Back to Journals » Infection and Drug Resistance » Volume 14
Clinical Characteristics and Risk Factors for Critically Ill Patients with Carbapenem-Resistant Klebsiella pneumoniae (CrKP): A Cohort Study from Developing Country
Authors Luan YY, Chen YH, Li X, Zhou ZP , Huang JJ, Yang ZJ, Zhang JJ, Wu M
Received 9 October 2021
Accepted for publication 12 December 2021
Published 20 December 2021 Volume 2021:14 Pages 5555—5562
DOI https://doi.org/10.2147/IDR.S343489
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Ying-Yi Luan,1,* Yan-Hong Chen,2,* Xue Li,3 Zhi-Peng Zhou,2 Jia-Jia Huang,2,4 Zhen-Jia Yang,2,4 Jing-Jing Zhang,2,5 Ming Wu2,4,6
1Department of Central Laboratory, Beijing Obstetrics and Gynecology Hospital, Capital Medical University, Beijing, 100026, People’s Republic of China; 2Department of Critical Care Medicine and Hospital Infection Prevention and Control, Shenzhen Second People`s Hospital & First Affiliated Hospital of Shenzhen University, Health Science Center, Shenzhen, 518035, People’s Republic of China; 3Department of Emergency, the Eighth Affiliated Hospital, Sun Yat-sen University, Shenzhen, 518033, People’s Republic of China; 4Shantou University Medical College, Shantou, 515041, People’s Republic of China; 5Department of Critical Care Medicine, Pingshan District People’s Hospital of Shenzhen, Shenzhen, 518118, People’s Republic of China; 6Guangxi University of Chinese Medicine, Nanning, 530200, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Ming Wu Tel +86 755 83676149
Email [email protected]
Background: Increasing evidence indicates carbapenem-resistant Klebsiella pneumoniae (CrKP) is increasingly prevalent in intensive care unit (ICU), but its clinical characteristics and risk factors remain unknown.
Aim: The aim of the present study was to evaluate clinical characteristics, risk factors in critically ill patients with CrKP infection.
Methods: A retrospective study was included in patients from January 2013 to October 2019. Clinical data were collected from CrKP patients on the day of specimen collection admitted to ICU. Multivariable logistic regression was used for risk factors. Receiver operating curve (ROC) and the area under the curve (AUC) with DeLong method of MedCalc software were used. Two-way repeated-measures ANOVA analysis was used to analyze the characteristics of independent risk factors over time.
Findings: A total of 147 adult patients with CrKP were screened, among them, 89 (median age 64.0 years, 66 (74.15%) males) patients with CrKP were finally included, of which 38 patients (42.7%) were non-survival group. Multivariate logistic regression analysis indicated that lactic acid (OR3.04 95% CI 1.38– 6.68, P = 0.006), APACHE II score (OR 1.20, 95% CI 1.09– 1.33, P < 0.001), tigecycline combined with fosfomycin treatment (OR0.15, 95% CI 0.04– 0.65, P = 0.011) are independent risk factors for 28-day mortality in patients with CRKP infection (P< 0.05). Combined lactic acid with APACHE II score could predict 28-day mortality, of which AUC value was 0.916 (95% CI, 0.847– 0.985), with sensitivity 0.76 and specificity 0.98. ANOVA analysis showed that APACHE II score and lactic acid between the two groups at three-time points were statistically significant, which interactive with time and showed an upward and downward trend with time (P < 0.05).
Conclusion: Therapeutic strategy based on improving lactic acid and APACHE II would contribute to the outcome in patients with CrKP infection. Tigecycline combined with fosfomycin could reduce the 28-day mortality in patients with CrKP infection in developing country.
Keywords: carbapenem resistant Klebsiella pneumoniae, lactic acid, APACHE II score, tigecycline, fosfomycin, mortality
Introduction
Klebsiella pneumoniae (KPN) is a kind of intestinal bacteria in our surrounding environment, and is colonized in human oral cavity, respiratory tract, gastrointestinal tract and urinary tract. It is generally considered that KPN, as an opportunistic pathogen, can lead to life-threatening infection in infants or children with low immunity and elderly patients or patients taking immunosuppressants for a long time, including sepsis caused by pulmonary infection, urinary infection and blood flow infection.1 KPN mainly obtains drug resistance through the production of antibiotic inactivating enzymes, active efflux mechanism and the formation of biofilm to resist the bactericidal activity of antibiotics. KPN produced carbapenem enzymes (classes A, B and D) and AmpC enzymes/ESBLs combined with deletion or down-regulation of outer membrane protein, which made it resistant to carbapenem antibiotics.2 The international drug resistance network monitoring organization (INFORM) collected 45,335 strains of Gram-negative bacteria from 18 European countries/regions from 2013 to 2017, and screened 9546 isolated strains β- Lactamase. Carbapenemase was found in 3.4% of intestinal bacteria collected in Greece, but KPN was the most common (accounting for 10.5% of the collected bacteria).3 Simultaneously, the infection rate of carbapenem-resistant Klebsiella pneumoniae (CrKP) in China has increased from 6% in 2012 to 10.1% in 2018, and the infection rate continues to rise slowly, and the resistance rate of CrKP is differentially distributed in China.
Previous studies reported that patients infected with CrKP usually had various types of chronic diseases or immunosuppressive status, which is also the reason for the high mortality of CrKP patients.4–7 A cross-sectional survey on the anti-infection treatment of carbapenem resistant gram-negative bacteria was conducted in 115 hospitals in Europe and America through Internet questionnaire. It was found that compared with single drug, antibiotic combination therapy can improve the treatment effect and prevent the emergence of bacterial drug resistance.8 Since 2013, CrKP began to appear in our hospital (the teaching Hospital of coastal open cities in China), and it has become an upward trend. The current study characterized the independent risk factor of CrKP infection and determined whether the combined risk factor equation was independently associated with an increased risk for CrKP and 28-day mortality. We further investigated the effect of the risk factors for 28-day mortality among CrKP infected critically ill patients.
Materials and Methods
Study Design and Population
A retrospective cohort study was designed in the ICU affiliated to the university hospital from January 2013 to October 2019. We reviewed the case records by retrieving the medical record system electronically the case records of 147 patients were reviewed using the clinical diagnosis criteria for CrKP.9 Among them, 13 patients with incomplete data, 25 patients with CrKP colonization according to clinical data, 3 cases with malignant tumors or malignant hematological diseases, 7 cases died or discharged automatically within 48 hours after sample submission, and 10 cases with other refractory microbial infections. The remaining 89 patients were included in the primary analysis of the current study (Figure 1).
 |
Figure 1 Diagram of patient eligibility and flow. |
Clinical Variables
Clinical and laboratory data were obtained daily throughout hospitalization and recorded on standardized data collection forms. Data included laboratory examinations (eg, the biochemical indexes on the day of submitting bacterial culture samples for inspection, the day of the results of bacterial culture drug sensitivity test report and the fourth day after the drug sensitivity test report, whichever is the worst), acute physiology and chronic health evaluation II (APACHE-II) score, sequential organ failure assessment (SOFA) score, Charlson comorbidity index (CCI).
Bacterial Identification and Drug Sensitivity Test
Merrier VITEK 2 compact automatic bacterial identification system and supporting drug sensitivity identification card were used to detect the drug sensitivity of common antibiotics (cefepime, amikacin, ciprofloxacin, ceftazidime, piperacillin tazobactam, meropenem, tobramycin, gentamicin, etc.). The VITEK 2 is an automated microbiology system utilizing growth-based technology. The system is available in three formats (VITEK 2 compact, VITEK 2, and VITEK 2 XL) that differ in increasing levels of capacity and automation. The drug sensitivity test results were judged according to the standards of clinical and Laboratory Standards Institute (CLSI) 2013–2019 editions.
Statistical Analysis
Quantitative parameters are presented as the means±standard deviations or medians and interquartile ranges (IQR), and qualitative parameters are expressed as numbers and percentages. Continuous variables were compared using the independent two-sample t-test or Mann–Whitney U-test. Categorical variables were compared using the chisquare test or Fisher’s exact test. Binary logistic regression multivariate model was used to analyze the risk factors of 28 day mortality of patients, and the receiver operating characteristic curve (ROC curve) was drawn to predict the best cut-off point of risk factors for 28 day mortality of CrKP patients. MedCalc software was used to test whether there was statistical difference in the area difference under each ROC curve by DeLong method. Repeated measurement analysis of variance was used to explore the relevant characteristics of risk factors over time (On the day of sampling (Day0), 3 days after sampling (Day4), 7 days after sampling (Day8)). All tests were two-sided, and P < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS 22.0 (SPSS Inc., Chicago, IL, USA), Empower(R) (http://www.empowerstats.com, X&Y solutions, Inc., Boston, MA) and R (http://www.R-project.org) software.
Results
Baseline Characteristics
A total of 89 patients were enrolled in this study (66 males). Patient age ranged from 19 to 95 years, and their mean age was 64.0 years. Thirty of the 51 CrKP patients (57.30%) survived. Thirty-three (37%) CrKP patients received tigecycline combined with fosfomycin anti-infection treatment. There were 6 kinds of infection sites in CrKP patients, including 12 cases of blood flow infection (13.48%), 49 cases of lung infection (55.05%), 2 cases of abdominal infection (2.24%), 1 case of central nervous system infection (1.2%), 16 cases of urinary system infection (17.97.3%), 2 cases of skin and soft tissue infection (2.24%), and 7 cases of mixed infection (Table 1, Figure 1 and S1).
 |
Table 1 Baseline Demographics and Clinical Characteristics of Patients with CRKP Infection |
There was no significant difference in C-reactive protein, leukocyte count, lymphocyte count, neutrophil count, erythrocyte count, neutrophil lymphocyte ratio between survival group and non-survival group. Compared with the non-survival group, the total bilirubin, unconjugated bilirubin, the glutamic oxaloacetic transaminase, urea nitrogen, lactic acid, troponin, myoglobin, APACHEII, and SOFA score median in the survival group were lower (P < 0.05) (Table 2).
 |
Table 2 Comparisons of Laboratory Findings Between Survival Group and Non-Survival Group |
Risk Factors Associated with 28-Day Mortality Caused by Klebsiella pneumoniae
Several variables in the univariate analysis were significantly associated with an increased risk for mortality immediately after CrKP. A stepwise regression method was used simultaneously to exclude variable correlation and multicollinearity in logistic regression. Age (years), gender, in baseline were adjusted in fully adjusted model. Multivariable analysis revealed the following predictors as independent risk factors for 28-day mortality after CRKP: lactic acid (OR = 3.25, 95% confidence interval [CI]: 1.58–6.71, P < 0.01), APACHE II score (OR = 0.018, 95% CI: 0.037–0.73, P < 0.05), tigecycline combined with fosfomycin anti-infection program (OR = 1.2, 95% CI: 1.08–1.34, P = 0.01) (Table 3).
 |
Table 3 Risk Factors of 28-Day Mortality in Patients with CrKP Infection |
Sensitivity Analysis
The current study, in multivariable logistic analysis, lactate combined with APACHE II score (logistic regression combined with ROC curve, LRCWR=0.204*APACHEII score+1.035*LACT-7.086) predicts the 28-day mortality prediction model of CrKP patients. The ROC curve shows that the AUC value of the prediction model is 91.6% (95% CI: 0.847–0.985; P < 0.01), the sensitivity were 0.763 and the specificity were 0.98, respectively (Figure 2). The combined risk factor equation predicts that the 28-day mortality rate of CrKP patients is stronger than lactic acid. The difference between the risk factor combination equation and the area under the ROC curve of the APACHE II score was not statistically different, proving that lactic acid has a weak predictive power for 28-day mortality in CrKP patients.
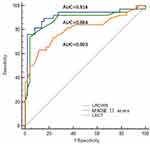 |
Figure 2 Combined model, lactate and APACHE II score for predicting 28-day in CRKP patients. |
Next, the two-way repeated-measures ANOVA analysis of variance showed that the difference between the APACHE II scores of then on non-survival group and the survival group at different times was statistically significant (P < 0.05, Figure S2), and the APACHE II score level of the death group at three-time points (Day0, Day4, and Day8). The APACHE II score level had an interactive effect with time; APACHE II score level did not increase significantly in the survival group at Day0, Day4, and Day8. The lactic acid level of the death group was higher than that of the surviving group at Day0, Day4, and Day8 time points, and showed a downward trend with time (P < 0.05, Figure S3). There was an interactive effect with time; the lactic acid level in the survival group did not increase significantly at Day0, Day4, and Day8.
Discussion
We found a significant association between lactic acid, APACHE II score and CrKP. Notably, tigecycline combined with fosfomycin can reduce the 28 day mortality of patients with CrKP infection.
With the progress of intensive care and treatment measures, it is generally believed that APACHE II score may be high for the estimated mortality of critically ill patients,9–12 but some researchers believe that APACHE II score has the ability to better evaluate the disease severity of patients in various types of patients,13–15 and higher APACHE II score often represents the possibility of higher in-hospital mortality. Studies have shown that APACHE II score is better than NEWS score and REMS score in predicting the prognosis of critically ill patients. Thanapaisal et al16 conducted a retrospective study and found that APACHE II score has a high predictive effect on the prognosis of patients with severe trauma. However, some researchers believe that APACHE II score has the ability to better evaluate the disease severity of patients in various types of patients,13 and higher APACHE II score often represents higher in-hospital mortality.17 As indicated in our data, lactate and APACHE II score was related to the 28-day prognosis of CrKP patients. At present, many clinical studies have shown that lactic acid may have a certain correlation in predicting the prognosis of infected patients.18,19 In the 1-hour cluster treatment in the campaign to save sepsis 2018, it is also mentioned that when the lactic acid level of sepsis patients is over 4mmol/L, fluid resuscitation treatment should be used quickly,20 and it is generally believed that the reduction of lactic acid value is closely related to the prognosis of patients.21 Thus, the generation and metabolism mechanism of lactic acid is complex, which is related to glucose metabolism, liver function level and treatment methods, and the optimal lactate clearance rate is still controversial. Prospective randomized clinical trials have shown that compared with sepsis treatment guided by central venous oxygen saturation, sepsis treatment guided by lactate clearance does not improve the short-term prognosis of sepsis patients.22 Although several studies have shown that the lactate clearance rate within 24 hours is related to the prognosis of infected patients,23 repeated measurement was not used to study the changes of lactate in patients with different prognosis at different time points. In our retrospective analysis, we found that the predictive efficiency of lactate in predicting 28 day mortality of CrKP patients was worse than that of the combined model. In the repeated measurement and analysis of lactic acid at Day0, Day4, and Day8, the lactic acid value in the death group showed a downward trend, suggesting that the lactic acid level on the day of sample submission (Day0) can be used to predict the 28-day prognosis of CrKP patients. However, there was no significant correlation between the lactic acid level on the day (Day4) of bacterial culture drug sensitivity test report, the fourth day (Day8) after drug sensitivity test report and the 28-day prognosis of CrKP patients.
In terms of clinical practice, CrKP is highly sensitive to polymyxin, tigecycline, fosfomycin and ceftazidime avibactam,24–29 and these antibiotics are limited to their pharmacokinetics and pharmaceutical properties, infection site, liver and kidney toxic and side effects. There are many reports on the combined therapy of CrKP, including the effect of polymyxin, tigecycline, fosfomycin, ceftazidime, avibactam, carbapenem antibiotics and aminoglycoside antibiotics on the prognosis of patients. Mikhail et al30 showed that the anti-infection effect of ceftazidime avibactam combined with meropenem was better than other anti-infection schemes, and ceftazidime avibactam combined with amikacin, meropenem and aztreonam showed synergistic effect, and the mic value of Klebsiella pneumoniae to the above drugs decreased. Similarly, the studies conducted by Liang et al31 showed that the combination therapy based on polymyxin B reduced the 30-day mortality of patients with bloodstream infection after CrKP. Furthermore, polymyxin combined with meropenem or amikacin has bactericidal effect on CRKP, but only bacteriostatic effect when polymyxin is used alone.
This study has some limitations. It was a single-center retrospective cohort study, which eliminate the selective bias of patients. The hospital’s laboratory performed all of the clinical biochemical measurement methods, and the medical electronic system was reviewed. The time span of this study is large, which was affected by different diagnosis and treatment concepts, organ support treatment and drug level in different periods, it may bring bias to the research results. Therefore, future studies should include multicenter cohort to expand the sample size to achieve higher-level clinical outcomes.
Conclusions
The prediction of lactate and APACHE II score at the day of sampling are independent risk factors for the 28-day prognosis of CrKP patients. Therapeutic strategy based on improving lactic acid and APACHE II would contribute to the outcome in patients with CrKP infection. Tigecycline combined with fosfomycin could reduce the 28-day mortality in patients with CrKP infection in developing country.
Data Sharing Statement
The data used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Statement of Ethics
The study was conducted in accordance with the Declaration of Helsinki. This study was approved by the Research Ethics Committee of the Shenzhen Second People’s Hospital (20200422008). Considering the retrospective study design and depersonalization of the data, the Ethics Committee agreed to waive the requirement for written informed consent but required that the patients be informed of the study details during the telephone follow-up.
Funding
This work was supported by grants from the Sanming Project of Medicine in Shenzhen (SZSM20162011), the National Natural Science Foundation of China (Nos. 81873943), Shenzhen Science and Technology Innovation Commission (JCYJ20190806163603504), and Shenzhen Second People’s Hospital Clinical Research Fund of Guangdong Province High-level Hospital Construction Project (Grant No20173357201815, No20193357003, No 20203357014).
Disclosure
The authors declare that they have no known competing financial interests or personal relationships that could have influenced the work reported in this paper.
References
1. Bengoechea José A, Joana SP. Klebsiella pneumoniae infection biology: living to counteract host defences. FEMS Microbiol Rev. 2018;43(2):123–144.
2. Kazmierczak KM, De Jonge B, Stone GG, et al. Longitudinal analysis of ESBL and carbapenemase carriage among Enterobacterales and Pseudomonas aeruginosa isolates collected in Europe as part of the International Network for Optimal Resistance Monitoring (INFORM) global surveillance programme, 2013-17. J Antimicrob Chemother. 2020;75(5):1165–1173.
3. Pagano L, Caira M, Trecarichi EM, et al. Carbapenemase-producing Klebsiella pneumoniae and hematologic malignancies. Emerg Infect Dis. 2014;20(7):1235–1236.
4. Huang M, He P, Munir S, et al. Ecology and etiology of bacterial top rot in maize caused by Klebsiella pneumoniae KpC4. Microb Pathog. 2020;139:103906.
5. Karlsson M, Stanton RA, Ansari U, et al. Identification of a carbapenemase-producing hypervirulent Klebsiella pneumoniae isolate in the United States. Antimicrob Agents Chemother. 2019;63(7):e00519.
6. Arena F, Henrici De Angelis L, D’Andrea MM, et al. Infections caused by carbapenem-resistant Klebsiella pneumoniae with hypermucoviscous phenotype: a case report and literature review. Virulence. 2017;8(8):1900–1908.
7. Zhong XS, Li YZ, Ge J, et al. Comparisons of microbiological characteristics and antibiotic resistance of Klebsiella pneumoniae isolates from urban rodents, shrews, and healthy people. BMC Microbiol. 2020;20(1):12.
8. Niu G, Li W. Next-generation drug discovery to combat antimicrobial resistance. Trends Biochem Sci. 2019;44(11):961–972.
9. Soares M, Dongelmans DA. Why should we not use APACHE II for performance measurement and bench marking? Rev Bras Ter Intensiva. 2017;29:268–270.
10. Lee H, Lim CW, Hong HP, et al. Efficacy of the APACHE II score at ICU discharge in predicting post-ICU mortality and ICU readmission in critically ill surgical patients. Anaesth Intensive Care. 2015;43(2):175–186.
11. Czajka S, Ziębińska K, Marczenko K, Posmyk B, Szczepańska AJ, Krzych KJ. Validation of APACHE II, APACHE III and SAPS II scores in in-hospital and one year mortality prediction in a mixed intensive care unit in Poland: a cohort study. BMC Anesthesiol. 2020;20(1):296.
12. Breslow MJ, Badawi O. Severity scoring in the critically ill: part 1–interpretation and accuracy of outcome prediction scoring systems. Chest. 2012;141(1):245–252.
13. Serpa Neto A, Assunção MS, Pardini A, Silva E. Feasibility of transitioning from APACHE II to SAPS III as prognostic model in a Brazilian general intensive care unit. A retrospective study. Sao Paulo Med J. 2014;133(3):199–205.
14. Williams JM, Greenslade JH, Chu K, Eliézer S. Severity scores in emergency department patients with presumed infection: a prospective validation study. Crit Care Med. 2016;44(3):539–547.
15. Hashmi M, Asghar A, Shamim F, Khan FH. Validation of acute physiologic and chronic health evaluation II scoring system software developed at the Aga Khan University, Pakistan. Saudi J Anaesth. 2016;10(1):45–49.
16. Thanapaisal C, Saksaen P. A comparison of the Acute Physiology and Chronic Health Evaluation (APACHE) II score and the Trauma-Injury Severity Score (TRISS) for outcome assessment in Srinagarind Intensive Care Unit trauma patients. J Med Assoc Thai. 2012;95(Suppl 11):S25–33.
17. Moreno RP, Júnior N, Paulo A. Is APACHE II a useful tool for clinical research. Rev Bras Ter Intensiva. 2017;29(3):264–267.
18. Sun YS, Yu JL. Clinical value of blood lactate in predicting the mortality of neonatal sepsis. Chin J Contemp Ped. 2019;21(7):629–634.
19. Verhaeghe M, Saïd H-I. Blood lactate and lactate kinetics as treatment and mortality markers for tissue hypoperfusion. Acta Clin Belg. 2020;75(1):1–8.
20. Levy MM, Evans LE, Rhodes A. The surviving sepsis campaign bundle: 2018 update. Intensive Care Med. 2018;44(6):925–928.
21. Vincent JL, Quintairos E, Silva A, Couto L
22. Jones Alan E, Shapiro Nathan I, Stephen T, et al. Lactate clearance vs central venous oxygen saturation as goals of early sepsis therapy: a randomized clinical trial. JAMA. 2010;303(8):739–746.
23. Ryoo SM, Lee J, Lee YS, et al. Lactate level versus lactate clearance for predicting mortality in patients with septic shock defined by Sepsis-3. Crit Care Med. 2018;46(6):e489–e495.
24. Amladi AU, Abirami B, Devi SM, et al. Susceptibility profile, resistance mechanisms & efficacy ratios of fosfomycin, nitrofurantoin & colistin for carbapenem-resistant Enterobacteriaceae causing urinary tract infections. Indian J Med Res. 2019;149(2):185–191.
25. Abe R, Hagiya H, Akeda Y, Sudarsanam TD, Kandasamy S, Kekre N. Bactericidal efficacy of meropenem in combination with cefmetazole against IMP-producing carbapenem-resistant Enterobacteriaceae. BMC Res Notes. 2019;12(1):740.
26. Abbas HA, Kadry AA, Shaker GH, Goda RM. Impact of specific inhibitors on metallo-β-carbapenemases detected in Escherichia coli and Klebsiella pneumoniae isolates. Microb Pathog. 2019;132:266–274.
27. Zhang J, Yu L, Fu Y, et al. Tigecycline in combination with other antibiotics against clinical isolates of carbapenem-resistant Klebsiella pneumoniae in vitro. Ann Palliat Med. 2019;8(5):622–631.
28. Zhou Y, Wang J, Guo Y, et al. Discovery of a potential MCR-1 inhibitor that reverses polymyxin activity against clinical mcr-1-positive Enterobacteriaceae. J Infect. 2019;78(5):364–372.
29. Ulloa ER, Dillon N, Tsunemoto H, et al. Avibactam sensitizes carbapenem-resistant NDM-1–producing Klebsiella pneumoniae to innate immune clearance. J Infect Dis. 2019;220(3):484–493.
30. Mikhail S, Singh NB, Mikhail RK, et al. Evaluation of the synergy of ceftazidime-avibactam in combination with meropenem, amikacin, aztreonam, colistin, or fosfomycin against well-characterized multidrug-resistant Klebsiella pneumoniae and Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2019;63(8):00779–00819.
31. Liang Q, Huang M, Xu Z. Early use of polymyxin B reduces the mortality of carbapenem-resistant Klebsiella pneumoniae bloodstream infection. Braz J Infect Dis. 2019;23(1):60–65.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
