Back to Journals » Infection and Drug Resistance » Volume 13
Clinical Characteristics of Pneumocystis Pneumonia After Parental Renal Transplantation
Authors Li T , Shi J, Xu F, Xu X
Received 9 October 2019
Accepted for publication 25 December 2019
Published 8 January 2020 Volume 2020:13 Pages 81—88
DOI https://doi.org/10.2147/IDR.S234039
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Eric Nulens
Tiantian Li,* Junqin Shi,* Fei Xu, Xiaoling Xu
Respiratory and Critical Care Medicine, Affiliated Provincial Hospital to Anhui Medical University, Hefei, Anhui, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiaoling Xu
Respiratory and Critical Care Medicine, Affiliated Provincial Hospital to Anhui Medical University, No. 17 Lujiang Road, Hefei, Anhui 230001, People’s Republic of China
Tel +86 189 6378 9002
Email [email protected]
Purpose: To analyze the clinical characteristics of Pneumocystis pneumonia (PCP) in renal transplant recipients, identify early sensitivity indicators, and optimize clinical strategies.
Patients and Methods: We retrospectively analyzed clinical data for 24 patients with confirmed PCP who underwent renal transplantation (RT) between 2010 and 2019, encompassing a mean follow-up of 29 (range, 11– 49) d.
Results: A 71% incidence was observed for PCP during the first 6 months after RT. Progressive dyspnea (79%) was the most common symptom, followed by fever (75%) and dry cough (67%). In the initial phase of PCP, the most frequent computerized tomography (CT) finding was the presence of symmetric, apically distributed ground-glass opacities. Nine of 11 patients (82%) were diagnosed by induced sputum testing, 14 of 17 (82%) by bronchoalveolar lavage, and 1 of 24 (4%) by sputum smear. The 1,3-β-D-glucan level was elevated (mean, 259.16 ± 392.34 pg/mL) in 80% of patients, while 75% had elevated C-reactive protein levels (median, 37.85 mg/L). Two of 18 patients (11%) were positive for cytomegalovirus. All patients were treated with trimethoprim-sulfamethoxazole (3 doses of 1– 6 g/kg) and third-generation cephalosporin or moxifloxacin monotherapy to prevent bacterial infection. The methylprednisolone dose (40– 400 mg/d) varied according to illness. Most patients were treated using a nasal cannula or oxygen mask, and 2 by mechanical ventilation. CT showed improved lesions after treatment, and completely absorbed lesions or residual fibrosis at follow-up. The mean hospitalization cost was 14,644.73 ± 11,101.59 RMB.
Conclusion: Peak PCP incidence occurred during the first 6 months after surgery. Progressive dyspnea, fever, and dry cough are important indicators for PCP. Bilateral and diffuse ground-glass opacities involving both lung apexes are often the first indication for PCP diagnosis. Induced sputum testing may be the method-of-choice for pathogen detection. The cure rate can be improved through early antipathogen, glucocorticoid, and preventive anti-infection therapies, as well as respiratory support.
Keywords: renal transplantation, Pneumocystis jirovecii, clinical characteristics, optimize clinical strategies
Introduction
Pneumocystis jirovecii is an opportunistic pathogenic fungus that causes Pneumocystis pneumonia (PCP) in humans. Pneumocystis jirovecii primarily infects immunocompromised individuals, causing damage to lung tissues. Pneumocystis pneumonia is one of the leading causes of death in HIV-positive patients. Several studies have shown that PCP is more prevalent in susceptible HIV-negative patients, including patients with solid tumors, those receiving solid organ transplants, especially kidney transplants, and hematopoietic stem cell transplant patients or those with lymphoproliferative disorders.1–6 Allogeneic renal transplantation (RT) is recognized as the treatment of choice for end-stage renal disease. Although the use of immunosuppressive agents can greatly improve the success rate of RT, recipients are at high risk of infection. Pneumocystis pneumonia is a significant cause of morbidity and mortality in RT recipients, with incidence varying between 0.3% and 2.6%,7 while mortality rates can be as high as 50%.8
There is a preliminary consensus on the diagnosis and treatment of PCP in HIV-positive patients. However, the sensitivity of diagnostic indicators for PCP after RT is not well defined, and a gold standard test is lacking, making diagnosis relatively difficult. A retrospective study of PCP in adults in the UK over a decade found that the number of reported PCP-related deaths exceeded that of laboratory-confirmed cases.1 A lack of effective management leads to the abuse of broad-spectrum antibiotics, and premature use of ventilator-assisted breathing or extracorporeal membrane oxygenation (ECMO). This wastes medical resources, greatly increases the economic burden and length of hospital stays for patients, increases mortality rates, and reduces long-term prognosis.
Therefore, we retrospectively analyzed the clinical symptoms, imaging data, laboratory test results, and therapeutic efficacy for 24 patients in our hospital with PCP confirmed after parental-donor RT. We aimed to identify indicators of sensitivity and optimize clinical management strategies, thereby providing a basis for preemptive treatment of PCP.
Materials and Methods
Study Population and Methods
Data for PCP patients diagnosed after undergoing RT from 2010 to 2019 were retrospectively analyzed by searching the information archived and coded by the Anhui Provincial Hospital. The inclusion criteria were: (1) negative for HIV antibodies; (2) receiving triple immunosuppressive therapy after transplantation (mycophenolate mofetil + tacrolimus/cyclosporine + glucocorticoids); (3) no preventive treatment. The Medical Research Ethics Committee of the Anhui Provincial Hospital approved the study. Because this was a retrospective study and is traceable, the committee exempted informed consent. The hospital is committed to protecting patient privacy and complying with the Helsinki Declaration.
The diagnosis of PCP was confirmed by detection of Pneumocystis cysts or trophozoites in bronchoalveolar lavage (BAL) fluid or sputum samples using Gomori’s methenamine silver staining (GMS). Imaging was mainly obtained by chest computerized tomography (CT) scanning using a 64-row spiral Optima CT680 scanner (GE Healthcare, USA). Each patient underwent a whole-lung, routine dose exposure, spiral CT scan with 5-mm slices.
The information recorded included gender, age, coexisting diseases, donor source, time of first surgery, clinical symptoms, CT findings and laboratory test results at admission and after treatment, etiology, therapy, treatment outcomes, hospitalization days, and total cost after follow-up.
Statistical Analysis
All statistical data were analyzed by IBM SPSS software v.16.0 (Chicago, IL, USA). Normally distributed data are expressed as means ± standard deviation, and non-normally distributed data as medians (interquartile range).
Results
Demographic Characteristics
As of March 2019, a total of 24 patients met the above study conditions. The average age of the subjects was 39 ± 7 years, and ranged from 29 to 57 years (Table 1). Of the 24 patients, 16 were males (67%) and 8 females (33%).
 |
Table 1 Demographic Characteristics, Symptoms, Auxiliary Examination, and Treatment of 24 Patients with PCP After RT |
Clinical Presentations
All the patients underwent parental-donor renal transplantation; 17 of them (71%) had a time of PCP onset within 6 months after RT, 5 (21%) within 6 to 12 months, and 2 (8%) longer than 1 year. The most common symptom was progressive dyspnea (19/24, 79%), followed by fever (18/24, 75%), dry cough (16/24, 67%), and production of white phlegm (7/24, 29%) (Table 1).
Imaging Features
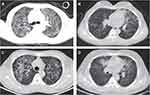
Among all the patients, CT scans revealed increased diffuse, patchy, and cord-like density shadows in both lungs, showing a ground-glass-like morphology, and a symmetrical and apical distribution in the initial phases. Meanwhile, 4 patients (17%) presented lesions with air bronchogram, 4 (17%) with pleural effusion, 1 (4%) with pleural thickening, and 1 (4%) with multiple nodules in the mediastinum (Table 1, Figures 1 and 2).
Etiology
Cysts and trophozoites were detected in 9 of 11 patients who underwent induced sputum testing, as detected by GMS. Of the 17 patients subjected to BAL, 14 (82%) were positive for PCP. Although all the patients were sent for sputum smears at the time of admission, PCP was detected in only one (4%) patient.
Laboratory Tests
Table 1 shows the results of laboratory tests at the time of patient admission to the hospital. It is worth clarifying that not all patients recorded the following laboratory tests on admission. Sixteen of 20 patients (80%) showed elevated 1,3-β-D-glucan levels with a mean of 259.16 ± 392.34 pg/mL. The median C-reactive protein (CRP) value was 37.85 (52.55) mg/L, and was elevated in 15 of 20 patients (75%). The percentage of neutrophils increased in 15 of 21 patients (71%), with a median of 82.4% (15.9). Five of 21 patients (24%) had an increased white blood cell count, with a mean value was 8.11 ± 4.30 × 109/L. The median procalcitonin (PCT) value was 0.16 (0.49) ng/mL, and only 5 of 15 patients (33%) presented abnormal values. Of 23 patients, only 1 (4%) showed a decreased percentage of CD3+ T cells (mean, 76.98 ± 11.08%), and 5 (22%) a decreased percentage of CD4+ T cells (mean, 34.6 ± 11.9%). Two out of 18 patients (11%) tested positive for cytomegalovirus (CMV). In addition, 2 out of 16 patients (13%) were positive for Klebsiella pneumoniae, 1 (6%) was positive for Acinetobacter baumannii, and 1 (6%) was positive for Pseudomonas aeruginosa.
Treatment and Clinical Outcomes
All 24 patients were treated with trimethoprim–sulfamethoxazole (TMP–SMZ) (1–6 mg/kg, divided into 3 doses) as antipathogen therapy and received sodium bicarbonate tablets to alkalize the urine, accompanied by third-generation cephalosporin or moxifloxacin monotherapy to prevent bacterial infection. Some patients had adverse reactions, such as nausea and vomiting, but these symptoms improved following dose reduction. The methylprednisolone dose (40–400 mg/d) varied depending on the illness. Most patients were treated using a nasal cannula (19/24, 79%) or oxygen mask (2/24, 8%) early during hospitalization. In addition, of the 24 patients, 1 (4%) was given non-invasive ventilation, 1 was applied intubation, and 1 was given ECMO. After treatment, CT scans indicated that the lesions were absorbed, and index values such as those for CRP and 1.3-β-D-glucan levels, were decreased. A total of 4 patients (17%) died. One patient (4%) died of renal allograft dysfunction, 1 of bloodstream infection, and 2 (8%) of respiratory failure. The mean hospitalization time was 17 ± 7 d. Eight patients (33%) were hospitalized within 2 weeks, and 10 (42%) within 3 weeks. Six patients (25%) were hospitalized for more than 3 weeks. Excluding patients who died, the mean hospitalization cost was 14,644.73 ± 11,101.59 RMB. Eight patients (33%) had a total cost of less than 10,000 RMB, and 10 (42%) between 10,000 and 20,000 RMB. CT scans for 20 of the 24 patients (83%) showed complete absorption or residual fibrosis of the lesion during 1–2 months of follow-up (Table 1).
Discussion
In this study, we restrospectively analyzed the characteristics of 24 PCP patients after RT. The analysis showed that physicians must be highly alert to PCP if clinical symptoms of progressive dyspnea, fever, and dry cough occur within 6 months of RT. Computerized tomography showed diffuse, patchy, ground-glass opacities at the apexes of both lungs in the initial phases, low white blood cell count and PCT levels, as well as elevated 1.3-β-D-glucan levels. Once suspected, immediate preemptive antimicrobial treatment should be undertaken while waiting for the results of mycological investigations.9
The incidence of PCP after RT is related to the degree of immunosuppression. Because the immunosuppressive regimen is strongest within 6 months of RT, the incidence of PCP is also highest during this period.10 When the immune function is normal, the body can actively phagocytose and kill pathogens through macrophage activity, and T lymphocytes, especially CD4+ T lymphocytes, secrete cytokines that promote pathogen clearance. However, when the immune system is impaired, P. jirovecii can multiply and produce trophozoites that adhere to type I alveolar epithelial cells and destroy the alveolar wall. This induces inflammation, expansion and widening of the alveolar space, and type II alveolar cell reactive hyperplasia and thickening. This is accompanied by lymphocyte and plasmocyte infiltration, resulting in increased alveolar–capillary blood–gas exchange dysfunction, especially impaired oxygen diffusion, which eventually leads to pulmonary fibrosis.11 Consequently, patients can develop progressive dyspnea or even respiratory failure. Analysis of CT scans showed diffuse injury in both lungs, consistent with the results of this study. It is good practice to start using adjuvant corticosteroid therapy within 72 h of commencing PCP-specific therapy when hypoxemia (PaO2 <70 mmHg) occurs, as updated by the American Society of Transplantation; this can significantly improve prognosis, including avoidance of endotracheal intubation and reduced mortality.12 All the patients in this study were treated with different doses of methylprednisolone at the early stage of the disease to suppress inflammation and reduce lung injury.
PCP in HIV-negative patients reportedly does not present abnormal features on chest radiographs in the early stage of the disease owing to acute onset, and some patients seem almost normal and have poor diagnosis sensitivity. In contrast, in CT scans, PCP infections present as crazy paving patterns, ground-glass opacities, crescent-shaped patterns, and single or multiple nodules. Of these, ground-glass opacities are the most common.13 In addition to PCP, CMV is also a major complication arising after solid organ transplantation. The CT profile is similar to that of PCP, but additionally features micronodules and/or air cavities with ground-glass shadows. Differential diagnosis can be achieved through quantitative nucleic acid detection, serology, and histopathology. In this study, 2 of 18 (11%) patients were positive for CMV and were treated with ganciclovir antiviral therapy. Moreover, we also found that all the patients were treated with third-generation cephalosporin or moxifloxacin monotherapy to prevent infection. Because patients may develop new coughs and/or fever, and CT patterns of interstitial pneumonia may be detected, physicians must be alert to the possibility of community-acquired pneumonia.
There are currently no clinically specific laboratory indicators for monitoring PCP infection after RT. Although leukocytes and neutrophils are involved in non-specific cellular immunity, detection using these hematological parameters is susceptible to interference from antibiotics. The CRP level is a highly sensitive indicator of tissue damage or inflammatory responses to various pathogens. However, it lacks specificity and cannot distinguish between infection types. In our study, the number of CD3+ and CD4+ T lymphocytes decreased in only a few patients, and most showed values within the normal range, or even slightly increased values. This indicates that T cell subsets can not be used as an early marker for PCP onset after RT. We also found that the levels of 1.3-β-D-glucan in most patients (16/20, 80%) increased to varying degrees. This polysaccharide is present on the cell wall of P. jirovecii and can be released into peripheral blood or plasma after phagocytosis. Therefore, an increase in the level of circulating 1,3-β-D-glucan is important laboratory evidence for PCP. This endorsement is based on sensitivity and negative predictive values of ≥90% and ≥97%,14–16 respectively, as determined by several meta-analyses. It has been reported that the values for the test were usually >500 pg/mL.17 1,3-β-D-glucan is commonly used as a highly sensitive and specific predictor of aspergillosis in clinical practice. Aspergillosis is mainly caused by Aspergillus fumigatus. Similar to PCP patients, aspergillosis patients often exhibit clinical symptoms such as a dry cough and dyspnea. Some patients have hemoptysis, and respiratory failure may occur in severe cases. However, there are characteristic imaging differences, including a halo sign and air crescent sign, which can distinguish aspergillosis from PCP. Therefore, we believe that elevated 1,3-β-D-glucan levels, combined with the imaging features of interstitial pneumonia, requires a high degree of suspicion of PCP. It should be stressed that 1,3-β-D-glucan is not a PCP-specific diagnostic marker, and a negative or non-significant increase in 1,3-β-D-glucan levels cannot completely rule out the possibility of PCP. However, other factors may be excluded, such as receiving antipathogen treatment before admission.
Detecting the pathogen under a microscope is the traditional method of PCP diagnosis, and this evidence is sufficient for diagnosis.7 Therefore, after collecting respiratory tract specimens from all patients with a high degree of clinical suspicion for PCP, we only used GMS for confirmation of the presence of P. jirovecii. Invasive techniques include BAL and transbronchial biopsy. Using these techniques, samples can be collected directly, and the detection rate is high. However, invasive techniques carry greater risks and a heavy economic burden. Non-invasive techniques mainly include expectorated sputum and induced sputum, among others. These methods are simple and samples are easy to obtain, making them commonly used methods for determining the presence of respiratory pathogens in clinical practice. However, sufficiently large specimens are required for testing. Most patients with PCP after RT present with a dry cough. In our study, only 7 patients (29%) produced a small amount of white phlegm. The American Society of Transplantation recommends initial screening of multiple sputum specimens, and BAL can help with rapid diagnosis.12 But for some patients with severe PCP, especially those on ventilators, invasive BAL techniques may not be tolerated for extended periods. In this study, the detection rate for induced sputum was not substantially different from that for BAL. Based on multifactor comparison, we recommend induced sputum as the specimen of choice.
TMP–SMZ is the recommended first-line treatment for PCP.12 At present, oral TMP–SMZ therapy is widely used in clinical practice, although intravenous TMP–SMZ administration is reportedly preferred for patients with mild to severe PCP.9 Treatment time is approximately 2–3 weeks, and clinical symptoms usually improve after 4–8 d.12 However, TMP–SMZ can frequently cause adverse reactions, such as drug allergy, myelosuppression, hemolytic anemia, hepatic or renal dysfunction, nausea and vomiting, and central nervous system toxicity. Notably, response to therapy should be closely monitored, and blood, hepatic and renal function, and other related indicators should be regularly tested. In this study, all the patients were given oral TMP–SMZ therapy (1–6 g/kg, divided into 3 doses), supplemented with orally administered sodium bicarbonate to alkalize urine. Although some patients had adverse reactions, including nausea and vomiting, the symptoms gradually disappeared after dose reduction, and there was no withdrawal of SMZ–CO owing to adverse drug reactions. TMP–SMZ is also recommended for the prevention of PCP after RT.18 However, because of the possibility of drug resistance and the toxic effects of sulfonamides on the kidneys, none of our patients received preventive treatment.
Most patients that develop PCP after RT are characterized by progressive dyspnea, and receiving ventilation in the early stages of the disease can greatly improve symptoms. All the patients in this study were given a nasal catheter or mask for oxygen inhalation at admission. Mechanical ventilation is an indicator of poor prognosis,9 and can easily cause lung injury and ventilator-associated pneumonia, resulting in increased mortality. In our study, of the 4 patients (17%) that died, 2 (8%) used ventilators, while 1 (4%) underwent ECMO and then died of a bloodstream infection. Therefore, we believe that ventilator use, especially invasive mechanical ventilation, should be avoided in PCP treatment.
Twenty patients (83%) exhibited improved clinical symptoms and laboratory test results after the above treatment. Reexamination by CT showed lesion absorption after treatment, and then complete absorption or residual fibrotic lesions during 1–2 months of follow-up. The average hospitalization time was 17 d, and only 6 patients (25%) were hospitalized for more than 3 weeks. The total cost for 18 patients (75%) was less than 20,000 RMB. Therefore, an optimized PCP treatment plan can greatly reduce hospitalization costs, as well as improve the cure rate and long-term quality of life after RT.
Conclusion
In summary, PCP should be highly suspected if clinical symptoms of progressive dyspnea, fever, and dry cough occur within 6 months after RT, and if symmetric, apically distributed, ground-glass opacities are detected in both lungs with CT. Preemptive treatment should be immediately applied, including TMP–SMZ as antipathogenic therapy, glucocorticoids as anti-inflammatories, third-generation cephalosporin or moxifloxacin monotherapy to prevent bacterial infection, and ventilation to improve hypoxemia. Induced sputum can be used as a preferred method for pathogen detection. Further studies are needed to determine the mechanisms involved in PCP-induced acute lung injury after RT, and to intervene in the inflammatory response and reduce mortality.
Abbreviations
PCP, Pneumocystis pneumonia; RT, renal transplantation; CT, computerized tomography; ECMO, extracorporeal membrane oxygenation; BAL, bronchoalveolar lavage; GMS, Gomori’s methenamine silver staining; CRP, C-reactive protein; PCT, procalcitonin; CMV, cytomegalovirus; TMP–SMZ, trimethoprim-sulfamethoxazole.
Ethical Approval and Informed Consent
This study was approved by the Medical Research Ethics Committee of the Anhui Provincial Hospital (approval number: 2019-P-050). Because this was a retrospective study and is traceable, the committee exempt ed informed consent. The hospital is committed to protecting patient privacy and complying with the Helsinki Declaration. Copies of these approval documents are available for review.
Authors’contributions
All authors contributed towards data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agreed to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Maini R, Henderson KL, Sheridan EA, et al. Increasing Pneumocystis pneumonia, England, UK, 2000–2010. Emerg Infect Dis. 2013;19(3):386–392. doi:10.3201/eid1903.121151
2. Patterson L, Coyle P, Curran T, Verlander NQ, Johnston J. Changing epidemiology of Pneumocystis pneumonia, Northern Ireland, UK and implications for prevention, 1 July 2011–31 July 2012. J Med Microbiol. 2017;66(11):1650–1655. doi:10.1099/jmm.0.000617
3. Pegorie M, Denning DW, Welfare W. Estimating the burden of invasive and serious fungal disease in the United Kingdom. J Infect. 2017;74(1):60–71. doi:10.1016/j.jinf.2016.10.005
4. Buchacz K, Lau B, Jing Y, et al. Incidence of AIDS-defining opportunistic infections in a multicohort analysis of HIV-infected persons in the United States and Canada, 2000–2010. J Infect Dis. 2016;214(6):862–872. doi:10.1093/infdis/jiw085
5. Williams KM, Ahn KW, Chen M, et al. The incidence, mortality and timing of Pneumocystis jiroveci pneumonia after hematopoietic cell transplantation: a CIBMTR analysis. Bone Marrow Transplant. 2016;51(4):573–580. doi:10.1038/bmt.2015.316
6. Avino LJ, Naylor SM, Roecker AM. Pneumocystis jirovecii pneumonia in the non-HIV infected population. Ann Pharmacother. 2016;50(8):673–679. doi:10.1177/1060028016650107
7. Iriart X, Bouar M, Kamar N, Berry A. Pneumocystis pneumonia in solid-organ transplant recipients. J Fungi (Basel). 2015;1(3):293–331. doi:10.3390/jof1030293
8. Gonzalez Santiago TM, Wetter DA, Kalaaji AN, Limper AH, Lehman JS. Pneumocystis jiroveci pneumonia in patients treated with systemic immunosuppressive agents for dermatologic conditions: a systematic review with recommendations for prophylaxis. Int J Dermatol. 2016;55(8):823–830. doi:10.1111/ijd.2016.55.issue-8
9. White PL, Price JS, Backx M. Therapy and management of Pneumocystis jirovecii infection. J Fungi (Basel). 2018;4(4):E127. doi:10.3390/jof4040127
10. Thomas CF, Limper AH. Pneumocystis pneumonia. N Engl J Med. 2004;350(24):2487–2498. doi:10.1056/NEJMra032588
11. Walzer PD. Pneumocystis carinii: recent advances in basic biology and their clinical application. AIDS. 1993;7(10):1293–1305. doi:10.1097/00002030-199310000-00001
12. Martin SI, Fishman JA; AST Infectious Diseases Community of Practice. Pneumocystis pneumonia in solid organ transplantation. Am J Transplant. 2013;13(4):272–279. doi:10.1111/ajt.12119
13. Ebner L, Walti LN, Rauch A, et al. Clinical course, radiological manifestations, and outcome of Pneumocystis jirovecii Pneumonia in HIV patients and renal transplant recipients. PLoS One. 2016;11(11):e0164320. doi:10.1371/journal.pone.0164320
14. Karageorgopoulos DE, Qu JM, Korbila IP, Zhu YG, Vasileiou VA, Falagas ME. Accuracy of β-D-glucan for the diagnosis of Pneumocystis jirovecii pneumonia: a meta-analysis. Clin Microbiol Infect. 2013;19(1):39–49. doi:10.1111/j.1469-0691.2011.03760.x
15. Onishi A, Sugiyama D, Kogata Y, et al. Diagnostic accuracy of serum 1,3-β-D-glucan for pneumocystis jiroveci pneumonia, invasive candidiasis, and invasive aspergillosis: systematic review and meta-analysis. J Clin Microbiol. 2012;50(1):7–15. doi:10.1128/JCM.05267-11
16. Li WJ, Guo YL, Liu TJ, Wang K, Kong JL. Diagnosis of Pneumocystis pneumonia using serum (1-3)-β-D-Glucan: abivariate meta-analysis and systematic review. J Thorac Dis. 2015;7(12):2214–2225. doi:10.3978/j.issn.2072-1439.2015.12.27
17. Damiani C, Le Gal S, Da Costa C, Virmaux M, Nevez G, Totet A. Combined quantification of pulmonary Pneumocystis jirovecii DNA and serum (1->3)-β-D-glucan for differential diagnosis of pneumocystis pneumonia and Pneumocystis colonization. J Clin Microbiol. 2013;51(10):3380–3388. doi:10.1128/JCM.01554-13
18. Kasiske BL, Zeier MG, Chapman JR, et al. KDIGO clinical practice guideline for the care of kidney transplant recipients: a summary. Kidney Int. 2010;77(4):299–311. doi:10.1038/ki.2009.377
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.