Cerquozzi S, Owen C. Azacitidine in the treatment of blastic plasmacytoid dendritic cell neoplasm: a report of 3 cases. Leuk Lymphoma. 2016;57(11):2720–2722. doi:10.3109/10428194.2016.1160084
72. Laribi K, Denizon N, Ghnaya H, et al. Blastic plasmacytoid dendritic cell neoplasm: the first report of two cases treated by 5-azacytidine. Eur J Haematol. 2014;93(1):81–85. doi:10.1111/ejh.12294
73. Marmouset V, Joris M, Merlusca L, et al. The lenalidomide/bortezomib/dexamethasone regimen for the treatment of blastic plasmacytoid dendritic cell neoplasm. Hematol Oncol. 2019;37(4):487–489. doi:10.1002/hon.2671
74. Iversen KF, Holdgaard PC, Preiss B, Nyvold CG, Plesner T. Daratumumab for treatment of blastic plasmacytoid dendritic cell neoplasm. A single-case report. Haematologica. 2019;104(9):e432–e433. doi:10.3324/haematol.2018.214635
75. Mirgh S, Sharma A, Folbs B, et al. Daratumumab-based therapy after prior Azacytidine-Venetoclax in an octagenerian female with BPDCN (blastic plasmacytoid dendritic cell neoplasm) - a new perspective. Leuk Lymphoma. 2021;62(12):3039–3042. doi:10.1080/10428194.2021.1941938
76. Betrian S, Guenounou S, Luquet I, et al. Bendamustine for relapsed blastic plasmacytoid dendritic cell leukaemia. Hematol Oncol. 2017;35(2):252–255. doi:10.1002/hon.2252
77. Ulrickson ML, Puri A, Lindstrom S, Cassaday RD, De Padova N, Becker PS. Gemcitabine and docetaxel as a novel treatment regimen for blastic plasmacytoid dendritic cell neoplasm. Am J Hematol. 2017;92(5):E75–E77. doi:10.1002/ajh.24696
78. Arranto C, Tzankov A, Halter J. Blastic plasmacytoid dendritic cell neoplasm with transient response to pralatrexate. Ann Hematol. 2017;96(4):681–682. doi:10.1007/s00277-016-2907-4
79. Leitenberger JJ, Berthelot CN, Polder KD, et al. CD4+ CD56+ hematodermic/plasmacytoid dendritic cell tumor with response to pralatrexate. J Am Acad Dermatol. 2008;58(3):480–484. doi:10.1016/j.jaad.2007.12.012
80. Sato S, Tanaka E, Tamai Y. Blastic plasmacytoid dendritic cell neoplasm with response to pralatrexate. Ann Hematol. 2019;98(3):801–803. doi:10.1007/s00277-019-03611-3
81. Pemmaraju N, Wilson NR, Khoury JD, et al. Central nervous system involvement in blastic plasmacytoid dendritic cell neoplasm. Blood. 2021;138(15):1373–1377. doi:10.1182/blood.2021011817
82. Feuillard J, Jacob MC, Valensi F, et al. Clinical and biologic features of CD4(+)CD56(+) malignancies. Blood. 2002;99(5):1556–1563. doi:10.1182/blood.v99.5.1556
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.