Back to Journals » Infection and Drug Resistance » Volume 17
Clinical Distribution and Drug Susceptibility Characterization of Invasive Candida Isolates in a Tertiary Hospital of Xinjiang Province
Authors Zhang S, Zhang L, Yusufu A, Hasimu H, Wang X , Abliz P
Received 6 December 2023
Accepted for publication 25 March 2024
Published 4 April 2024 Volume 2024:17 Pages 1345—1356
DOI https://doi.org/10.2147/IDR.S450933
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Sandip Patil
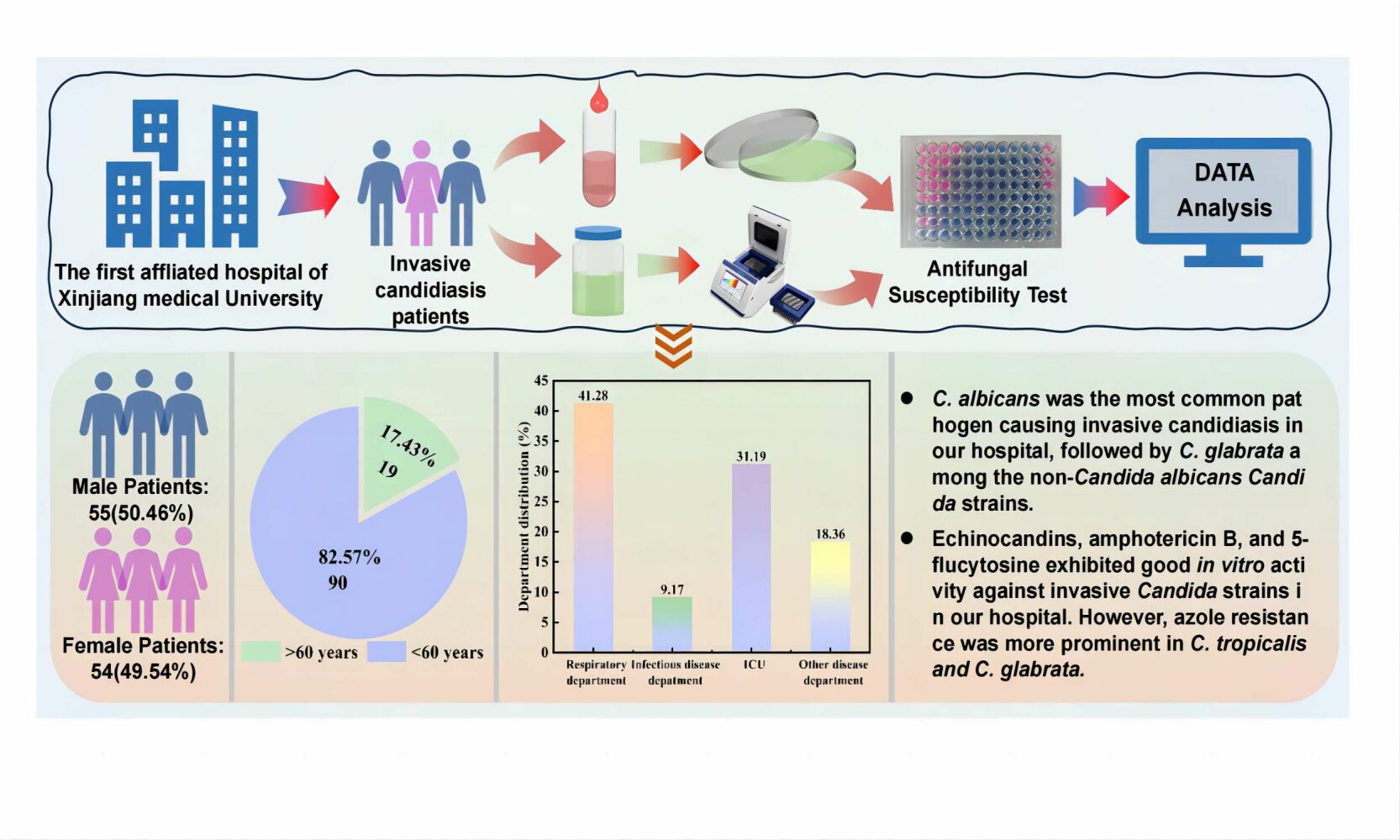
Songdi Zhang,* Lijuan Zhang,* Aikedai Yusufu,* Hadiliya Hasimu, Xiaodong Wang, Paride Abliz
Department of Dermatology, The First Affiliated Hospital of Xinjiang Medical University, Urumqi, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiaodong Wang; Paride Abliz, Department of Dermatology, The First Affiliated Hospital of Xinjiang Medical University, No. 393, Xinyi Road, Urumqi, Xinjiang, People’s Republic of China, Email [email protected]; [email protected]
Objective: This study aims to investigate the clinical distribution characteristics and drug susceptibility profiles of invasive Candida isolates in a tertiary hospital in Urumqi.
Methods: The examination was conducted on samples obtained from patients who were clinically diagnosed with invasive candidiasis in this hospital. A total of 109 strains of Candida strains were identified through the use of internal transcribed spacer (ITS) sequencing and fungal cultivation methods.The clinical distribution of the strains was analyzed. Antifungal drug susceptibility tests were performed using the Sensititre YO10 fungal drug susceptibility plate based on the micro-broth dilution method.
Results: Candida albicans had the highest percentage (51.38%) among 109 Candida isolates, followed by C. glabrata (18.35%) and C. tropicalis (15.60%). The isolates were predominantly found in the respiratory department (41.28%), intensive care unit (ICU) (31.19%), and infection department (9.17%).The results of drug susceptibility tests indicated that amphotericin B, 5-fluorocytosine, and echinocandins exhibited good in vitro antifungal activity, with a susceptibility rate of over 96%. However, the azoles demonstrated low antifungal activity, especially posaconazole and voriconazole, which had high resistance rates of 64.71% for C. tropicalis and 70% for C. glabrata, respectively.
Conclusion: In our hospital, Candida albicans was identified as the primary causal agent of invasive candidiasis. In terms of in vitro antifungal activity, echinocandins, amphotericin B, and 5-fluorocytosine demonstrated efficacy against invasive Candida infections. However, it was important to note that C. glabrata and C. tropicalis exhibited low susceptibility to azoles.
Keywords: Candida isolates, clinical distribution, antifungal drug susceptibility tests, drug resistance
Graphical Abstract:
Introduction
The incidence of invasive fungal infections is increasing, attributed to the wide usage of glucocorticoids, broad-spectrum antibiotics, immunosuppressants, as well as advances in invasive manipulation techniques and novel therapeutic approaches.1,2 Among these infections, invasive candidiasis is the most common, with Candida albicans being the predominant causative agent; however, due to the widespread use of antifungal medications, there is an epidemiological shift in the prevalence of strains from Candida albicans to non-Candida albicans Candida species (NCAC).3 The epidemiology and drug sensitivity of invasive Candida strains vary based on different regions, patients, and doctors’ medication practices.Understanding the local distribution of strains and their drug susceptibility brings numerous advantages in terms of prompt detection and therapy, thereby improving the cure rate and reducing drug resistance and adverse reactions caused by long-term empiric and preventive use of antifungal drugs.
This research aimed to analyze the distribution of Candida strains obtained from patients with invasive candidiasis within a prominent tertiary care hospital situated in Urumqi, the capital city of Xinjiang Province. Furthermore, we evaluated the characteristics of drug resistance to establish a credible scientific foundation for improving the diagnosis and treatment strategies for invasive Candida infections in the local area.
Material and Methods
Fungal Isolates Collection and Identification
Samples were obtained from the respiratory tract, digestive tract and other parts of patients who were clinically diagnosed with invasive candidiasis in all the wards in the First Affiliated Hospital of Xinjiang Medical University from June 2022 to June 2023. A total of 109 Candida strains were detected, ensuring that any replication of strains from a single patient was excluded. The identification of strains involved the use of Candida chromogenic media (CHROMagar, France) for morphological identification and ITS sequencing for molecular biological identification.DNA samples from Candida strains were PCR amplified using fungal universal primers ITS1/ITS4.ITS 1:5’-TCCGTAGGTGAACCTGCGG-3’, ITS4:5’-TCCTCCGCTTATTGATATGC-3’.PCR programme included: 94°C (5 min); 94° C (30 sec); 55°C(30 sec) and 72°C (90 sec) for 35 cycles; and then a final extension of 72°C (8 min).The obtained sequences were aligned with the corresponding sequences of reference Candida strains in GenBank. GenBank accession numbers of ITS1/ITS4 sequences are listed in Supplementary Table 1.
Antifungal Susceptibility Tests
The in vitro drug susceptibility test was conducted according to the Sensititre Yeast One YO10 product specification. The minimal inhibitory concentration (MIC) of amphotericin B, 5-fluorocytosine, anidulafungin, caspofungin, micafungin, fluconazole, itraconazole, posaconazole, and voriconazole was determined using Sensitizer YO10 fungal sensitization plates (Thermo Fisher Scientific, USA) based on the micro bouillon dilution method. MIC results for Candida isolates were read after 24 hours of culture, with the positive control well serving as the reference. The drug susceptibility results of different strains were interpreted based on the latest M60-Ed2 document4 of the Clinical and Laboratory Standards Institute (CLSI) for clinical breakpoints (CBPs). For strains without clinical breakpoints, the epidemiological cutoff values (ECVs) of CLSI M59-Ed35 were used for interpretation. The quality control strains used were C. parapsilosis ATCC22019 and C. Krusei ATCC6258. Measured ITS sequences were compared using mafft software6, and the phylogenetic tree was constructed through Fast Tree software7 using the maximum likelihood method along with 1000 bootstrap tests. The drug resistance index (log (Drug Concentration)) was calculated using the logarithm of the MIC value obtained from the drug susceptibility test. The drug resistance index was then represented as a heat map on the periphery of the phylogenetic tree. Visualization was performed using the online tool iTol.8
Statistical Analysis
All data analyses and graphs were performed using SPSS (version 22, Chicago, USA) and Origin (version 2018, Northampton, USA) software. Count data were presented as frequencies or constituent ratios. A P value < 0.05 was considered statistically significant for all statistical analyses.
Results
Clinical Distribution Characteristics of Candida Isolates
A total of 109 Candida isolates were obtained from the samples sent for analysis. The isolates were incubated in Candida chromogenic medium and identified by sequencing the ITS region (Figure 1). The total 109 isolates that were examined, 55 (50.46%) were obtained from male patients while the remaining 54 (49.54%) were obtained from female patients. The patients ranged in age from 25 to 93 years, with a mean age of 74 (65,81) years. Among the isolates, 90 were from patients over 60 years of age, while 19 were from patients under 60 years of age. In terms of department distribution, 45 (41.28%) isolates were obtained from the respiratory department, 34 (31.19%) were from the intensive care unit (ICU), and 10 (9.17%) were from the infectious diseases department (Figure 2).
 |
Figure 2 Distribution of Candida isolates in the clinical department. |
Distribution of Candida Isolates
A total of seven Candida species were identified among the 109 Candida strains. Candida albicans was found to be the most prevalent species, accounting for 51.38% (56/109) of the strains. Non-Candida albicans Candida (NCAC) species accounted for 48.62% of the strains, with C. glabrata being the most dominant at 18.35% (20/109), followed by C. tropicalis at 15.60% (17/109), and C. krusei at 7.34% (8/109). Candida isolates were obtained from various parts of the respiratory tract, urinary tract, gastrointestinal tract, and others. The majority of isolates (77.98%, 85/109) were from sputum, followed by urine (15.60%, 17/109), and bronchoalveolar lavage fluid (BALF)(3.67%, 4/109) (Table 1).
 |
Table 1 Specimen Distribution of Candida Isolates [n(%)] |
In vitro Antifungal Susceptibility Profiles
In this study, we analyzed the drug susceptibility profiles of Candida species with interpretive standards (CBPs or ECVs) to nine antifungal drugs commonly used in clinical settings. For the sake of simplicity, we refer to phenotypic strains that were susceptible (S) and wild-type (WT) as ”susceptible strains”, while resistant strains (R)and non-wild-type (NWT) were referred to as ”resistant strains”.
In vitro Antifungal Susceptibility to Azoles
A total of 3.57% (2/56), 7.14% (4/56) 7.14% (4/56) and 10.71% (6/56) of Candida albicans showed resistance to voriconazole (VOR), Fluconazole (FLC), itraconazole (ITC), and Posaconazole (POS), respectively. Compared to C. albicans, NCAC species exhibited variable levels of resistance. Only 35.29% (6/17) of Candida tropicalis were susceptible to all azoles, while 64.71% (11/17), 23.53% (4/17), 17.65% (3/17), and 11.76% (2/17) strains demonstrated resistance to POS, FLC, VOR and ITC, respectively. Within the C. glabrata isolates, one strain showed resistance to FLC, whereas the other 19 strains showed a susceptible dose-dependent phenotype (SDD). Furthermore, 70% (14/20), 45% (9/20), and 5% (1/20) of C. glabrata isolates showed resistance to VOR, POS, and ITC, respectively. One isolate of C. krusei exhibited cross-resistance to all three azoles, while one C. parapsilosis demonstrated resistance to 5-Flucytosine (5-FC) and FLC. Both isolates of C. lusitania were sensitive to all azoles (Table 2).
 |
Table 2 In vitro Antifungal Susceptibility Profiles |
In vitro Antifungal Susceptibility to Echinocandins, AMB, and 5-FC
Most of the Candida isolates in this study were highly susceptible to echinocandins, with an overall susceptibility rate of over 95%. No echinocandin-resistant strains were found, particularly in C. albicans, C. tropicalis, C. krusei, C. parapsilosis, and C. lusitaniae. Only two C. glabrata strains demonstrated resistance to echinocandins, one of which was resistant to CAS and the other was cross-resistant to Anidulafungin (AND),Micafungin (MCF), and Caspofungin (CAS).In this study, Amphotericin B (AmB) and 5-Flucytosine (5-FC) displayed good in vitro antifungal activity against most of the Candida species, with a susceptibility rate of over 99%. (Table 2).
Multiple Drug Resistance and Cross-Drug Resistance of Candida Isolates
Multi-resistant strains are defined as strains that are not susceptible to at least 2 categories of antifungal drugs, while cross-resistant strains are strains that are not susceptible to at least 2 drugs in the same category of antifungal drugs.9 In this study, we identified a total of four isolates of C. albicans cross resistant to azoles. Out of these, two isolates exhibited cross resistance to POS, ITC, and FLC, whereas the other two were resistant to all four azoles. Additionally, we discovered five strains of C. tropicalis resistant to azoles. One isolate displayed cross-resistant to POS and VOR, while two isolates were cross-resistant to POS and FLC, and the other two isolates were resistant to all four azoles. Among these, one isolate was a resistant strain that was resistant to all azoles and AmB. Furthermore, we observed nine azoles cross-resistant strains of C. glabrata. Eight of these strains were cross-resistant to POS and VOR, and one strain was resistant to all four azoles. Additionally, we identified two multi-resistant strains of C. glabrata. One strain was resistant to VOR and 5-FC, while the other strain was resistant to POS, VOR, and CAS. Interestingly, one isolate of C. glabrata was found to be resistant to all three echinocandins simultaneously. We also discovered that isolate of C. krusei exhibited cross-resistance to POS, VOR, and ITC. Lastly, one isolate of C. parapsilosis was identified as a multi-resistant strain resistant to 5-FC and FLC (Tables 3 and 4).
 |
Table 3 Multi-Drug Resistance and Cross-Resistance of Candida Strains |
 |
Table 4 Azoles Cross-Resistance of Candida Strains |
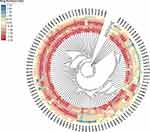
Phylogenetic Tree and Heat Map of Drug Resistance Levels Constructed Based on ITS Sequences
The heat map displayed drug susceptibility for different Candida strains to antifungal drugs.The outermost circles indicated strain numbers, with each column representing a strain and each row representing an antifungal agent.The heat map values indicated the drug resistance index, which was calculated as the logarithm of the MIC value obtained from drug susceptibility testing.The color from blue to red indicated the log (MIC) value from large to small, and the more obvious the blue-red color contrast indicated the greater the difference in the minimum inhibitory concentration of the drug. In the picture, the color of the azoles area was closer to blue and the echinocandins was closer to red.The heat map of resistance index showed that Candida isolates were more sensitive to echinocandins and had a more obvious resistance trend to azoles (Figure 3).
Discussion
The incidence of invasive candidiasis is increasing worldwide, largely due to the continuous development of contemporary medicine and nosocomial infections.10 This is further exacerbated by the growing resistance of Candida species, which is the result of the widespread empirical and prophylactic use of antifungal drugs. In particular, the emergence of multidrug-resistant species like Candida auris11 and Candida vulturna12 has posed a significant challenge to clinical treatment. Hence, it is crucial to understand the regional prevalence of invasive Candida strains and their susceptibility to antifungal drugs. Acquiring these information will greatly contribute to the prevention, control, and management of invasive candidiasis.
The findings of this research demonstrated that the invasive Candida species were distributed primarily in the respiratory department, ICU, hepatology department, and other departments, with a high prevalence among patients over 60 years of age. This correlation could potentially be attributed to the severe illness of patients in these departments, the administration of broad-spectrum antibiotics and glucocorticoids, and the frequent utilization of invasive procedures and medical equipment. The increased susceptibility of elderly patients may be linked to age-related physiological changes, multiple underlying diseases, and the complexity of medication use.13 Previous studies14 have indicated that more than 90% of invasive Candida species comprise C. albicans, C. glabrata, C. tropicalis, C. krusei and C. parapsilosis, with C. albicans being the most commonly identified pathogenic species. In recent times, the proportion of NCAC species has increased significantly, even exceeding the proportion of C. albicans in some areas.15,16 A global SENTRY antifungal surveillance program17 revealed that C. albicans accounted for the highest number of isolates in all monitoring regions from 1997 to 2016. Additionally, C. glabrata was the most common NCAC species in the Asia-Pacific, Europe, and North America, while Latin America frequently reported C. parapsilosis and C. tropicalis. The most prevalent NCAC species in Canada was found to be C. glabrata, according to a multi-center study.18 Similarly, another SENTRY antifungal surveillance program19 identified C. glabrata as the most common NCAC species in patients over 65 years of age with invasive candidiasis. However, in some large-sample, multi-center studies in China, C. parapsilosis was found to be the most frequently isolated NCAC strain.20,21 Within the scope of this study, C. albicans constituted the most frequently isolated pathogen, followed by C. glabrata, C. tropicalis, and C. krusei. Some multi-center studies of Urumqi reported by Yi22 and Abibai23 found that the most common Candida species of invasive candidiasis were C. tropicalis and C. parapsilosis,respectively. Similarly, a study conducted in a tertiary hospital in Anhui province and reported by Xia24 identified C. glabrata as the most common isolates in invasive candidiasis. These findings indicated that the epidemiologic distribution of invasive Candida strains varied by region and time and was influenced by factors such as the source of the sample, the affected population, and the use of medications. Therefore, it is crucial to actively monitor the epidemiology of invasive Candida strains in local areas.
In our research, the majority of Candida strains showed high sensitivity to AmB and 5-FC, with a sensitivity rate of over 99%. There was only one isolate of C. tropicalis that demonstrated NWT to AMB, and one isolate of C. glabrata that showed NWT to 5-FC. This suggests that these drugs may help treat invasive candidiasis invasive candidiasis in our hospital. Regarding C. albicans strains, they were relatively sensitive to azoles; however, the resistance rate to FLC was higher compared to global data17 and the average rate in our country21 (7.1% vs 0.3% and 4.1%, respectively). This could be attributed to the relatively high usage of FLC in our institution. Previous studies25,26 have found that NCAC species, such as C. glabrata and C. tropicalis, naturally exhibit lower sensitivity to azoles. Some researchers20,21,27 have summarized the drug susceptibility of Candida strains in mainland China over 10 years from 2011 to 2021. They discovered that the resistance rate of C. glabrata and C. tropicalis to FLC exceeded 20%, particularly for C. tropicalis, which showed an increase in resistance rate from less than 6% to over 30% between 2015 and 2017. The susceptibility rate of C. tropicalis to voriconazole and posaconazole in our study was only 35.29%, which was lower than that reported in Guangdong28 and Sichuan Province29 in the same period.Similar to reports from Beijing,15 our study revealed that C. glabrata had the highest rate of resistance to VOR, with 70.5% and 45% of C. glabrata isolates being resistant to VOR and POS respectively. Azole resistance in C. glabrata is more common in Xinjiang than in Anhui30 and Hebei31 Province.Therefore, it is essential to carefully consider the selection of POS and VRC for the treatment of C. glabrata and C. tropicalis infections in our hospital. The overall resistance rate of Candida strains to POS and VOR was high in this study (25% and 18.5% respectively). Notably, among the 27 strains (27/109, 24.8%) that were not susceptible to POS, 19 strains (19/27, 70.4%) exhibited cross-resistance to other azoles. This highlights the need to be cautious about the potential cross-resistance to other drugs when choosing POS for treatment.
In this study, only two isolates of C. glabrata were found to be resistant to echinocandins, which is consistent with the findings of Song15 and Xiao.20 C. glabrata is considered to be the most frequently resistant species to echinocandins, as reported by Alexander.32 Our study revealed that the overall resistance rate of Candida strains to echinocandins was less than 2%. Based on the recommendations of existing guidelines33,34 and considering the drug susceptibility of our hospital, echinocandins can be considered as the preferred first-line therapeutic agents for invasive candidiasis in our hospital. In addition, it is noteworthy that we identified four multidrug-resistant Candida isolates. Given the limited availability of antifungal agents for patients infected with these multidrug-resistant strains, it is vital to improve the management of antifungal drug usage and monitor resistance to minimize the emergence and spread of multidrug-resistant strains caused by the inappropriate use of antifungal drugs.
The limitation of this research is that it is a single-center study, which may not be representative of the patients in the general population. To enhance the comprehensiveness of future research, we will further include samples from multiple centers and investigate the resistance mechanisms of drug-resistant candida strains. Furthermore, incorporating clinical information about patients, including risk factors, baseline conditions, previous medication, prognosis and outcome, would provide a more detailed scientific foundation for the diagnosis and treatment of invasive candidiasis in Xinjiang region of China. In conclusion, the departments with high incidence of invasive Candida infections were the respiratory department, ICU, and hepatology department. C. albicans was found to be the most common pathogen causing invasive candidiasis in our hospital, followed by C. glabrata in the NCAC strains Echinocandins, amphotericin B, and 5-flucytosine have good in vitro activity against invasive Candida strains in our hospital. However, azoles resistance is more prominent in C. tropicalis and C. glabrata. It is important to note that our hospital has identified multidrug-resistant strains that are resistant to two classes of antifungal drugs. Clinicians should highly prioritize these findings and implement timely, dynamic, and continuous drug susceptibility monitoring as well as standardized antifungal treatment. Although this study was conducted in a single-center setting, it is important to note that our hospital is the largest tertiary care hospital in the Xinjiang region. Therefore, we believe that these data can provide meaningful clinical basis for the prevention and management of invasive candidiasis.
Ethics Statement
The study was conducted in accordance with the Declaration of Helsinki. Ethical approval for the study was obtained from the Ethics Committee of the First Affiliated Hospital of Xinjiang Medical University. All patients consented to being involved in this study. (Approval number: K202305-04).
Acknowledgments
This paper is the result of a thesis by the author Songdi Zhang, titled “Analysis of distribution and drug susceptibility characteristics of invasive Candida isolates in a hospital in Xinjiang”.Dissertation, Xinjiang Medical University.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research was financed by National Key Research and Development Program of China (No.2022YFC2504802),National Natural Science Foundation of China (No.81960366), the Xinjiang Nature Science Foundation of China(No.2021D01E30), Key research and development program of Xinjiang Uygur Autonomous Region (No.2021B03001-3).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Lamoth F. Novel therapeutic approaches to invasive candidiasis: considerations for the clinician. Infect Drug Resist. 2023;16:1087–1097. doi:10.2147/IDR.S375625
2. Lee Y, Puumala E, Robbins N, Cowen LE. Antifungal drug resistance: molecular mechanisms in candida albicans and beyond. Chem Rev. 2021;121(6):3390–3411. doi:10.1021/acs.chemrev.0c00199
3. Koehler P, Stecher M, Cornely OA, et al. Morbidity and mortality of candidaemia in Europe: an epidemiologic meta-analysis. Clin Microbiol Infect. 2019;25(10):1200–1212. doi:10.1016/j.cmi.2019.04.024
4. CLSI M60. Performance Standards for Antifungal Susceptibility Testing of Yeasts. M60Ed2E.pdf.2020.
5. CLSI M59. Epidemiological cutoff values for antifungal susceptibility testing,3rd edition, M59 Ed3E.pdf. 2020.
6. Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013;30(4):772–780. doi:10.1093/molbev/mst010
7. Price MN, Dehal PS, Arkin AP. FastTree: computing large minimum evolution trees with profiles instead of a distance matrix. Mol Biol Evol. 2009;26(7):1641–1650. doi:10.1093/molbev/msp077
8. Letunic I, Bork P. Interactive Tree Of Life (iTOL) v5: an online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021;49(W1):W293–W296. doi:10.1093/nar/gkab301
9. Arendrup MC, Patterson TF. Multidrug-resistant candida: epidemiology, molecular mechanisms, and treatment. J Infect Dis. 2017;216(suppl_3):S445–S451. doi:10.1093/infdis/jix131
10. Leroy O, Bailly S, Gangneux JP, et al. Systemic antifungal therapy for proven or suspected invasive candidiasis: the AmarCAND 2 study. Ann Intensive Care. 2016;6(1):2. doi:10.1186/s13613-015-0103-7
11. Wang Y, Zou Y, Chen X, et al. Innate immune responses against the fungal pathogen candida auris. Nat Commun. 2022;13(1):3553. doi:10.1038/s41467-022-31201-x
12. Du H, Bing J, Xu X, et al. Candida vulturna outbreak caused by cluster of multidrug-resistant strains, China. Emerg Infect Dis. 2023;29(7):1425–1428. doi:10.3201/eid2907.230254
13. Dekkers BGJ, Veringa A, Marriott DJE, et al. Invasive candidiasis in the elderly: considerations for drug therapy. Drugs Aging. 2018;35(9):781–789. doi:10.1007/s40266-018-0576-9
14. Barantsevich N, Barantsevich E. Diagnosis and Treatment of Invasive Candidiasis. Antibiotics. 2022;11(6):718. doi:10.3390/antibiotics11060718
15. Song Y, Chen X, Yan Y, Wan Z, Liu W, Li R. Prevalence and antifungal susceptibility of pathogenic yeasts in China: a 10-year retrospective study in a teaching hospital. Front Microbiol. 2020;11:1401. doi:10.3389/fmicb.2020.01401
16. Pappas PG, Lionakis MS, Arendrup MC, Ostrosky-Zeichner L, Kullberg BJ. Invasive candidiasis. Nat Rev Dis Primers. 2018;4(1):18026. doi:10.1038/nrdp.2018.26
17. Pfaller MA, Diekema DJ, Turnidge JD, Castanheira M, Jones RN. Twenty years of the SENTRY antifungal surveillance program: results for candida species from 1997–2016. Open Forum Infect Dis. 2019;6(Suppl 1):S79–S94. doi:10.1093/ofid/ofy358
18. McTaggart LR, Cabrera A, Cronin K, Kus JV. Antifungal susceptibility of clinical yeast isolates from a large Canadian reference laboratory and application of whole-genome sequence analysis to elucidate mechanisms of acquired resistance. Antimicrob Agents Chemother. 2020;64(9):e00402–20. doi:10.1128/AAC.00402-20
19. Pfaller MA, Carvalhaes CG, DeVries S, Huband MD, Castanheira M. Elderly versus nonelderly patients with invasive fungal infections: species distribution and antifungal resistance, SENTRY antifungal surveillance program 2017–2019. Diagn Microbiol Infect Dis. 2022;102(4):115627. doi:10.1016/j.diagmicrobio.2021.115627
20. Xiao M, Sun ZY, Kang M, et al. Five-year national surveillance of invasive candidiasis: species distribution and azole susceptibility from the china hospital invasive fungal surveillance net (CHIF-NET) study. J Clin Microbiol. 2018;56(7):e00577–18. doi:10.1128/JCM.00577-18
21. Xiao M, Chen SCA, Kong F, et al. Distribution and antifungal susceptibility of candida species causing candidemia in China: an update from the CHIF-NET study. J Infect Dis. 2020;221(Suppl 2):S139–S147. doi:10.1093/infdis/jiz573
22. Huixia Y, Na S, Yumei L, et al. Distribution and drug resistance of clinical Candida infection. Int J Lab Med. 35(21):2997–2998. Chinese.
23. Aibibai A, Zhihua M, Daiqin X, Peiru X. Clinical characteristics and risk factors for bloodstream infection analysis of invasive candidiasis in children in Urumqi. Chin J Contemp Pediatr. 2017;19(4):414–418. Chinese.
24. Xia J, Huang W, Lu F, Li M, Wang B. Comparative analysis of epidemiological and clinical characteristics between invasive Candida infection versus colonization in critically Ill patients in a tertiary hospital in Anhui, China. Infect Drug Resist. 2022;15:3905–3918. doi:10.2147/IDR.S368792
25. Kullberg BJ, Arendrup MC, Campion EW. Invasive Candidiasis. N Engl J Med. 2015;373(15):1445–1456. doi:10.1056/NEJMra1315399
26. Perlin DS, Rautemaa-Richardson R, Alastruey-Izquierdo A. The global problem of antifungal resistance: prevalence, mechanisms, and management. Lancet Infect Dis. 2017;17(12):e383–e392. doi:10.1016/S1473-3099(17)30316-X
27. Fan X, Xiao M, Liao K, et al. Notable Increasing trend in azole non-susceptible Candida tropicalis causing invasive candidiasis in China (August 2009 to July 2014): molecular epidemiology and clinical azole consumption. Front Microbiol. 2017;8:464. doi:10.3389/fmicb.2017.00464
28. Zeng X, Peng M, Liu G, et al. Strain distribution and drug susceptibility of invasive fungal infection in clinical patients with systemic internal diseases. Front Bioeng Biotechnol. 2021;8:625024. doi:10.3389/fbioe.2020.625024
29. Ao K, Deng J, Liu Y, et al. Distribution and drug susceptibility analysis of pathogenic bacteria for fungal bloodstream infection in 19tertiary first-class general hospitals in Sichuan. China Trop Med. 2022;22(12):1188–1193. Chinese.
30. Xia J, Wang Z, Li T, et al. Immunosuppressed patients with clinically diagnosed invasive fungal infections: the fungal species distribution, antifungal sensitivity and associated risk factors in a tertiary hospital of Anhui province. Infect Drug Resist. 2022;15:321–333. doi:10.2147/IDR.S351260
31. Mu N, Zhen N, Wang Y, et al. Analysis of the distribution and drug resistance of pathogenic bacteria in deep fungal infections in Hengshui City from 2017 to 2019. Chin J Disinfection. 2021;38(3):182–188. Chinese.
32. Alexander BD, Johnson MD, Pfeiffer CD, et al. Increasing echinocandin resistance in Candida glabrata: clinical failure correlates with presence of FKS mutations and elevated minimum inhibitory concentrations. Clin Infect Dis. 2013;56(12):1724–1732. doi:10.1093/cid/cit136
33. Pappas PG, Kauffman CA, Andes DR, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the infectious diseases society of America. Clin Infect Dis. 2016;62(4):e1–50. doi:10.1093/cid/civ933
34. Martin-Loeches I, Antonelli M, Cuenca-Estrella M, et al. ESICM/ESCMID task force on practical management of invasive candidiasis in critically ill patients. Intensive Care Med. 2019;45(6):789–805. doi:10.1007/s00134-019-05599-w
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.