Back to Journals » Infection and Drug Resistance » Volume 16
Clinical Characteristics of Enterococcus-Associated Peritonitis in Patients with Peritoneal Dialysis
Authors Ni J, Zhou L, Wang H , Yu J, Tong M, Yu D
Received 30 January 2023
Accepted for publication 26 May 2023
Published 30 May 2023 Volume 2023:16 Pages 3399—3405
DOI https://doi.org/10.2147/IDR.S406437
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Jun Ni, Liusha Zhou, Hua Wang, Jin Yu, Mengli Tong, Dongrong Yu
Department of Nephrology, Hangzhou Hospital of Traditional Chinese Medicine (Hangzhou TCM Hospital Affiliated to Zhejiang Chinese Medical University), Hangzhou, Zhejiang, 310007, People’s Republic of China
Correspondence: Hua Wang, Department of Nephrology, Hangzhou Hospital of Traditional Chinese Medicine, No. 453 Tiyuchang Road, Hangzhou, Zhejiang, People’s Republic of China, Tel +86 13858061992, Email [email protected]
Background: This study aims to investigate the clinical characteristics of enterococcus-associated peritonitis in patients with peritoneal dialysis (PD).
Methods: In this retrospective study, patients with PD-associated enterococcal peritonitis (Group E) who were treated in our center between January 2010 and September 2020 were included. Patients with PD-associated streptococcus peritonitis (Group S) and patients with coagulase-negative staphylococcus peritonitis (Group CNS) were matched 1:1 as cohort-control groups. The clinical characteristics and prognosis of these patients were analyzed.
Results: A total of 21 peritonitis episodes were noted in nine males and nine females, with an average age of 60.33± 14.79 years and an average dialysis duration of 63.56± 35.23 months. Mixed infection was observed in 7 out of 21 cases. A total of 22 strains of enterococci were identified in bacterial culture, all sensitive to vancomycin. There were significant differences in white blood cell (WBC) count and blood urea nitrogen (BUN) level among three groups on admission (p< 0.05). In all three groups, WBC count on the second and third day post-treatment was higher in Group E than in other groups (p< 0.05). The cure rate in Group E was lower than in other groups (p< 0.01). The mortality rate in Group E was slightly higher than in other groups (p> 0.05). Kaplan–Meier analysis revealed a significant difference in the cumulative survival among three groups (p< 0.05).
Conclusion: Enterococcus peritonitis is a rare and severe complication of peritoneal dialysis. Although vancomycin is effective for the treatment of Enterococcus infection, Enterococcus peritonitis still has a high rate of treatment failure, poor response to treatment, and poor prognosis as compared to CNS and streptococcus-related infections.
Keywords: peritoneal dialysis, peritonitis, enterococcus, vancomycin, staphylococcus peritonitis, streptococcus peritonitis
Background
Peritonitis is a critical complication of peritoneal dialysis (PD), and also the leading cause of treatment failure in PD patients. PDOPPS1 study suggests that Gram-positive bacteria, such as Staphylococcus and Streptococcus, are the leading cause of peritonitis, whilst Enterococcus-associated peritonitis (EAP) is relatively rare. Enterococcus is a symbiotic bacterium in human intestine and also has pathogenic potential. It is a natural colonizing bacterium in the gastrointestinal tract of animals and human beings.2 The genomic plasticity of Enterococcus enables it to adapt to harsh environments and increases the ability of some strains to colonize in the gastrointestinal tract and/or spread beyond the intestine.2 EAP in PD patients has a poor prognosis and high risks of intubation, hemodialysis, and even death.3,4 In the present retrospective study, patients with EAP were investigated to elucidate the clinical and pathogenic characteristics of EAP, which will be helpful for the accurate diagnosis and treatment of EAP in the future.
Methods
Design and Participants
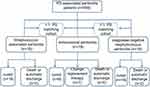
Between January 2010 and September 2021, 18 patients were diagnosed with enterococcal peritonitis among 655 PD-associated peritonitis patients and included in this study (Group E). In addition, patients with Streptococcus-associated peritonitis (Group S) and coagulase-negative staphylococcus peritonitis (Group CNS) were matched with Group E at 1:1 as cohort-control groups. All the patients were diagnosed with ESKD and received PD. The duration of dialysis was at least 3 months for all patients enrolled in this study. The flow diagram of patient recruitment in three Groups is shown in Figure 1.
All patients met the diagnostic criteria for peritonitis, according to the International Society for Peritoneal Dialysis guidelines.5
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Hangzhou Hospital of Traditional Chinese Medicine, Hangzhou TCM Hospital Affiliated to Zhejiang Chinese Medical University (No. 2021KY052). The informed consent was waived because this was a retrospective study (No. 2021KY052).
Data Collection
Clinical and demographic characteristics such as gender, age, duration of dialysis, body temperature, heart rate (HR), and blood pressure (BP) were collected from medical records. Laboratory findings including white blood cell (WBC) count, hemoglobin (Hb), high-sensitivity C-reactive protein (HS-CRP), serum albumin (ALB), potassium (K+), sodium (Na+), creatinine (SCR), urea nitrogen (BUN), serum uric acid (SUA), creatine kinase (CK), and lactate dehydrogenase (LDH) were also collected. Additionally, WBC count of dialysis effluent and results from peritoneal dialysis fluid culture and drug susceptibilities were also recorded.
Statistical Analysis
Statistical analyses were performed with SPSS version 25.0 (SPSS Inc., Chicago, IL, USA). Data with normal distribution are expressed as mean± standard deviation (SD), and those without normal distribution are expressed as median (interquartile range). The clinical and pathological characteristics were compared with t-test between groups or analysis of variance (ANOVA) among groups for quantitative variables or with nonparametric tests for qualitative variables. Categorical variables were expressed as percentages and compared with Pearson’s Chi-squared test or Fisher’s exact test. A value of P less than 0.05 was considered statistically significant. Kaplan–Meier survival curves were used to analyze the cumulative survival from the time of biopsy to the endpoints.
Results
Clinical Characteristics at Baseline
Baseline clinical characteristics are shown in Table 1. There were no significant differences in the duration of PD dialysis, number of patients with initial infection, and vital signs among three groups (P>0.05). Twenty-one EAP episodes were observed in 18 patients (Group E), accounting for 3.2% of patients hospitalized for PD-related peritonitis. Of these, three patients had repeat peritonitis, and six had relapsing peritonitis. The primary renal diseases in Group E were as follows: chronic nephritis (n=9), IgA nephropathy (IgAN; n=2), diabetic nephropathy (n=2), lupus nephritis (n=2), and obstructive nephropathy (n=1). The remaining two patients were diagnosed with unexplained renal failure (one had concomitant diabetes). In addition, 6 patients had coronary heart disease, 2 had sepsis on admission, and 14 patients with 17 episodes had a history of peritonitis.
 |
Table 1 Comparison of General Data of Three Groups of Patients |
The sources of infection in Group E were as followed: infection originating from gastrointestinal tract was noted in five patients, and two patients had concomitant urinary tract infection. However, the source of infection was unknown in two patients.
Fifteen patients (71.43%) had HS-CRP level >100 mg/L and 16 (76.2%) had serum albumin level <30 g/L (in Group E). Except for WBC and BUN, there were no significant differences in other baseline parameters among three groups (P>0.05) (Table 2). Count of WBC in the dialysis fluid on day 1 was not higher in Group E than in Group S and Group CNS (P>0.05); however, it was much higher in Group E on the second day and third day after the initiation of treatment as compared to other groups (P<0.05).
 |
Table 2 Comparison of Physical and Chemical Indexes Among the Three Groups |
Etiology
In 21 episodes of EAP in the Group E, 14 patients experienced single enterococcal infection, and 7 showed mixed infection. Among patients with mixed infection, infection was caused by two kinds of enterococci in one patient, by enterococci and staphylococcus in two patients, by concomitant Gram-negative bacterium in three patients, and by concomitant fungal infection in one patient. A total of 22 strains of Enterococcus were identified by bacterial culture: Enterococcus faecalis was found in 13 cases, Enterococcus faecium in 7 cases, Enterococcus avium in 1 case, Enterococcus casseliflavus in 1 case, and Enterococcus gallinaceous in 1 case. All strains of Enterococcus were sensitive to vancomycin.
Treatments and Clinical Outcome
Treatments and clinical outcomes are shown in Table 3. Eighteen patients in Group E were treated with vancomycin (vancomycin was switched to linezolid in three patients), one patient was treated with cefazolin and amikacin, one patient was treated with levofloxacin, and one patient was treated with levofloxacin and ceftriaxone. In the Group CNS, vancomycin was used in eight cases; cefazolin and amikacin were used in four cases, and levofloxacin was used in six cases. In Group S, vancomycin was used in seven cases; cefazolin and amikacin were used in three cases, and levofloxacin was used in eight cases. Four out of 18 patients died of peritonitis or refused to receive treatments and were discharged due to disease progression. PD catheter was extubated in six patients who then received hemodialysis (including one patient died). One patient recovered from repeat peritonitis and received reintubation of PD catheter; one patient recovered from repeat peritonitis and then received kidney transplantation 5 months later. Remaining six patients recovered from EAP. In Group CNS and Group S, 16 patients recovered while two died of peritonitis or refused to receive further treatments and were discharged due to disease progression. The remission rate in Group E was 33.3%, which was significantly lower than in Group CNS and Group S (both 88.9%) (P<0.01). The mortality rate in Group E was 27.8%, which was slightly higher than in Group CNS and Group S (both 11.1%) (P>0.05) (Table 3). The endpoint was death or failure to withdrawing from PD (excluding modified kidney transplantation). Patients were followed up until December 2021, and the duration of follow-up ranged from 1 month to 107 months. The Kaplan–Meier survival curve is shown in Figure 2. Kaplan–Meier analysis revealed significant difference in the cumulative survival among three groups (log rank χ2=6.072, P=0.048) (Figure 2).
 |
Table 3 Comparison of Prognosis Among the Three Groups |
 |
Figure 2 Kaplan-Meier survival analysis in three groups. |
Discussion
Gram-positive bacteria are still the main infectious causes of PD-associated peritonitis. Common Gram-positive bacteria that cause peritonitis include staphylococcus aureus, coagulase-negative staphylococcus, and streptococcus. In general, their clinical characteristics are similar, and the etiology may well be touch contamination.
Enterococcus is different from the above Gram-positive bacteria. Edey et al3 showed that Enterococcus accounted for only 3% of PD-associated peritonitis in an Australian population. Yip et al4 reported that single enterococcal peritonitis accounted for 2% whilst single enterococcal and mixed infections accounted for 3.45% in total in Hong Kong. Similarly, our study showed that single enterococcal peritonitis accounted for 2.44%, which was consistent with what was previously reported.3,4 The overall rate of enterococcal infection was 3.2% in PD-associated peritonitis. Edey et al3 reported that the ratio of diabetes in PD patients with EAP was 38%, suggesting an association between gastrointestinal symptoms and diabetes. In our study, one out of six patients had diabetic nephropathy or concomitant diabetes due to small number of diabetes patients in PD patients in our center.
In terms of routes of infection, many previous studies have reported that enterococcal peritonitis mainly originates from the intestine, and it may also be caused by direct contamination in several cases. It has been reported that colonoscopy might also cause enterococcal infection.6 In our study, three cases showed repeat peritonitis, and another five cases showed relapsing peritonitis six times. The high recurrence rate may be related to complex factors in the digestive tract, such as intestinal compartment, ulcer, and cholelithiasis. Enterococci coexisting with other organisms can cause polymicrobial infection. In our study, the proportion of mixed infection caused by enterococcus and other pathogens was 33.3%, and the mortality rate was 27.8%, which suggested worse outcomes than those after Enterococcus peritonitis caused by a single organism. Meanwhile, enterococci sometimes enter the slime layer of intra-abdominal portion of PD catheter and form a biofilm on the catheter. This may increase the hospitalization rate, alteration of antibiotics used, and risks for surgical exploration, mortality, and treatment failure. The poor prognosis in our study also confirmed the results from studies in Australia and Hong Kong.3,4
Imaging examinations such as computed tomography (CT) may be beneficial for the diagnosis and prognosis of enterococcal peritonitis. Studies have confirmed that around 41% of patients with hemodynamic instability need to be admitted to intensive care units.7 Mixed infections, fungal peritonitis, relapsing peritonitis, and refractory peritonitis often manifest as abdominal abnormalities on imaging, including intestinal obstruction, biliary tract abnormalities, and encapsulated fluid accumulation in the abdominal cavity.7 In addition, more advanced techniques such as second-generation genome sequencing may be beneficial for the diagnosis of peritonitis caused by rare pathogens.8
Hypoalbuminemia in PD patients is associated with early-onset peritonitis.9 Hypoalbuminemia can lead to tissue and intestinal edema, thereby causing the translocation of intestinal flora to the abdominal cavity and secondary peritonitis. In this study, there were no significant differences in the serum ALB and HS-CRP among Group E, Group CNS, and Group S, suggesting that hypoalbuminemia and high HS-CRP level are mainly related to peritonitis rather than different Gram-positive bacteria. Our study showed that the WBC count in Group E was much lower than in Group CNS and Group S on admission, suggesting that early inflammatory response to Enterococcus infection is weaker than that to Streptococcus or Staphylococcus infection. Some studies have shown that WBC count10 and decline rate of WBC11 in the dialysis effluent on the third day are related to the prognosis of peritonitis. In our study, there was no marked difference in the count of WBC in the dialysis effluent on admission among three groups, but it was significantly different on the second day and third day post-admission among groups. The count of WBC in the dialysis effluent of Group E was significantly higher than in Group CNS and Group S, suggesting that the response to treatment of enterococcal peritonitis is poor, which attributed to the poor prognosis in Group E.
The drug resistance of Enterococcus is related to a variety of factors. The use of vancomycin can enhance the transmission of drug-resistant Enterococcus from patients to the environment and healthy people and also increase its concentration and detection rate in the feces of patients.12 In this study, all enterococci were sensitive to vancomycin. The overall low rate of vancomycin-resistant Enterococcus (VRE) in China may be related to the relatively rare use of oral vancomycin in the treatment of Clostridium difficile. Enterococcus can form biofilms in response to virulent factors.13 In our study, one patient recovered and the dialysis catheter was refreshed. After 10-month follow-up, recurrence of peritonitis was not observed, suggesting that replacement of dialysis catheter may be a protective factor against relapse of enterococcal peritonitis. The prevention of relapsing enterococcal peritonitis may be achieved by using intestinal flora regulating drugs in PD patients, especially for Enterococcus faecalis.14 In our study, the long-term mortality and treatment failure of Enterococcus peritonitis were higher than those of Streptococcus and Staphylococcus peritonitis, suggesting a worse long-term prognosis for Enterococcus peritonitis patients.
In conclusion, although vancomycin is effective for the treatment of EAP in our study, EAP still has a high rate of treatment failure, poor response to treatments, and poor prognosis as compared to CNS and streptococcus-related infections.
Abbreviations
PD, peritoneal dialysis; WBC, white blood cells, Hb, hemoglobin, HS-CRP, high-sensitivity C-reactive protein; ALB, serum albumin; K+, potassium; Na+, sodium; SCR, serum creatinine; BUN, blood urea nitrogen; SUA, serum uric acid; CK, creatine kinase; LDH, lactate dehydrogenase; CT, computed tomography; VRE, Vancomycin-resistant Enterococcus.
Data Sharing Statement
The datasets used and analyzed during the present study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Hangzhou Hospital of Traditional Chinese Medicine, Hangzhou TCM Hospital Affiliated to Zhejiang Chinese Medical University (No. 2021KY052). The informed consent was waived because this was a retrospective study (No. 2021KY052). The data of all patients in this study were kept confidential according to the patients’ wishes.
Funding
There is no funding to report.
Disclosure
The authors declared that they have no competing interests.
References
1. Perl J, Fuller DS, Bieber BA, et al. Peritoneal dialysis-related infection rates and outcomes: results from the peritoneal dialysis outcomes and practice patterns study (PDOPPS). Am J Kidney Dis. 2020;76(1):42–53. doi:10.1053/j.ajkd.2019.09.016
2. Arias CA, Murray BE. The rise of the Enterococcus: beyond vancomycin resistance. Nat Rev Microbiol. 2012;10(4):266–278. doi:10.1038/nrmicro2761
3. Edey M, Hawley CM, McDonald SP, et al. Enterococcal peritonitis in Australian peritoneal dialysis patients: predictors, treatment and outcomes in 116 cases. Nephrol Dial Transplant. 2010;25:1272–1278. doi:10.1093/ndt/gfp641
4. Yip T, Kai-Chung T, Flora N, et al. Clinical course and outcomes of single-organism Enterococcus peritonitis in peritoneal dialysis patients. Perit Dial Int. 2011;31(5):522–528. doi:10.3747/pdi.2009.00260
5. Li PK, Kai Ming C, Yeoungjee CH, et al. ISPD peritonitis guideline recommendations: 2022 update on prevention and treatment. Perit Dial Int. 2022;42(2):110–153. doi:10.1177/08968608221080586
6. Lin YC, Lin WP, Huang JY, et al. Polymicrobial peritonitis following colonoscopic polypectomy in a peritoneal dialysis patient. Intern Med. 2012;51(14):1841–1843. doi:10.2169/internalmedicine.51.7485
7. Trinh E, Bargman JM. Utility of abdominal imaging in peritoneal dialysis patients presenting with peritonitis. Can J Kidney Health Dis. 2020;7:2054358120964115. doi:10.1177/2054358120964115
8. van Hougenhouck-Tulleken WG, Lebre PH, Said M, et al. Bacterial pathogens in peritoneal dialysis peritonitis: insights from next-generation sequencing. Perit Dial Int. 2020;40(6):581–586. doi:10.1177/0896860820908473
9. Wu H, Huang R, Yi C, et al. Risk factors for early-onset peritonitis in southern Chinese peritoneal dialysis patients. Perit Dial Int. 2016;36(6):640–646. doi:10.3747/pdi.2015.00203
10. Cho Y, Johnson DW. Peritoneal dialysis-related peritonitis: towards improving evidence, practices, and outcomes. Am J Kidney Dis. 2014;64(2):278–289. doi:10.3747/pdi.2015.00203
11. Zhang Z, Jiang N, Fang W, et al. Analysis of risk factors for prognosis of peritoneal dialysis-related bacterial peritonitis. Chin J Nephrol. 2015;31(9):647–651.
12. Donskey CJ, Chowdhry TK, Hecker MT, et al. Effect of antibiotic therapy on the density of vancomycin-resistant enterococci in the stool of colonized patients. N Engl J Med. 2000;343(26):1925–1932. doi:10.1056/NEJM200012283432604
13. Ch’ng JH, Chong KKL, Lam LN, et al. Biofilm-associated infection by enterococci. Nat Rev Microbiol. 2019;17(2):82–94. doi:10.1038/s41579-018-0107-z
14. Argentino G, Borrelli S. Prevention of recurrence of enteric peritonitis in peritoneal dialysis with Escherichia coli Nissle 1917: a case-series study. Clin Nephrol. 2021;96(1):17–21. doi:10.5414/CN110271
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.