Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 18
Clinical and Radiological Features Between Patients with Stable COPD from Plateau and Flatlands: A Comparative Study
Authors Jiang Z , Wang X, Zhang L , Yangzom D, Ning Y, Su B, Li M, ChuTso M, Chen Y, Liang Y, Sun Y
Received 18 November 2022
Accepted for publication 30 April 2023
Published 12 May 2023 Volume 2023:18 Pages 849—858
DOI https://doi.org/10.2147/COPD.S397996
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Russell
Zhihan Jiang,1,* Xiaosen Wang,1,* Lijiao Zhang,1,* Drolma Yangzom,2,* Yanping Ning,2 Baiyan Su,3,4 Meijiao Li,5 Meilang ChuTso,2 Yahong Chen,1,6 Ying Liang,1,2,6 Yongchang Sun1,6
1Department of Respiratory and Critical Care Medicine, Peking University Third Hospital, Beijing, 100191, People’s Republic of China; 2Department of Respiratory and Critical Care Medicine, Tibet Autonomous Region People’s Hospital, Lhasa, 850000, People’s Republic of China; 3Radiology Department, Peking Union Medical College Hospital, Beijing, 100730, People’s Republic of China; 4Radiology Department, Tibet Autonomous Region People’s Hospital, Lhasa, 850000, People’s Republic of China; 5Radiology Department, Peking University Third Hospital, Beijing, 100191, People’s Republic of China; 6Research Center for Chronic Airway Diseases, Peking University Health Science Center, Beijing, 100083, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Ying Liang; Yongchang Sun, Department of Respiratory and Critical Care Medicine, Peking University Third Hospital, North Garden Road 49, Haidian District, Beijing, People’s Republic of China, Tel +86 138 1096 4766 ; +86 139 1097 9132, Email [email protected]; [email protected]
Background: COPD patients living in Tibet are exposed to specific environments and different risk factors and probably have different characteristics of COPD from those living in flatlands. We aimed to describe the distinction between stable COPD patients permanently residing at the Tibet plateau and those in flatlands.
Methods: We conducted an observational cross-sectional study that enrolled stable COPD patients from Tibet Autonomous Region People’s Hospital (Plateau Group) and Peking University Third Hospital (Flatland Group), respectively. Their demographic information, clinical features, spirometry test, blood routine and high-resolution chest CT were collected and evaluated.
Results: A total of 182 stable COPD patients (82 from plateau and 100 from flatland) were consecutively enrolled. Compared to those in flatlands, patients in plateau had a higher proportion of females, more biomass fuel use and less tobacco exposure. CAT score and frequency of exacerbation in the past year were higher in plateau patients. The blood eosinophil count was lower in plateau patients, with fewer patients having an eosinophil count ≥ 300/μL. On CT examination, the proportions of previous pulmonary tuberculosis and bronchiectasis were higher in plateau patients, but emphysema was less common and milder. The ratio of diameters of pulmonary artery to aorta ≥ 1 was more often in plateau patients.
Conclusion: Patients with COPD living at Tibet Plateau had a heavier respiratory burden, lower blood eosinophil count, less emphysema but more bronchiectasis and pulmonary hypertension. Biomass exposure and previous tuberculosis were more common in these patients.
Keywords: chronic obstructive pulmonary disease, plateau, phenotype, computed tomography, emphysema
Introduction
Chronic obstructive pulmonary disease (COPD) is a common, preventable and treatable disease that is characterized by persistent respiratory symptoms and airflow limitation.1 As a chronic airway disease which accounts for more than 3 million deaths annually, the number of deaths due to COPD in 2017 increased 23% compared to 1990.2 The overall prevalence of COPD in China is 8.6% and the prevalence is 13.7% among the adults aged 40 or older, estimating that there are approximately 99.9 million adults with COPD in mainland China.3
COPD is a heterogeneous disease. Phenotypes of COPD, which refer to “a single or combination of disease attributes that describe differences between individuals with COPD as they relate to clinically meaningful outcomes (symptoms, exacerbations, response to therapy, rate of disease progression, or death)”, are proposed to describe such heterogeneity,4,5 including clinical and physiological manifestations, radiological characteristics, and airway and systematic inflammation. Phenotyping the patients with COPD is of clinical significance for treatment optimization.6
People living at high altitude are exposed to a specific environment which is characterized by low barometric pressure, increased ultra violet radiation as well as biomass fuel exposure, which are harmful to the health status of plateau residents.7 Previous studies have demonstrated the risk factors, prevalence and higher mortality of COPD patients living at high altitude and highlighted the importance of biomass fuel exposure and household air pollution in facilitating the development and progression of COPD.8–11 Our study carried out in Tibet Plateau also found that biomass exposure and previous pulmonary tuberculosis were very common in patients with COPD and that they had a heavy symptom burden, although the emphysema phenotype was less common.12 From these findings, we hypothesized that the clinical characteristics of COPD in Tibet Plateau might differ from those in flatlands. To address this question, we performed a cross-sectional study on two well-defined cohorts of COPD patients living at high altitude and in flatlands respectively, which revealed remarkable differences in disease characteristics between these two populations.
Methods
Study Subjects
During January 2018 to March 2021, we conducted an observational, cross-sectional comparative study in the outpatients of Respiratory Medicine in Tibet Autonomous Region People’s Hospital and Peking University Third Hospital, respectively. All the patients met the diagnosis of COPD according to Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines and had definite airflow limitation with a post-bronchodilator forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) <0.7. The patients enrolled from Tibet Autonomous Region People’s Hospital were permanent residents at Tibet Plateau. They resided at an altitude of exceeding 3000 meters above the sea and were designated as the plateau group. Their data were extracted from our database of COPD patients at plateau and the processes were described previously,12 with some data overlapping in the current study. The patients recruited from Peking University Third Hospital permanently lived in flatlands and were designated as the flatland group.
Exclusion criteria included: 1) age <40 years; 2) subjects with airway diseases other than COPD; 3) acute exacerbation of COPD in the past 3 months; 4) active tuberculosis; 5) cardiovascular or cerebrovascular events in the past 3 months; 6) cognitive dysfunction such as vascular dementia or Alzheimer’s disease; 7) refusal to participate in this study; 8) incomplete or invalid information.
The study protocol was approved by the Ethics Committee of Tibet Autonomous Region People’s Hospital (ME-TBHP-20-KJ-036) and the Independent Ethics Committee of the Peking University Third Hospital (IRB00006761-M2020430). All interviewers underwent a centralized training session prior to the formal investigation. Written informed consents were obtained from the patients or their close relatives. This study was conducted in accordance with the Declaration of Helsinki. Each subject was recruited once and data were analyzed anonymously.
The study protocol has been registered on the ClinicalTrials.gov website (NCT04964076).
Data Collection
Medical history and other information including demographic data, education level, tobacco exposure and biomass fuel use were collected. The COPD Assessment Test (CAT) score and the modified Medical Research Council (mMRC) dyspnea scale were performed to assess the respiratory symptoms.13,14 Frequency of exacerbation in the past year was recorded. Spirometry test, blood routine test and high-resolution chest computed tomography (CT) were completed around 3 months of the visit and the data were evaluated for validity.
Spirometry Test
Standardized spirometry tests were performed according to current ATS/ERS recommendations.15 Spirometry tests performed in Tibet Autonomous Region People’s Hospital used a MasterScreen PFT Analyzer Unit (Jaeger, Hoechberg, Germany), while those performed in Peking University Third Hospital used a MasterScreen SeS lung function testing device (Vyaire Medical GmbH, Hoechberg, Germany). Post-bronchodilator (400 μg of salbutamol) FEV1%predicted, FVC %predicted, and ratio of FEV1/FVC were documented.
Blood Routine Test
Blood routine test was completed in the clinical laboratory of the respective hospital. Counts of white blood cells, red blood cells and platelets, hemoglobin, count and percentage of eosinophils were collected.
High-Resolution Chest CT Examination
In Tibet Autonomous Region People’s Hospital, high-resolution chest CT (HRCT) examination was administrated by using SOMATOM Drive (Siemens, Erlangen, Germany) and the lungs were imaged in horizontal position with breath-holding at full inspiration. CT images were reconstructed at section thickness of 1–1.25 mm and the lung parenchyma was extracted by using the software package SYNAPSE 3D (FUJIFILM, Japan). In Peking University Third Hospital, HRCT was performed using GE Discovery 750 HD Device (General Electric, Boston, USA), with the lung parenchyma extraction by the software package Airway Analysis (General Electric, USA).
Low attenuation area (LAA) defined by a CT attenuation value of less than −950 Hu on inspiratory scans was considered to be emphysema.16 LAA% calculated by the ratio of LAA and total pulmonary volumes was used for assessment of severity of emphysema. LAA% ≥6% was classified as emphysema.17
Bronchiectasis was considered present if HRCT showed abnormal dilation of bronchi with the ratio of the diameter of bronchus to that of the accompanying pulmonary artery being >1.1 (signet ring sign) or the lack of tapering of bronchi (tramline sign).18 We separately estimated the extent and severity of bronchiectasis in each of the five lung lobes as well as the lingula and calculated the summed score from six parts. The extent of bronchiectasis in each lobe was graded according to the grading system proposed by Smith et al (Smith score).19 The score was 0 if there was no evidence of bronchiectasis, and bronchiectasis in <25%, in 25% to 50%, in 50% to 75% and in ≥75% were scored as 1, 2, 3 and 4. According to the Bhalla scoring system (Bhalla score),20 the severity of bronchiectasis was scored as 0 for no involvement; scored as 1 (mild) if the luminal diameter of dilated bronchi was slightly greater than but did not exceed twice the diameter of the adjacent vessel; scored as 2 (moderate) if the luminal diameter was two or three times the diameter of the adjacent vessel; and scored as 3 (severe) if the luminal diameter exceeded three times the diameter of the adjacent vessel.
The diameters of the main pulmonary artery (PA) and the ascending aorta (A) were measured at the level of the bifurcation of the main pulmonary artery. The ratio of PA/A was calculated and PA/A ratio ≥1 indicated the presence of pulmonary hypertension.21
Any presence of previous pulmonary tuberculosis on chest CT scans was recorded, including discrete linear or reticular fibrotic scars, or dense nodules locating in the upper lobes and/or superior segment of lower lobes, with or without calcification in the lesions or in the local lymph nodes.22
Two radiological specialists unaware of the clinical data of the patients performed the imaging analysis separately.
Statistical Analysis
Data analyses were performed with the use of SPSS software, version 26.0. Continuous variables were recorded as mean ± standard deviation and unpaired t-test was administrated for difference evaluation. Categorical variables were recorded as numbers (%) and Chi-square or Fisher exact test was used for difference comparation. P-value <0.05 was considered statistically significant.
Results
Clinical Characteristics Between Patients from Plateau and Flatland
Two hundred and forty-two subjects with a diagnosis of COPD or probable COPD visited the outpatients during the study period, of whom 35 refused to participate in the study, and 23 were excluded as their post-bronchodilator FEV1/FVC was ≥70% and another 2 patients were excluded because they did not have available radiological data. A total of 182 patients with stable COPD were enrolled in the final analysis, of whom 82 were from Tibet Plateau (Plateau Group) and 100 from Beijing (Flatland Group, Figure 1).
 |
Figure 1 Flow chart of the study. |
Demographic characteristics and risk factors relevant to COPD are presented in Table 1. Age and body mass index showed no statistical difference between the two groups. Patients from plateau had a higher percentage of females (41.5%) and almost all were Tibetans. They had a relatively lower education level, with more than 75% having primary school education or lower. Although the proportion of current and former smokers and the smoking index were lower, the percentage of exposure to biomass fuel was dramatically higher in the Plateau Group. In terms of the comorbidities of COPD, approximately 58.5% of the patients in the Plateau Group had at least one comorbidity, which was higher than those in the Flatland Group. Hypertension and heart failure were more common in the former, while ischemic heart disease was more frequent in the latter group.
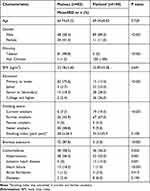 |
Table 1 Demographic Characteristics, Relevant Risk Factors and Comorbidities Between COPD Patients from Plateau (n = 82) and Flatland (n = 100) |
Assessments of the respiratory symptoms and risk of exacerbation of the patients are presented in Table 2. The CAT score was significantly higher in patients from plateau, who were more likely to have a CAT score ≥10 and ≥20. More patients from plateau had mMRC dyspnea score ≥2. Spirometry test showed no difference regarding post-bronchodilator FEV1/FVC ratio and FEV1%predicted. Distribution of GOLD stage was not different between two groups. Patients in plateau had a higher frequency of exacerbation in the past year, and the percentage of those experiencing ≥2 exacerbations was higher accordingly.
 |
Table 2 Clinical Characteristics Between COPD Patients from Plateau (n = 82) and Flatland (n = 100) |
Peripheral Blood Cell Characteristics Between Patients from Plateau and Flatland
Peripheral blood cell count characteristics are presented in Table 3. Red blood cell count, hemoglobin, hematocrit and platelet count were higher in patients from plateau, while white blood cell, neutrophil and lymphocyte showed no difference. The mean eosinophil count in patients from plateau was 163.79/μL, which was lower than that in patients from flatland (258.85/μL, P=0.046). Fewer patients living in plateau had a count of eosinophil ≥300/μL, although the P value was 0.052.
 |
Table 3 Blood Routine Characteristics Between COPD Patients from Plateau (n = 82) and Flatland (n = 100) |
HRCT Characteristics Between Patients from Plateau and Flatland
The imaging characteristics evaluated by HRCT are presented in Table 4. 29.3% patients in plateau had emphysema, in contrast to the 71.0% of patients in flatland showing emphysema. In COPD patients with emphysema, LAA% was significantly lower in patients living at plateau. Bronchiectasis was more common in patients from plateau although the P value was 0.051. However, Smith score was lower in patients from plateau. The Bhalla score showed no significant difference. The diameter of main pulmonary artery in patients from plateau was larger than that of patients in flatland, and PA/A ratio in patients from plateau was larger. 40.2% patients in plateau had a PA/A ratio ≥1, which was much higher than those in flatland. Besides, signs of previous pulmonary tuberculosis were more common in patients living at high altitude.
 |
Table 4 HRCT Characteristics Between COPD Patients from Plateau (n = 82) and Flatland (n = 100) |
Discussion
The present study highlighted the differences of disease characteristics between the patients with stable COPD residing permanently in super high altitude (Tibet Plateau, ≥3000m above the sea level) and those living in flatlands. The risk factors for COPD, the symptom burden and exacerbation risk, the characteristics of peripheral blood cells and the chest CT features in COPD patients living at Tibet Plateau were distinct from their counterparts living in flatlands. Our data provide more evidence for further understanding the heterogeneity of COPD in different populations.
Biomass fuel exposure is a major risk factor for the development and progress of COPD in residents in plateau.10,23 In our study, over 80% of the COPD patients living at Tibet Plateau used biomass fuels for cooking and heating for a long-term, which differed from the patients living in the flatland. Compared to cigarette smoking, exposure to biomass fuel was a more common risk factor for COPD in residents living at the Tibet Plateau. Moreover, the higher proportion of female patients in Tibet Plateau was consistent with previous studies, in which the male–female ratio was nearly equal among non-smokers with COPD.24 Inhaled particles from biomass fuels are less deposited in the lungs, while particles from cigarette smoke tend to deposit in the distal airways, which may partly explain the different characteristics between COPD related to biomass fuel or cigarette smoke.25 In addition, we found that more patients in plateau had signs of previous pulmonary tuberculosis on chest CT, which may be another factor accounting for the discrepancy. Previous pulmonary tuberculosis can cause permanent damage to the lung and has been recognized as an important risk factor for the higher prevalence of COPD in low and middle-income countries.26,27 Biomass exposure also increases susceptibility to pulmonary tuberculosis, by facilitating the transmission of Mycobacterium tuberculosis, even though high altitude environment with excessive ultraviolet light inhibits this pathogen.28,29 Switching to clean energy and controlling the spread of pulmonary tuberculosis are of clinical significance in reducing the prevalence of COPD among the population residing at the Tibet Plateau.
Although spirometry measurements and the GOLD stage did not show marked difference between the two groups, patients in plateau had higher CAT score, heavier dyspnea and more frequent exacerbations. Our data were consistent with a previous study in which 76.8% participants living at high altitude who were not diagnosed as having COPD yet had a CAT score of 10 or higher.8 Patients with biomass-related COPD had more severe respiratory symptoms and lower partial pressure of blood oxygen.30 In terms of the comorbidities of COPD, 58.5% patients in plateau had at least one comorbidity and this proportion was higher than those in flatland. The most common comorbidities were hypertension and other concomitant cardiovascular diseases, such as heart failure and ischemic heart disease. Interestingly, although hypertension and heart failure were more common in patients in plateau, ischemic heart disease was more frequent in those in flatland. The reason for this discrepancy was unclear.
We compared the HRCT findings between the two groups and found distinct radiological characteristics in patients living in plateau. First, there was a much lower proportion of emphysema and lower severity of emphysema in plateau patients. Previous studies also demonstrated that emphysema was more prevalent in tobacco-induced COPD than in biomass-induced COPD,24,30,31 probable due to the fact that more cigarette smoke particles deposit in distal airways and tend to induce inflammation and structure damage in the lung parenchyma.25 Second, the proportion of bronchiectasis was higher in patients living in plateau, but the extent of bronchi involved was less because of the lower Smith score, which was hard to explain. We speculated that airway lesions induced by pulmonary tuberculosis was mostly regional, commonly located in the upper lobes or superior segments of the lower lobes, while more extensive airways were involved in cigarette smoking-related COPD.
PA/A ratio correlates strongly with mean pulmonary artery pressure,32 with PA/A ratio ≥1 indicating high probability of pulmonary hypertension (PH) and the specificity being up to 92%.33 In our study, patients living in the plateau had larger diameter of main pulmonary artery and the proportion of PA/A ratio ≥1 was significantly higher, suggesting that PH was more likely to emerge in COPD patients living at the Tibet Plateau. Aguirre-Franco et al found that 56.3% COPD patients living at high altitude (2640m) had PH and this proportion increased with GOLD stage.34 The proportion in our study was 40.2%, which was slightly lower. The spirometry parameters were similar between the two groups in our study, while emphysema which can cause diffusing capacity impairment was less frequent in plateau patients. The causes for PH in these patients may be related to the hypoxic environment. Hypoxic stimulus can cause constriction of pulmonary arteries, which plays an important role in matching the ratio of ventilation and perfusion but induces the formation of PH in COPD patients,35 especially when the alveolar pressure of oxygen decreases to 60 mmHg or lower. COPD patients accompanied with PA/A ratio ≥1 had higher risk of exacerbations, as well as poor prognosis,21,34 which could partly explain the higher frequency of exacerbations in our patients living in the plateau.
Our study also demonstrated that the eosinophilic phenotype was less frequent in patients in the plateau. The eosinophil count can serve as a treatable trait for inhaled corticosteroids (ICS), as recommended by the GOLD guideline, with the threshold of ≥300/μL as an indication of initial therapy with ICS.1,36,37 In context of the more severe symptom burden and more signs of previous pulmonary tuberculosis in plateau patients, long-acting bronchodilators, including long-acting muscarinic antagonists (LAMAs) and LAMA plus long-acting β2 agonists (LAMA+LABA), may be more appropriate in alleviating respiratory symptoms and averting the risk of tuberculosis in this specific population.38,39
There were some limitations in our study. First, as a cross-section study, we could not compare the clinical outcomes between the cohorts in this study. Second, the patients with COPD in flatland and plateau were enrolled in their own single center, and the representativeness of the patients was limited. Lastly, airway remodeling was not assessed due to the restriction of the software package, though comparison of airway remodeling would be of interest for addressing in more detail the radiological characteristics of COPD patients in plateau.
In conclusion, COPD patients living at the Tibet Plateau had unique disease manifestations, characterized by more respiratory symptoms, lower blood eosinophil counts, less emphysema, more bronchiectasis and potential pulmonary hypertension, as compared to those living in the flatland. These findings highlight the heterogeneity of COPD in different populations, and the underlying mechanisms warrant further investigation.
Abbreviations
COPD, chronic obstructive pulmonary diseases; GOLD, Global Initiative for Chronic Obstructive Lung Disease; CT, computed tomography; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; HRCT, high-resolution chest CT; BMI, body mass index; CAT, COPD Assessment Test; LAA, low attenuation area; PA/A, the ratio of the diameter of pulmonary artery and the diameter of aorta; PH, pulmonary hypertension; LABA, long-acting β2 agonist; ICS, inhaled corticosteroids; LAMA, long-acting muscarinic antagonist.
Data Sharing Statement
The data that supports the findings of this study will not be shared openly with other third parties due to contractual statements related to intellectual property, confidentiality, and proprietary rights.
Ethics Approval and Informed Consent
The study protocol was approved by the Ethics Committee of Tibet Autonomous Region People’s Hospital (ME-TBHP-20-KJ-036) and the Independent Ethics Committee of the Peking University Third Hospital (IRB00006761-M2020430). Written informed consents were obtained from the patients or their close relatives. This study was conducted in accordance with the Declaration of Helsinki. Each subject was recruited once and data were analyzed anonymously.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
This work was supported by Natural Science Foundation of Tibet Autonomous Region [XZ2021ZR-ZY19(Z)] and the National Natural Science Foundation of China [No. 81700039].
Disclosure
The authors report no conflicts of interest in this work.
References
1. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2021 report. Available from: http://www.goldcopd.org/.
2. Li X, Cao X, Guo M, Xie M, Liu X. Trends and risk factors of mortality and disability adjusted life years for chronic respiratory diseases from 1990 to 2017: systematic analysis for the Global Burden of Disease Study 2017. BMJ. 2020;368:m234. doi:10.1136/bmj.m234
3. Wang C, Xu J, Yang L, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study. Lancet. 2018;391(10131):1706–1717. doi:10.1016/S0140-6736(18)30841-9
4. Agusti A, Calverley PM, Celli B, et al. Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respir Res. 2010;11:122. doi:10.1186/1465-9921-11-122
5. Han MK, Agusti A, Calverley PM, et al. Chronic obstructive pulmonary disease phenotypes: the future of COPD. Am J Respir Crit Care Med. 2010;182(5):598–604. doi:10.1164/rccm.200912-1843CC
6. Siafakas N, Corlateanu A, Fouka E. Phenotyping before starting treatment in COPD? COPD. 2017;14(3):367–374. doi:10.1080/15412555.2017.1303041
7. Burtscher M. Effects of living at higher altitudes on mortality: a narrative review. Aging Dis. 2013;5(4):274–280. doi:10.14336/AD.2014.0500274
8. Guo Y, Xing Z, Shan G, et al. Prevalence and risk factors for COPD at high altitude: a large cross-sectional survey of subjects living between 2100–4700 m above sea level. Front Med. 2020;7:581763. doi:10.3389/fmed.2020.581763
9. Horner A, Soriano JB, Puhan MA, et al. Altitude and COPD prevalence: analysis of the PREPOCOL-PLATINO-BOLD-EPI-SCAN study. Research. 2017;18(1):62. doi:10.1186/s12931-017-0643-5
10. Brakema EA, Tabyshova A, Kasteleyn MJ, et al. High COPD prevalence at high altitude: does household air pollution play a role? Eur Respir J. 2019;53(2):1801193. doi:10.1183/13993003.01193-2018
11. Olloquequi J, Silva OR. Biomass smoke as a risk factor for chronic obstructive pulmonary disease: effects on innate immunity. Innate Immun. 2016;22(5):373–381. doi:10.1177/1753425916650272
12. Liang Y, Yangzom D, Tsokyi L, et al. Clinical and radiological features of COPD patients living at ≥3000 m above sea level in the Tibet plateau. Int J Chron Obstruct Pulmon Dis. 2021;16:2445–2454. doi:10.2147/copd.S325097
13. Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD assessment test. Eur Respir J. 2009;34(3):648–654. doi:10.1183/09031936.00102509
14. Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54(7):581–586. doi:10.1136/thx.54.7.581
15. Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi:10.1183/09031936.05.00034805
16. Wang Z, Gu S, Leader JK, et al. Optimal threshold in CT quantification of emphysema. Eur Radiol. 2013;23(4):975–984. doi:10.1007/s00330-012-2683-z
17. Occhipinti M, Paoletti M, Bartholmai BJ, et al. Spirometric assessment of emphysema presence and severity as measured by quantitative CT and CT-based radiomics in COPD. Respir Res. 2019;20(1):101. doi:10.1186/s12931-019-1049-3
18. Hansell DM, Bankier AA, MacMahon H, McLoud TC, Muller NL, Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008;246(3):697–722. doi:10.1148/radiol.2462070712
19. Smith IE, Jurriaans E, Diederich S, Ali N, Shneerson JM, Flower CD. Chronic sputum production: correlations between clinical features and findings on high resolution computed tomographic scanning of the chest. Thorax. 1996;51(9):914–918. doi:10.1136/thx.51.9.914
20. Bhalla M, Turcios N, Aponte V, et al. Cystic fibrosis: scoring system with thin-section CT. Radiology. 1991;179(3):783–788. doi:10.1148/radiology.179.3.2027992
21. Wells JM, Washko GR, Han MK, et al. Pulmonary arterial enlargement and acute exacerbations of COPD. N Engl J Med. 2012;367(10):913–921. doi:10.1056/NEJMoa1203830
22. American Thoracic Society. Diagnostic standards and classification of tuberculosis in adults and children. This official statement of the American Thoracic Society and the Centers for Disease Control and Prevention was adopted by the ATS Board of Directors, July 1999. This statement was endorsed by the Council of the Infectious Disease Society of America, September 1999. Am J Respir Crit Care Med. 2000;161(4 Pt 1):1376–1395. doi:10.1164/ajrccm.161.4.16141
23. van Gemert F, Kirenga B, Chavannes N, et al. Prevalence of chronic obstructive pulmonary disease and associated risk factors in Uganda (FRESH AIR Uganda): a prospective cross-sectional observational study. Lancet Glob Health. 2015;3(1):e44–e51. doi:10.1016/S2214-109X(14)70337-7
24. Salvi SS, Brashier BB, Londhe J, et al. Phenotypic comparison between smoking and non-smoking chronic obstructive pulmonary disease. Respir Res. 2020;21(1):50. doi:10.1186/s12931-020-1310-9
25. Nicolaou L, Checkley W. Differences between cigarette smoking and biomass smoke exposure: an in silico comparative assessment of particulate deposition in the lungs. Environ Res. 2021;197:111116. doi:10.1016/j.envres.2021.111116
26. Kamenar K, Hossen S, Gupte AN, et al. Previous tuberculosis disease as a risk factor for chronic obstructive pulmonary disease: a cross-sectional analysis of multicountry, population-based studies. Thorax. 2021;77(11):1088–1097. doi:10.1136/thoraxjnl-2020-216500
27. Ravimohan S, Kornfeld H, Weissman D, Bisson GP. Tuberculosis and lung damage: from epidemiology to pathophysiology. Eur Respir Rev. 2018;27(147):170077. doi:10.1183/16000617.0077-2017
28. Mishra VK, Retherford RD, Smith KR. Biomass cooking fuels and prevalence of tuberculosis in India. Int J Infect Dis. 1999;3(3):119–129. doi:10.1016/s1201-9712(99)90032-2
29. Saito M, Pan WK, Gilman RH, et al. Comparison of altitude effect on Mycobacterium tuberculosis infection between rural and urban communities in Peru. Am J Trop Med Hygiene. 2006;75(1):49–54. doi:10.4269/ajtmh.2006.75.49
30. Meneghini AC, Koenigkam-Santos M, Pereira MC, et al. Biomass smoke COPD has less tomographic abnormalities but worse hypoxemia compared with tobacco COPD. Braz J Med Biol Res. 2019;52(5):e8233. doi:10.1590/1414-431x20198233
31. Camp PG, Ramirez-Venegas A, Sansores RH, et al. COPD phenotypes in biomass smoke- versus tobacco smoke-exposed Mexican women. Eur Respir J. 2014;43(3):725–734. doi:10.1183/09031936.00206112
32. Devaraj A, Wells AU, Meister MG, Corte TJ, Wort SJ, Hansell DM. Detection of pulmonary hypertension with multidetector CT and echocardiography alone and in combination. Radiology. 2010;254(2):609–616. doi:10.1148/radiol.09090548
33. Ng CS, Wells AU, Padley SP. A CT sign of chronic pulmonary arterial hypertension: the ratio of main pulmonary artery to aortic diameter. J Thorac Imaging. 1999;14(4):270–278. doi:10.1097/00005382-199910000-00007
34. Aguirre-Franco C, Torres-Duque CA, Salazar G, Casas A, Jaramillo C, Gonzalez-Garcia M. Prevalence of pulmonary hypertension in COPD patients living at high altitude. Pulmonology. 2022. doi:10.1016/j.pulmoe.2021.12.006
35. Dunham-Snary KJ, Wu D, Sykes EA, et al. Hypoxic Pulmonary Vasoconstriction: from Molecular Mechanisms to Medicine. Chest. 2017;151(1):181–192. doi:10.1016/j.chest.2016.09.001
36. Pascoe S, Locantore N, Dransfield MT, Barnes NC, Pavord ID. Blood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: a secondary analysis of data from two parallel randomised controlled trials. Lancet Respir Med. 2015;3(6):435–442. doi:10.1016/s2213-2600(15)00106-x
37. Watz H, Tetzlaff K, Wouters EFM, et al. Blood eosinophil count and exacerbations in severe chronic obstructive pulmonary disease after withdrawal of inhaled corticosteroids: a post-hoc analysis of the WISDOM trial. Lancet Respir Med. 2016;4(5):390–398. doi:10.1016/s2213-2600(16)00100-4
38. Lee CH, Kim K, Hyun MK, Jang EJ, Lee NR, Yim JJ. Use of inhaled corticosteroids and the risk of tuberculosis. Thorax. 2013;68(12):1105–1112. doi:10.1136/thoraxjnl-2012-203175
39. Martinez FJ, Fabbri LM, Ferguson GT, et al. Baseline symptom score impact on benefits of glycopyrrolate/formoterol metered dose inhaler in COPD. Chest. 2017;152(6):1169–1178. doi:10.1016/j.chest.2017.07.007
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
