Back to Journals » Journal of Inflammation Research » Volume 17
Clinical Implications of Naples Prognostic Score for Patients with Resected Cholangiocarcinoma: A Real-World Experience
Authors Xu B, Zhu J, Wang R, Pang X, Wang X, Lian J, Lu H
Received 26 October 2023
Accepted for publication 16 January 2024
Published 2 February 2024 Volume 2024:17 Pages 655—667
DOI https://doi.org/10.2147/JIR.S446735
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tara Strutt
Benjie Xu,* Jiahao Zhu,* Ren Wang, Xiangyi Pang, Xin Wang, Jie Lian, Haibo Lu
Department of Outpatient Chemotherapy, Harbin Medical University Cancer Hospital, Harbin, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jie Lian; Haibo Lu, Harbin Medical University Cancer Hospital, No. 150 Haping Road, Nangang District, Harbin, Heilongjiang Province, 150081, People’s Republic of China, Tel +86 451 85718309, Email [email protected]; [email protected]
Purpose: The Nutritional Prognostic Score (NPS) is a composite indicator that effectively reflects the preoperative nutritional and inflammation status of patients. Its prognostic value has been extensively confirmed in various types of tumors. Our study aims to investigate the clinical implications of the NPS in the postoperative patients with cholangiocarcinoma (CCA).
Patients and Methods: Data on clinicopathological characteristics were collected from CCA patients who underwent radical surgery between 2014 and 2019 at Harbin Medical University Cancer Hospital. NPS was calculated using relevant indicators to categorize the patients, and association of NPS with clinicopathological characteristics and survival outcomes were analyzed. To assess differences in survival rates between different groups, we utilized the Kaplan-Meier method. Independent prognostic risk factors were identified by Cox regression analysis. A CONomogram was created, and its accuracy in survival prediction was evaluated using receiver operating characteristic (ROC) curves. Independent verification was conducted in the validation group.
Results: For this study, a cohort of 232 patients was enlisted and subsequently divided into training group (N=162) and validation group (N=70). An evident correlation was detected between NPS and preoperative malnutrition. Patients with higher NPS exhibited a worse overall survival (OS), with 5-year OS rates of 79.1%, 33.1%, and 10.6%. Multivariate analysis revealed that NPS was an independent risk factor for OS in resected CCA patients (P< 0.001). The NPS-based Nomogram was developed to accurately assess the risk of patients.
Conclusion: The NPS was identified as a significant risk factor that impacts the prognosis of patients with resected CCA. In order to improve prognosis management, the NPS-based Nomogram has been demonstrated to be a precise and efficient tool.
Keywords: Naples prognostic score, cholangiocarcinoma, nomogram, overall survival, real-world experience
Introduction
Cholangiocarcinoma (CCA) is a highly aggressive malignancy that originates from the epithelium of the bile duct.1 It is a relatively uncommon type of gastrointestinal cancer, representing only 3% of all patients. During these years, there has been an increase in the incidence of CCA in various regions.2 Clinical symptoms of CCA are always non-specific, making it challenging to diagnose at early stages.3 Surgical resection remains the only curative treatment for CCA, but only 20% of patients are eligible for surgery. Furthermore, patients’ long-term survival was adversely affected by the high recurrence rate after surgery.4,5 The tumor-node-metastasis (TNM) system is the primary method used to predict survival in resected CCA patients.6 However, the traditional staging system has limitations as it can only be available for assessment after surgery. Thus, it is crucial to identify preoperative markers to improve survival management.
Previous studies have established a strong correlation between preoperative inflammation and nutritional status with the prognosis of cancer patients. Various indicators, such as albumin, total cholesterol, neutrophil to lymphocyte ratio (NLR) and prognostic nutritional index (PNI) have been proven to be useful in evaluating patient prognosis.7–10 However, a single marker is insufficient to comprehensively assess the patient’s preoperative status. The Naples prognostic score (NPS) is a novel and comprehensive index that incorporates serum albumin, total cholesterol, NLR, and lymphocyte to monocyte ratio (LMR). It was initially confirmed as an independent risk factor in colon cancers and further demonstrated its correlation with prognosis in esophageal cancer, pancreatic cancer, gastric cancer, and other types of cancers.11–14 However, the predictive significance of NPS in patients with CCA has not yet been confirmed.
The present study aims to explore the clinical implications of NPS in postoperative patients with CCA. Additionally, a Nomogram is constructed to guide the prognosis of CCA based on NPS and other clinical factors.
Materials and Methods
Study Population
This study included 803 patients with CCA in the Harbin Medical University Cancer Hospital from January 2014 to January 2019. Inclusion criteria of our study were as follows: 1. aged ≥ 18; 2. pathological diagnosis of CCA. Exclusion criteria: 1. patients had no available clinicopathology data; 2. patients did not undergo radical surgery; 3. patients with preoperative therapies (local or systemic therapy); 4. with multiple primary cancers. Finally, 232 patients were enrolled in our study (Figure 1). The study was approved by the ethics committee of Harbin Medical University Cancer Hospital, and obtained informed consent from patients.
 |
Figure 1 The flow diagram of the cholangiocarcinoma study. |
Variables Collection
Several variables were included in our study: sex, age, body mass index (BMI), hepatocirrhosis or Hepatitis B Virus (HBV), obstructive jaundice, preoperative carcinoembryonic antigen (CEA), carbohydrate antigen 19–9 (CA19-9), tumor size, tumor location, differentiation, perineural invasion, microvascular invasion, TNM stage, adjuvant chemotherapy, PNI, controlling nutritional status (CONUT) and NPS. Sex was categorized into male and female. Patients were categorized into two groups depending on whether they had chronic liver disease. The diagnostic criteria for obstructive jaundice included a total bilirubin (TBil) level greater than 34.2 μmol/L along with a noticeable rise in direct bilirubin. Tumor size was classified by utilizing a threshold of 5 cm. Based on the anatomical origins, CCA was classified as intrahepatic cholangiocarcinoma (iCCA) and extrahepatic cholangiocarcinoma (eCCA). Pathological differentiation was categorized into three groups. Perineural invasion and microvascular invasion were defined as either “positive” or “negative”. Postoperative pathological staging of CCA patients was conducted based on the TNM staging system. Furthermore, patients were classified into two groups based on whether they underwent adjuvant therapy after surgery. Preoperative hematological markers were collected, including lymphocyte, neutrophil, platelet counts, fibrinogen, monocyte, total cholesterol, TBil, albumin, CEA and CA19-9. The relevant indexes were calculated as follows: NLR = neutrophil divided by lymphocyte, LMR = lymphocyte divided by monocyte, PNI = albumin (g/L) + 5 × lymphocyte.
Statistical Analysis
The study endpoint is the OS of CCA patients. It referred to the duration from the surgery until either the occurrence of death or the latest follow-up. Statistical analyses were conducted using SPSS software (version 23.0) and GraphPad Prism 8. There is no consensus on an ideal ratio of the numbers of training and validation groups, however, a commonly used rule-of-thumb is to split the dataset into a 7:3 or 8:2 ratio for training and validation groups. In our study, we referenced several recently published clinical studies and randomized our cohort using a 7:3 ratio. After randomly grouping the data, the chi-square test was performed to evaluate whether there were distribution differences between the training group and the validation group. The random grouping ensured a balanced distribution of clinical variables in both the training group and validation group. Finally, we utilized the training group to construct the scoring model, which was subsequently evaluated in the validation group. When performing statistical descriptions, ratios were used to represent categorical variables. Kaplan-Meier curves were used to perform survival analysis, and the Log rank test was conducted. Cox regression analysis was performed to identify independent prognostic risk factors. We implemented Chi-square test or Fisher’s exact test for categorical variables. Additionally, the optimal cutoff values for continuous variables were carried out by utilizing the Youden index. To assess the predictive efficacy of various variables, receiver operating characteristic (ROC) curves were generated. P < 0.05 was used to indicate statistical difference.
Results
Baseline Characteristics of Patients
Finally, 232 patients with CCA were enlisted and randomly divided into the training group (n=163) and the validation group (n=70) in a 7:3 ratio. The duration of follow-up was 29.6 months on average and 107 patients had passed away (46.1%) in the end. The training group comprised 116 males (71.2%) and 44 females (28.8%). The average age was 59.4 years (range: 44–75). Among the training group, 23 cases (14.2%) had liver cirrhosis or HBV infection, while 101 cases (62.4%) had preoperative obstructive jaundice. According to the TNM staging system, patients with stage I, II and III were 28 (17.2%), 58 (35.6%) and 76 (47.8%), respectively. Approximately 40% patients with CCA were moderately differentiated and 52 patients (32.9%) received postoperative adjuvant therapy.
The average age of patients in the validation group was 59.2 years old (range: 36–78). The group consisted of 47 males (67.1%) and 23 females (32.9%). Patients with stage I, II and III were 12 (17.1%), 22 (31.4%) and 36 (51.5%), respectively. Additionally, 22 patients (31.4%) in the validation group received postoperative adjuvant therapy. In conclusion, the clinicopathologic features were evenly distributed between the training and validation groups (Table 1).
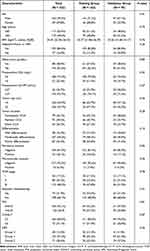 |
Table 1 Baseline Clinical and Pathological Characteristics of Patients with CCA |
The Prognostic Accuracy of NPS and Other Nutritional Indexes
NPS was composed of serum albumin, total cholesterol, NLR and LMR. The score was calculated by comparing the actual results with predefined thresholds. A positive relationship was observed between the patient’s preoperative malnutrition and the NPS. Previous studies have established the thresholds for each indicator as follows: serum albumin (40 g/L), total cholesterol (180 mg/dL), NLR (2.96), and LMR (4.44) (Supplemental Table 1). Patients were grouped based on their NPS score. These groups represented varying levels of preoperative malnutrition risk. Survival analysis demonstrated that the NPS-based group exhibited prognostic value in patients with CCA who underwent radical resection (P<0.001, Figure 2A). Significant differences in prognosis were observed among the various groups, with 5-year survival rates of 79.1%, 33.1%, and 10.06%, respectively.
The assessment of predictive accuracy for variables in survival analysis often involved the use of the ROC curves. Our study analyzed the predictive performance of several comprehensive nutritional indexes (NPS, CONUT, PNI, and BMI) for OS in resected CCA patients. The CONUT score is calculated based on albumin (35 g/L), lymphocyte counts (1.6), and total cholesterol (180 mg/dL). A total score of ≥3 indicates malnutrition. In the training group, the area under the curve (AUC) of NPS, CONUT, PNI, and BMI were 0.668 (95% CI 0.580–0.703), 0.565 (95% CI 0.482–0.603), 0.574 (0.498–0.623), and 0.530 (0.453–0.603), respectively (Figure 2C). Similar results were observed in the validation group (Figure 2B and D).
Association Between NPS and Clinicopathological Characteristics
In the training group, there were 20 (12.3%) patients in Group 0, 90 (55.2%) patients in Group 1, and Group 2 consisted of 52 patients (32.5%). The baseline characteristics of patients in different NPS groups are presented in Table 2. The chi-square test revealed significant differences in clinicopathological characteristics among these groups. The NPS was found to be associated with BMI (P<0.001), obstructive jaundice (P<0.01), preoperative CA19-9 (P<0.01), differentiation (P<0.001), perineural invasion (P<0.01), TNM stage (P<0.001), PNI (P<0.001), and CONUT (P<0.001).
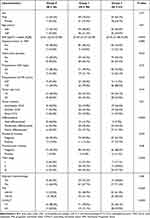 |
Table 2 Baseline Clinical and Pathological Characteristics of Patients with Different NPS Group |
Univariate and Multivariate Regression Analysis
We conducted Cox regression analyses to identify the prognostic risk factors for resected CCA patients (Table 3). In the univariate analysis, it was observed that BMI, CA19-9, tumor location, differentiation, perineural invasion, TNM stage, adjuvant therapy, PNI, CONUT and NPS had significant effects on OS of patients. Further multivariate analysis determined that NPS, differentiation, TNM stage, adjuvant chemotherapy and tumor location were independent prognostic factors for CCA patients. The 5-year OS rates for patients with different NPS groups were 79.1%, 33.1%, and 10.6%, respectively (P<0.001, Figure 3A). And significant differences in prognosis were observed among patients with highly, moderately, and poorly differentiated, with 5-year OS rates of 63.3%, 36.3%, and 26.9%, respectively (P<0.001, Figure 3B). Additionally, the prognosis differed based on different pathological stages, with 5-year OS rates of 65.1% (stage I), 41.7% (stage II), and 22.5% (stage III) (P<0.001, Figure 3C). Patients who received adjuvant chemotherapy for CCA had a better prognosis compared to those who did not, with a significant difference in 5-year survival rates (61.1% vs 19.4%) (P<0.001, Figure 3D). Survival analysis revealed that patients diagnosed with eCCA exhibited a more favorable 5-year OS rate in comparison to cases with iCCA (42.4% vs 28.9%) (P<0.05, Figure 3E).
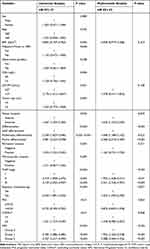 |
Table 3 Univariate and Multivariate Analysis of Clinical Variables |
Survival Analysis Based on NPS
There were significant differences in the prognosis of patients with CCA across different NPS groups (P<0.001, Supplemental Figure 1A). To further investigate the implications of NPS on prognosis in different pathological stages, we performed a subgroup analysis. The findings demonstrated that NPS also has prognostic value for patients with the same pathological stage (Supplemental Figure 1B–D).
Nomogram Development and Validation
We developed a Nomogram for survival prediction using the results of multivariate analysis. The Nomogram included five independent prognostic factors (NPS, stage, differentiation, tumor location and adjuvant chemotherapy) (Figure 4A). In the training group, the AUC of the Nomogram for predicting the 1-year, 3-year, and 5-year survival of resected CCA patients were 0.801, 0.829, and 0.808, respectively (Figure 4B). The C-index of the Nomogram was 0.717 (95% CI: 0.688–0.746). The calibration curves indicated that the Nomogram effectively predicted survival of 1-year, 3-year, and 5-year (Figure 5A–C). Additionally, the decision curves demonstrated that the Nomogram had a higher prognostic value compared to TNM system (Figure 4D). These findings were further confirmed in the validation group, with AUC of 0.719, 0.731, and 0.869 for predicting 1-year, 3-year, and 5-year survival, respectively (Figure 4C). The C-index was 0.695 (95% CI: 0.645–0.745). Both the calibration curves and the decision curves demonstrated a strong prognostic value of nomogram model (Figures 4E and 5D–F). In conclusion, the Nomogram based on NPS was a more accurate prognostic tool for resected CCA patients compared to the traditional staging system.
Discussion
The present study confirmed the predictive value of NPS on the prognosis of CCA patients. In clinical practice, the NPS groups had a significance correlation with the patient’s preoperative malnutrition. Survival analysis revealed that patients in Group 2 (NPS=3 or 4) had the lowest 5-year OS rate. Moreover, this study firstly constructed a Nomogram for OS prediction using NPS and other independent prognostic risk factors. According to the decision curves, the novel model outperformed the traditional TNM stage in accurately predicting the survival of resected CCA patients.
With the advancement of diagnosis and treatment technology, an increasing number of CCA patients have been able to undergo radical surgical. However, the mortality rate of CCA worldwide continues to rise. Therefore, it is imperative to identify appropriate prognostic markers for the stratified management of CCA patients. Inflammation and nutrition played a crucial role in the occurrence and development of malignancies. As research progresses, it has become evident that these factors were closely associated with tumor microenvironment and prognosis.15,16 Elevated serum level of NLR was associated to early tumor recurrence and shorter survival in patients with iCCA.17 According to another retrospective study, LMR has been identified as an independent risk factor for the prognosis of eCCA. The study found that higher level of preoperative LMR were associated with a better prognosis.18 However, a single marker was insufficient for risk stratification. NPS was a novel prognostic marker that incorporated both inflammation and nutritional indicators. Serum albumin and total cholesterol are indicators of nutritional status. Preoperative malnutrition had been found to be associated with early postoperative recurrence and poorer survival prognosis.19,20 Lymphocytes and neutrophils played a crucial role in the release of cytokines and inflammatory mediators, contributing to the anti-tumor immune response.21 Additionally, monocytes have been linked to tumor-associated macrophages (TAMs).22 In conclusion, the NPS is a comprehensive indicator that reflects both inflammatory and nutritional status. It has potential to serve as a substantial indicator for survival prediction in CCA patients. Recent studies have identified various prognostic indicators, including CONUT and PNI.23,24 Notably, NPS has been found to possess a better prognostic value compared to other markers in esophageal cancer and gastric cancer.12,13 In our study, the ROC curves demonstrated that NPS exhibited a higher prognostic value in both the training group and the validation group.
The heterogeneity of CCA is not adequately addressed by the conventional TNM stage, leading to varying prognoses for patients in the same pathological stage. To address this, the Nomogram incorporating multiple risk factors was developed to provide improved risk stratification and survival prediction.25,26 In this study, risk factors included in the Nomogram were identified by multivariate analysis. All the factors included in the Nomogram are clinical variables, facilitating its further adoption in clinical practice. Compared to the TNM stage, the Nomogram demonstrated a higher predictive value for survival in CCA patients.
As a representative of heterogeneous malignancies, CCA exhibits unique clinicopathological and survival outcome in different anatomical sites.27 It is important to note that chronic viral hepatitis or cirrhosis is more closely associated with iCCA, as supported by this study.28 On the other hand, primary sclerosing cholangitis is the main cause of eCCA.29 The clinical symptoms of CCA were often non-specific and insidious, leading to patients being diagnosed at an advanced stage. However, previous studies have shown that eCCA can be helpful for early diagnosis due to its clinical features of obstructive jaundice, which may lead to a better prognosis.27 The findings of this study supported this conclusion. Specifically, the prognosis of eCCA was better than that of iCCA.
Our study aimed to explore the applicability and limitations of NPS in the context of patients with CCA. NPS is a composite marker that includes nutritional and inflammatory indicators. We have confirmed its prognostic value in CCA, and all the indicators included in NPS are easily accessible clinical or hematological indicators. However, there are still some limitations that need to be addressed for the wider adoption and implementation of NPS in clinical practice. One limitation is the potential differences in nutritional and immune status among patients with different types of cancer. This study, which was conducted retrospectively at a single center, and unable to determine the optimal threshold based on the nutritional and immune characteristics of patients with CCA. It is worth noting that the threshold may change as the study includes a larger number of patients. Therefore, more convincing conclusions need to be verified in larger samples. Further studies are required to validate the impact of preoperative nutritional support and inflammation control on patients’ NPS and to enhance the prognosis of patients with CCA. Additionally, considering the heterogeneity of CCA, which exhibits differences in inflammation and nutritional status across different anatomical sites, we are currently conducting a multicenter retrospective study to determine the optimal threshold for CCA. Although the inflammation and nutritional indicators may be influenced by several conditions, the Nomogram based on NPS is still an effective tool for survival prediction in CCA patients after radical surgery.
Conclusion
In conclusion, NPS is an independent prognostic factor for patients with resected CCA. The high NPS group (Group 1 or 2) is associated with lower survival rates. Furthermore, the integration of NPS into a Nomogram with additional risk factors could accurately provide a survival prediction for CCA patients.
Data Sharing Statement
All clinical data collected in our study are available on request from the corresponding author Haibo Lu.
Ethical Approval
This retrospective study was approved by Harbin Medical University Cancer Hospital ethics committee and conducted in accordance with the Declaration of Helsinki.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the National Natural Science Foundation of China (grant no. U20A20376), Beijing Award Foundation, (grant no. YXJL-2020-0818-0478), Heilongjiang Province Postdoctoral Science Foundation (grant no. LBHZ21189), Harbin Medical University Innovative Science Research Funded Project (grant no. 2022-KYYWF-0289), China Postdoctoral Science Foundation (grant no.2022MD713747).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Valle JW, Kelley RK, Nervi B, et al. Biliary tract cancer. Lancet. 2021;397(10272):428–444. doi:10.1016/s0140-6736(21)00153-7
2. Banales JM, Marin JJG, Lamarca A, et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol. 2020;17(9):557–588. doi:10.1038/s41575-020-0310-z
3. Alvaro D, Hassan C, Cardinale V, et al. Italian clinical practice guidelines on cholangiocarcinoma – part I: classification, diagnosis and staging. Dig Liver Dis. 2020;52(11):1282–1293. doi:10.1016/j.dld.2020.06.045
4. Li J, Moustafa M, Linecker M, et al. ALPPS for locally advanced intrahepatic cholangiocarcinoma: did aggressive surgery lead to the oncological benefit? An international multi-center study. Ann Surg Oncol. 2020;27(5):1372–1384. doi:10.1245/s10434-019-08192-z
5. Pavicevic S, Reichelt S, Uluk D, et al. Prognostic and predictive molecular markers in cholangiocarcinoma. Cancers. 2022;14(4):1026. doi:10.3390/cancers14041026
6. Ronnekleiv-Kelly SM, Pawlik TM. Staging of intrahepatic cholangiocarcinoma. Hepatobiliary Surg Nutr. 2017;6(1):35–43. doi:10.21037/hbsn.2016.10.02
7. Saito H, Noji T, Okamura K, et al. A new prognostic scoring system using factors available preoperatively to predict survival after operative resection of perihilar cholangiocarcinoma. Surgery. 2016;159(3):842–851. doi:10.1016/j.surg.2015.10.027
8. Guo Y, Zhang Y-G, Li H-C, et al. Transiently elevated TC and sdLDL-C levels and falsely low LDL-C levels in patients with extrahepatic cholangiocarcinoma. Onco Targets Ther. 2021;14:1061–1071. doi:10.2147/ott.S285856
9. Z-q L, Ma C, Cao W-Z, et al. Prognostic significance of NLR, PLR, LMR and tumor infiltrating T lymphocytes in patients undergoing surgical resection for hilar cholangiocarcinoma. Front Oncol. 2022;12. doi:10.3389/fonc.2022.908907
10. Wang A, He Z, Cong P, et al. Controlling Nutritional Status (CONUT) score as a new indicator of prognosis in patients with hilar cholangiocarcinoma is superior to NLR and PNI: a single-center retrospective study. Front Oncol. 2021;10. doi:10.3389/fonc.2020.593452
11. Galizia G, Lieto E, Auricchio A, et al. Naples prognostic score, based on nutritional and inflammatory status, is an independent predictor of long-term outcome in patients undergoing surgery for colorectal cancer. Dis Colon Rectum. 2017;60(12):1273–1284. doi:10.1097/dcr.0000000000000961
12. Feng J-F, Zhao J-M, Chen S, et al. Naples prognostic score: a novel prognostic score in predicting cancer-specific survival in patients with resected esophageal squamous cell carcinoma. Front Oncol. 2021;11. doi:10.3389/fonc.2021.652537
13. Wang H, Fang T, Yin X, et al. Prognostic importance of the preoperative New-Naples prognostic score for patients with gastric cancer. Cancer Med. 2022;12(2):1358–1375. doi:10.1002/cam4.5017
14. Nakagawa N, Yamada S, Sonohara F, et al. Clinical implications of Naples prognostic score in patients with resected pancreatic cancer. Ann Surg Oncol. 2019;27(3):887–895. doi:10.1245/s10434-019-08047-7
15. Zitvogel L, Pietrocola F, Kroemer G. Nutrition, inflammation and cancer. Nat Immunol. 2017;18(8):843–850. doi:10.1038/ni.3754
16. Kiely M, Lord B, Ambs S. Immune response and inflammation in cancer health disparities. Trends Cancer. 2022;8(4):316–327. doi:10.1016/j.trecan.2021.11.010
17. Buettner S, Spolverato G, Kimbrough CW, et al. The impact of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio among patients with intrahepatic cholangiocarcinoma. Surgery. 2018;164(3):411–418. doi:10.1016/j.surg.2018.05.002
18. Lv M, Wang K, Zhang Z, et al. The predictive value of lymphocyte to monocyte ratio for overall survival in cholangiocarcinoma patients with hepatic resection. Cancer Med. 2023;12(8):9482–9495. doi:10.1002/cam4.5712
19. Huang H, Zhang L, Chen D-B, et al. Validation of prognosis value of cumulative prognostic scores based on serum high-density lipoprotein cholesterol and albumin levels in patients with colorectal cancer. J Cancer. 2019;10(1):35–42. doi:10.7150/jca.26637
20. Feng Z, Pang K, Tian M, et al. Sarcobesity, but not visceral fat, is an independent risk factor for complications after radical resection of colorectal cancer. Front Nutr. 2023;10. doi:10.3389/fnut.2023.1126127
21. Carnevale S, Di Ceglie I, Grieco G, et al. Neutrophil diversity in inflammation and cancer. Front Immunol. 2023;14. doi:10.3389/fimmu.2023.1180810
22. Caprara G, Allavena P, Erreni M. Intestinal macrophages at the crossroad between diet, inflammation, and cancer. Int J Mol Sci. 2020;21(14):4825. doi:10.3390/ijms21144825
23. Kuroda D, Sawayama H, Kurashige J, et al. Controlling Nutritional Status (CONUT) score is a prognostic marker for gastric cancer patients after curative resection. Gastric Cancer. 2017;21(2):204–212. doi:10.1007/s10120-017-0744-3
24. Chen L, Bai P, Kong X, et al. Prognostic Nutritional Index (PNI) in patients with breast cancer treated with neoadjuvant chemotherapy as a useful prognostic indicator. Front Cell Develop Biol. 2021;9. doi:10.3389/fcell.2021.656741
25. Lleo A, Colapietro F, Maisonneuve P, et al. Risk stratification of cholangiocarcinoma patients presenting with jaundice: a retrospective analysis from a tertiary referral center. Cancers. 2021;13(9):2070. doi:10.3390/cancers13092070
26. Zhang G, Chen B-W, Yang X-B, et al. Prognostic analysis of patients with combined hepatocellular-cholangiocarcinoma after radical resection: a retrospective multicenter cohort study. World J Gastroenterol. 2022;28(41):5968–5981. doi:10.3748/wjg.v28.i41.5968
27. Izquierdo-Sanchez L, Lamarca A, La Casta A, et al. Cholangiocarcinoma landscape in Europe: diagnostic, prognostic and therapeutic insights from the ENSCCA registry. J Hepatol. 2022;76(5):1109–1121. doi:10.1016/j.jhep.2021.12.010
28. Clements O, Eliahoo J, Kim JU, et al. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: a systematic review and meta-analysis. J Hepatol. 2020;72(1):95–103. doi:10.1016/j.jhep.2019.09.007
29. Montal R, Sia D, Montironi C, et al. Molecular classification and therapeutic targets in extrahepatic cholangiocarcinoma. J Hepatol. 2020;73(2):315–327. doi:10.1016/j.jhep.2020.03.008
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




