Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 18
Clinical Characteristics and Medical Utilization of Smokers with Preserved Ratio Impaired Spirometry
Authors Shin YY, Park S , Kim KJ, Rhee CK , Yoo KH , Jung KS, Lee JH
Received 2 August 2023
Accepted for publication 1 October 2023
Published 6 October 2023 Volume 2023:18 Pages 2187—2194
DOI https://doi.org/10.2147/COPD.S425934
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Russell
Yune-Young Shin,1,* Sojung Park,1,* Kyung Joo Kim,2 Chin Kook Rhee,2 Kwang Ha Yoo,3 Ki-Suck Jung,4 Jin Hwa Lee1
1Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, College of Medicine, Ewha Womans University, Seoul, Republic of Korea; 2Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, College of Medicine, Seoul St. Mary’s Hospital, The Catholic University of Korea, Seoul, Republic of Korea; 3Division of Pulmonary and Allergy Medicine, Department of Internal Medicine, Konkuk University School of Medicine, Seoul, Republic of Korea; 4Division of Pulmonary Medicine, Department of Internal Medicine, Hallym University Sacred Heart Hospital, Hallym University Medical School, Anyang, Gyeonggi-do, Republic of Korea
*These authors contributed equally to this work
Correspondence: Jin Hwa Lee, Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, College of Medicine, Ewha Womans University, 25 Magokdong-ro 2-gil Gangseo-gu, Seoul, 07804, Republic of Korea, Tel +82 2 6986 1631, Fax +82 2-2655-2076, Email [email protected]
Purpose: To investigate the clinical characteristics and medical utilization of smokers with preserved ratio impaired spirometry (PRISm).
Patients and Methods: We used data from the Korean National Health and Nutrition Examination Survey between 2007 and 2012, linked to the Health Insurance Review and Assessment Service. Clinical characteristics and medical utilization, including inpatient admission, emergency department visit, prescribed medication, and medical cost, were retrospectively compared among three groups: normal spirometry, PRISm, and chronic obstructive pulmonary disease (COPD).
Results: A total of 7115 smokers were included (4743 normal spirometry, 689 PRISm, and 1683 COPD subjects). The mean age was the highest in the COPD group, followed by the PRISm and normal groups, and the proportion of women was the highest in the PRISm group. The tobacco exposure, socioeconomic status (SES), and schooling level of the PRISm group were at levels between those of the normal and COPD groups. However, the PRISm group had the highest proportion of current smokers, highest body mass index (BMI), and lowest mean FEV1 and FVC % predicted. During the study period, the medical utilization of 92 smokers (13.4%) in the PRISm group and 436 smokers (25.9%) in the COPD group was related to respiratory diseases. Emergency department visit or hospitalization and overall medical cost of the PRISm group were comparable to those of the COPD group, except for outpatient clinic visit. Old age, women, low BMI, low SES, low schooling level, high amount of tobacco exposure, wheezing, and decreased FEV1 and FVC % predicted were factors associated with medical utilization in PRISm.
Conclusion: Medical utilization was comparable between the PRISm and COPD groups. Smokers with PRISm who were older, women, or heavy smokers with low BMI, low SES and schooling level, wheezing, or low FEV1 and FVC might need close observation and early treatment.
Keywords: emergency department, hospitalization, lung function, restrictive lung disease, wheezing
A Letter to the Editor has been published for this article.
A Response to Letter by Mr Hammad has been published for this article.
Introduction
Preserved ratio impaired spirometry (PRISm) is a spirometric impairment being defined as decreased forced expiratory volume in one second (FEV1) <80% predicted with FEV1/forced vital capacity (FVC) ≥0.7.1–5 The prevalence of PRISm is 9–20%; however, little is known about PRISm because of the exclusion of individuals with this spirometric impairment from previous obstructive lung disease studies.6–8
The natural history and prognosis of PRISm is highly variable.6,7,9 In the US COPDGene cohort, 22.2% of subjects with PRISm were at risk of chronic obstructive pulmonary disease (COPD) with Global Initiative for Obstructive Lung Disease (GOLD) stage 0, and 25.1% of them progressed to GOLD stages 1–4 COPD during the 5-year follow-up period.6 In a UK Biobank cohort, 12.2% of subjects with PRISm developed COPD.9 In addition, individuals with PRISm could exhibit increased respiratory symptoms, poor health-related quality of life, multiple comorbidities, and increased mortality compared with those with normal spirometry.6,8,10–12 However, few studies have investigated the disease burden and incidence of pulmonary complications in subjects with PRISm.
The aim of the present study was to investigate the clinical characteristics, real-world treatment pattern, medical cost, and medical utilization of smokers with PRISm using data obtained from the Korean National Health and Nutrition Examination Survey (KNHANES) linked to the Health Insurance Review and Assessment Service (HIRA). In addition, we compared differences in the clinical characteristics of smokers with PRISm according to medical utilization.
Materials and Methods
Study Population
This observational, retrospective cohort study investigated the data obtained from the KNHANES between 2007 and 2012, linked to the HIRA. The HIRA system includes medical reimbursement records for the entire Korean population. The KNHANES is a national cross-sectional surveillance system containing de-identified data on demographics, comorbidities, smoking history, and spirometry results.13 Sampling was conducted with a stratified multistage cluster sampling design.
We screened ex- or current smokers aged 40 year or older who underwent spirometry. Subjects who had ever smoked more than 100 cigarettes was defined as smokers. Ex-smokers were defined as smokers who quit smoking more than 3 months ago. Smokers who currently smoke or quit smoking within 3 months were defined as current smokers. Socioeconomic status was stratified into quartiles based on income level. The 2nd and 3rd quartiles were considered middle. Spirometry was performed using a rolling dry-seal spirometer (Vmax series 2130; SensorMedics Corp., Yorba Linda, CA, USA), according to the American Thoracic Society performance criteria.14 We divided the smokers into three groups based on spirometry results: normal spirometry, PRISm, and COPD. A FEV1/FVC ratio of <0.7 was used to define COPD.1 PRISm was defined as described above. Normal spirometry was defined as an FEV1/FVC ≥0.7 and an FEV1 ≥80% predicted.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All KNHANES participants signed an informed consent form. Open data are available at the KNHANES website (https://knhanes.cdc.go.kr). All data were anonymously managed in all stages. Ethical approval for this study was waived by the Institutional Review Board of Ewha Womans University Mokdong Hospital because this study used anonymously managed open data.
Study Design and Data Collection
We collected data on demographics, comorbidities, smoking history, and spirometry results from the KNHANES database. Based on the HIRA data between 2007 and 2012, the smoker’s hospitalization, emergency department (ED) visit, intensive care unit (ICU) admission, and prescription records were analyzed. We investigated differences in demographic characteristics, clinical characteristics, and prescribed medications related to obstructive lung disease among the three groups. Disease burden and medical utilization were evaluated using claims data on outpatient clinic visit, ED visit, hospitalization, ICU admission, and medical cost. In addition, we compared the demographic and clinical characteristics of the PRISm group divided into smokers with and without medical utilization. Smokers who visited an outpatient clinic with claim codes for the International Classification of Disease 10th edition (ICD-10) codes J43-44 except for J43.0 were included. We also included smokers with acute respiratory events who required an ED visit and hospitalization with the ICD-10 codes J43-J44 except for J43.0, J12-17, I26, I26.0, I26.9, R06.0, and J80. As we used the data available before the release of the dual long-acting muscarinic antagonist/ long-acting beta-2 agonist in South Korea, there were no data regarding on dual therapy.
Statistical Analysis
All statistical analyses were performed using SAS ver. 9.4 (SAS Institute, Cary, NC, USA). Data are expressed as means ± standard deviations or numbers (%). Continuous variables were analyzed by Student’s t-test or one-way analysis of variance. Categorical variables were analyzed by Pearson’s chi-square test. All tests for significance were two-sided, and all variables with p <0.05 were considered significant. Bonferroni correction (alpha = 0.05/3= 0.0167) was performed for multiple comparisons between the groups in post-hoc test.15
Results
Baseline Characteristics
A total of 7115 smokers were included in the present study, consisting of 4743 smokers (66.7%) in the normal group, 689 smokers (9.7%) in the PRISm group, and 1683 smokers (23.7%) in the COPD group. The mean age was 57.0 years, and 6344 smokers (89.2%) were men (Table 1). The PRISm group had the highest proportion of women and medical aid and showed the highest body mass index (BMI) and waist circumference. The number of smoking pack-years (PY) of the PRISm group (26.0 ± 20.2 PY) was significantly higher than that of the normal group (21.2 ± 18.0 PY) but lower than that of the COPD group (30.7 ± 22.9 PY, p <0.001). The proportion of smokers who reported intermittent wheezing was the second highest in the PRISm group; however, spirometry results revealed that the PRISm group had significantly reduced FEV1 and FVC % predicted.
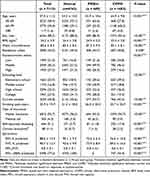 |
Table 1 Baseline Characteristics of Smokers with Normal Spirometry, Preserved Ratio Impaired Spirometry, and Obstructive Spirometry |
Treatment and Medical Utilization
During the study period, 92 smokers (13.4%) in the PRISm group and 436 smokers (25.9%) in the COPD group visited hospital for the treatment of respiratory diseases. The various types of respiratory medicines taken by the PRISm group were at a level between that of the normal and COPD groups (Table 2). The outpatient clinic visit rate and cost of the PRISm group were significantly lower than those of the COPD group (p <0.001) but were increased tendency compared with those of the normal group (Table 3). On the other hand, the rates of hospitalization, ICU admission, and ED visit were not significantly different between the PRISm and COPD groups; however, these rates were significantly higher in the PRISm group than in the normal group.
 |
Table 2 Treatment Regimens of Smokers with Normal Spirometry, Preserved Ratio Impaired Spirometry, and Obstructive Spirometry |
 |
Table 3 Medical Utilization of Smokers with Normal Spirometry, Preserved Ratio Impaired Spirometry, and Obstructive Spirometry |
Medical Utilization of the PRISm Group
Of 689 smokers in the PRISm group, 92 smokers (13.4%) visited the hospital. The mean FEV1% predicted (70.7 ± 9.1 vs 74.0 ± 5.6) and FVC % predicted (69.1 ± 10.7 vs 74.6 ± 8.4) were significantly lower in the PRISm group with medical utilization than in the PRISm group without medical utilization (Table 4). Hospital visit was associated with old age, women, low BMI, low socioeconomic status, low schooling level, higher amount of tobacco exposure, intermittent wheezing, osteoarthritis, and decreased FEV1 and FVC % predicted. Interestingly, a higher portion of smokers were hospitalized in the PRISm group (54/92, 58.7%) than in the COPD group (201/436, 46.1%) among PRISm and COPD smokers with medical utilization (p = 0.028).
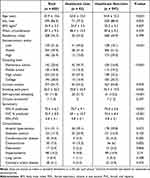 |
Table 4 Comparison of Clinical Characteristics of Smokers with Preserved Ratio Impaired Spirometry According to Medical Utilization |
Discussion
To the best of our knowledge, this is the first study to investigate the medical utilization of smokers with PRISm and their clinical characteristics. The present study showed that the frequency of clinically significant exacerbation requiring ED visit or hospitalization was comparable between the PRISm and COPD groups.
PRISm remains poorly understood.1 PRISm is commonly referred to as restrictive lung disease; however, 11.8% of smokers in the PRISm group reported wheezing, which suggests narrowed airways. In comparison with other ethnicities, East Asians seem to have a lower incidence of PRISm.16,17 In the present study, the PRISm group had the highest BMI, whereas the COPD group had the lowest BMI. Given the relationship between a low obesity rate and a low incidence of PRISm among East Asians as well as the high BMI in the PRISm group, obesity may play an important role in the pathogenesis of PRISm. Leptin, secreted in direct proportion to the adipose tissue mass, exerts proinflammatory effects.18,19 Although the functional effects of leptin on the respiratory system are less clear, elevated leptin levels may modulate the immune reaction in the airways by inciting a robust proinflammatory response or skewing the cellular response towards a type 1 helper phenotype.20,21 Indeed, Wan et al reported that a reduction in BMI was associated with transitioning from PRISm to normal spirometry.6
Previous studies reported that 15–40% of subjects with PRISm progressed to COPD, and 33–50% of subjects had persistent PRISm.2,3,6,17 We could not determine the number of smokers with PRISm who developed COPD in a few years; however, we found that PRISm also requires careful observation because of frequent exacerbation and concomitant medical utilization, comparable to those in COPD. The present study showed that the amount of tobacco exposure was associated with medical utilization. Smoking is a major factor in COPD exacerbation. Cumulative cigarette smoke exposure causes airflow limitation, accelerating the rate of decline in FEV1.22 Parekh et al reported that current smoking and frequent exacerbation were factors contributing to decreased quality of life, which may be associated with a high burden of respiratory symptoms in PRISm.23 Subjects with PRISm were relatively young and current smokers compared with those with COPD. Therefore, further evaluation is needed to determine whether smoking cessation can reduce the incidence of acute exacerbation or achieve normal lung function.
In this study, low FEV1 and FVC were associated with medical utilization in PRISm. No study has evaluated the relationship between lung function and the severity of symptoms or prognosis of PRISm. However, Guerra et al reported that individuals with PRISm with low FVC had persistent PRISm over time.2 A study of Peruvian adults showed that PRISm was associated with accelerated decline in FEV1; however, those with PRISm could be classified into high-, and low-risk groups that progressed to COPD or even achieved normal spirometry.6,24 Although Kim et al reported that lung function was not a predictive factor of COPD, decreased FEV1 and FVC might imply severe airflow limitation and respiratory symptoms, which would require close observation and early management.8
The present study has several limitations. First, spirometry was conducted through the pre-bronchodilator spirometry test even though the reliability of portable spirometry has been proven.25 The lack of post-bronchodilator spirometry may have resulted in an overestimate of the prevalence of both PRISm and COPD. However, although bronchodilator administration has been shown to reduce the prevalence of obstructive lung disease, its effect on the prevalence of PRISm is less well-established. Comorbidities such as heart failure, combined interstitial lung disease, and intermediate tuberculosis prevalence might contribute to the prevalence of PRISm and associated adverse respiratory outcomes; however, we could not examine these comorbidities comprehensively. Second, the European Respiratory Society/ American Thoracic Society technical standard on interpretative strategies for lung function tests recommend to use of z-score of FEV1/FVC to determine impaired lung function.26 We did not use the z-score, however, a previous study showed that both the fixed ratio and the z-score of FEV1/FVC showed comparable prediction performance for the 10-year respiratory and COPD mortalities in elderly patients.27 Third, we excluded never smokers; thus, the effects of genetic factors as well as exposure to second-hand smoke and other non-tobacco environmental exposures were not evaluated. Considering that PRISm subjects are known to be young and that a study reported an association between history of childhood asthma and PRISm, lung growth might be an important pathogenic factor underlying one of various phenotypes influencing symptoms, severity, and longitudinal trajectories.28 Fourth, other risk factors of exacerbation, such as the exacerbation history before the study period, comorbidities, infection, and compliance with medications, were not fully considered. Fifth, analyses were limited to the examination of the relationship between cross-sectional PRISm and clinical outcomes; thus, we could not determine whether distinct longitudinal trajectories within PRISm may be differentially associated with clinical outcomes.
Conclusion
The clinical characteristics and medical utilization of the PRISm group were comparable to those of the COPD group. Smokers with PRISm who were older, women, or heavy smokers with low BMI, low socioeconomic status and schooling level, wheezing, or low FEV1 and FVC might need close observation and early treatment. Further studies on appropriate management and medical treatment for individuals with PRISm are needed to improve their respiratory symptoms and prognosis.
Abbreviations
PRISm, preserved ratio impaired spirometry; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; COPD, chronic obstructive pulmonary disease; GOLD, Global Initiative for Obstructive Lung Disease; KNHANES, Korean National Health and Nutrition Examination Survey; HIRA, Health Insurance Review and Assessment Service; ED, emergency department; ICU, intensive care unit; ICD-10, International Classification of Disease 10th edition; BMI, body mass index; PY, pack-years.
Data Sharing Statement
KNHANES data is open data which is available at the KNHANES website (https://knhanes.cdc.go.kr).
Ethics Approval and Informed Consent
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All KNHANES participants signed an informed consent. In addition, it is open data which is available at the KNHANES website (https://knhanes.cdc.go.kr). All data were anonymously managed in all stages. Ethical approval for this study was waived by the Institutional Review Board of Ewha Womans University Mokdong Hospital because this study used anonymously managed open data.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Research Program funded Korea National Institute of Health (Fund CODE 2016ER670100, 2016ER670101, 2016ER670102, 2018ER67100, 2018ER67101, 2018ER67102, 2021ER120500 and 2021ER120501), grants from the Korean Environment Industry and Technology Institute through the Core Technology Development Project for Environmental Disease Prevention and Management, funded by the Korea Ministry of Environment (Grant number 2022003310008), and the National Research Foundation of Korea (NRF-2020R1A5A2019210).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Agustí A, Celli BR, Criner GJ, et al. Global initiative for chronic obstructive lung disease 2023 report: GOLD executive summary. Eur Respir J. 2023;61(4):2300329. doi:10.1183/13993003.00239-2023
2. Guerra S, Sherrill DL, Venker C, Ceccato CM, Halonen M, Martinez FD. Morbidity and mortality associated with the restrictive spirometric pattern: a longitudinal study. Thorax. 2010;65(6):499–504. doi:10.1136/thx.2009.126052
3. Iyer VN, Schroeder DR, Parker KO, Hyatt RE, Scanlon PD. The nonspecific pulmonary function test: longitudinal follow-up and outcomes. Chest. 2011;139(4):878–886. doi:10.1378/chest.10-0804
4. Wan ES, Castaldi PJ, Cho MH, et al. for COPDGene Investigators. Epidemiology, genetics, and subtyping of preserved ratio impaired spirometry (PRISm) in COPDGene. Respir Res. 2014;15(1):89. doi:10.1186/s12931-014-0089-y
5. Backman H, Eriksson B, Hedman L, et al. Restrictive spirometric pattern in the general adult population: methods of defining the condition and consequences on prevalence. Respir Med. 2016;120:116–123. doi:10.1016/j.rmed.2016.10.005
6. Wan ES, Fortis S, Regan EA, et al. for COPDGene Investigators. Longitudinal phenotypes and mortality in preserved ratio impaired spirometry in the COPDGene study. Am J Respir Crit Care Med. 2018;198(11):1397–1405. doi:10.1164/rccm.201804-0663OC
7. Marott JL, Ingebrigtsen TS, Çolak Y, Vestbo J, Lange P. Trajectory of preserved ratio impaired spirometry: natural history and long-term prognosis. Am J Respir Crit Care Med. 2021;204(8):910–920. doi:10.1164/rccm.202102-0517OC
8. Kim J, Lee C-H, Lee HY, Kim H. Association between comorbidities and preserved ratio impaired spirometry: using the Korean national health and nutrition examination survey IV–VI. Respiration. 2022;101(1):25–33. doi:10.1159/000517599
9. Higbee DH, Granell R, Smith GD, Dodd JW. Prevalence, risk factors, and clinical implications of preserved ratio impaired spirometry: a UK Biobank cohort analysis. Lancet Respir Med. 2022;10(2):149–157. doi:10.1016/S2213-2600(21)00369-6
10. Vaz Fragoso CA, McAvay G, Van Ness PH, et al. Phenotype of spirometric impairment in an aging population. Am J Respir Crit Care Med. 2016;193(7):727–735. doi:10.1164/rccm.201508-1603OC
11. Guerra S, Carsin A-E, Keidel D, et al. Health-related quality of life and risk factors associated with spirometric restriction. Eur Respir J. 2017;49(5):1602096. doi:10.1183/13993003.02096-2016
12. Heo IR, Kim HC, Kim T-H. Health-related quality of life and related factors in persons with preserved ratio impaired spirometry: data from the Korea National Health and nutrition examination Surve. Medicina. 2020;57(1):4. doi:10.3390/medicina57010004
13. Kweon S, Kim Y, Jang M-J, et al. Data resource profile: the Korea national health and nutrition examination survey (KNHANES). Int J Epidemiol. 2014;43(1):69–77. doi:10.1093/ije/dyt228
14. Celli BR, MacNee W, Agusti A; for ATS/ERS Task Force. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23(6):932–946. doi:10.1183/09031936.04.00014304
15. Haynes W; Bonferroni Correction. Encyclopedia of Systems Biology. New York: Springer; 2013:154.
16. Wan ES, Balte P, Schwartz JE, et al. Association between preserved ratio impaired spirometry and clinical outcomes in US adults. JAMA. 2021;326(22):2287–2298. doi:10.1001/jama.2021.20939
17. Washio Y, Sakata S, Fukuyama S, et al. Risks of mortality and airflow limitation in Japanese with preserved ratio impaired spirometry. Am J Respir Crit Care Med. 2022;206(5):563–572. doi:10.1164/rccm.202110-2302OC
18. Creutzberg EC, Wouters EF, Vanderhoven-Augustin IM, Dentener MA, Schols AM. Disturbances in leptin metabolism are related to energy imbalance during acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;162(4 Pt 1):1239–1245. doi:10.1164/ajrccm.162.4.9912016
19. Claycombe K, King LE, Fraker PJ. A role for leptin in sustaining lymphopoiesis and myelopoiesis. Proc Natl Acad Sci USA. 2008;105(6):2017–2021. doi:10.1073/pnas.0712053105
20. Mancuso P, Canetti C, Gottschalk A, Tithof PK, Peters-Golden M. Leptin augments alveolar macrophage leukotriene synthesis by increasing phospholipase activity and enhancing group IVC iPLA2 (cPLA2γ) protein expression. Am J Physiol Lung Cell Mol Physiol. 2004;287(3):L497–L502. doi:10.1152/ajplung.00010.2004
21. Dixon AE, Johnson SE, Griffes LV, et al. Relationship of adipokines with immune response and lung function in obese asthmatic and non-asthmatic women. J Asthma. 2011;48(8):811–817. doi:10.3109/02770903.2011.613507
22. Simmons MS, Connett JE, Nides MA, et al. Smoking reduction and the rate of decline in FEV1: results from the Lung Health Study. Eur Respir J. 2005;25(6):1011–1017. doi:10.1183/09031936.05.00086804
23. Parekh TM, Bhatia S, Cherrington A, et al. Factors influencing decline in quality of life in smokers without airflow obstruction: the COPDGene study. Respir Med. 2020;161:105820. doi:10.1016/j.rmed.2019.105820
24. Siddharthan T, Grigsby M, Miele CH, et al. Prevalence and risk factors of restrictive spirometry in a cohort of Peruvian adults. Int J Tuberc Lung Dis. 2017;21(9):1062–1068. doi:10.5588/ijtld.17.0101
25. Park HJ, Rhee CK, Yoo KH, Park YB. Reliability of portable spirometry performed in the Korea national health and nutrition examination survey compared to conventional spirometry. Tuberc Respir Dis. 2021;84(4):274–281. doi:10.4046/trd.2021.0016
26. Stanojevic S, Kaminsky DA, Miller MR, et al. ERS/ATS technical standard on interpretive strategies for routine lung function tests. Eur Respir J. 2022;60(1):2101499. doi:10.1183/13993003.01499-2021
27. Oh DK, Baek S, Lee SW, Lee JS, Lee S-D, Oh Y-M. Comparison of the fixed ratio and the Z-score of FEV1/FVC in the elderly population: a long-term mortality analysis from the third national health and nutrition examination survey. Int J Chron Obstruct Pulmon Dis. 2018;13:903–915. doi:10.2147/COPD.S148421
28. Kaise T, Sakihara E, Tamaki K, et al. Prevalence and characteristics of individuals with preserved ratio impaired spirometry (PRISm) and/or impaired lung function in Japan: the OCEAN study. Int J Chron Obstruct Pulmon Dis. 2021;16:2665–2675. doi:10.2147/COPD.S322041
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
