Back to Journals » Drug Design, Development and Therapy » Volume 13
Clinical And Bacteriological Impact Of Clarithromycin In Streptococcal Pharyngitis: Findings From A Meta-Analysis Of Clinical Trials
Received 23 February 2019
Accepted for publication 11 September 2019
Published 16 October 2019 Volume 2019:13 Pages 3551—3558
DOI https://doi.org/10.2147/DDDT.S205820
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Qiongyu Guo
Daryl J Hoban,1 Jos Nauta2
1Department of Medical Microbiology and Infectious Disease, Max Rady College of Medicine, University of Manitoba, Winnipeg, Manitoba, Canada; 2Department of Innovation & Development, Established Pharmaceuticals Division, Abbott Healthcare Products B.V., Weesp 1381 CP, The Netherlands
Correspondence: Jos Nauta
Department of Innovation & Development, Established Pharmaceuticals Division, Abbott Healthcare Products B.V., C.J. van Houtenlaan 36, Weesp 1381 CP, The Netherlands
Tel +31 294 47 7360
Email [email protected]
Introduction: Among the bacterial upper respiratory tract infections (UTRIs), the most medically significant is pharyngitis due to Group A beta-hemolytic Streptococci (GABHS). A 2012 meta-review and a 2016 Cochrane systematic review reported favorably on the comparative efficacy and safety of clarithromycin in pediatric patients with URTIs and in adults with GABHS pharyngitis. In this paper, the evidence base for clarithromycin in patients with URTIs is augmented by a meta-analysis of comparative studies in GABHS pharyngitis.
Methods: A series of five outpatient trials of clarithromycin for the treatment of streptococcal pharyngitis from an internal database were subjected to meta-analysis. Active comparators comprised penicillin VK and erythromycin.
Results: Rates of clinical cure or improvement were very similar in all treatment assignments, but the rates of bacteriological cure were numerically higher with clarithromycin than with comparator antibiotics. Adverse events data indicated that clarithromycin was generally well tolerated in these studies, with a relatively low incidence of adverse events and few severe incidents.
Discussion: Though currently not advised as a first-line therapy for URTI in most guidelines, the results of the meta-analysis indicate that clarithromycin is nevertheless a valid, effective and largely well-tolerated treatment option for GABHS pharyngitis patients who cannot benefit from other agents.
Keywords: clarithromycin, erythromycin, amoxicillin, Streptococcus, pharyngitis, metaanalysis
Introduction
Upper respiratory tract infections (URTIs) such as acute pharyngitis generate many primary care consultations and are regarded by patients, their families and many physicians as a condition with a high initial nuisance impact and potential for substantial and serious sequelae. Among those with a bacterial origin, the most prominent category, and arguably the most medically significant, is pharyngitis due to Group A beta-hemolytic Streptococci (GABHS) (S. pyogenes), which may account for ≥20% of sore throat-related clinic visits in pediatric patients aged >3 years and a smaller, but significant, percentage (5–15%) in adults.1
Symptoms of acute sore throat are often self-limiting and the impact of antibiotic therapy on resolution of bacteria-attributable pharyngitis symptoms is relatively limited, although more pronounced in cases confirmed to be associated with GABHS infection.2 Nevertheless, accurate diagnosis and effective treatment of streptococcal pharyngitis are important for preventing re-infection and spread and for the avoidance of longer-term complications of the initial infection, notably acute rheumatic fever, but also suppurative complications such as mastoiditis and cervical lymphadenitis, plus post-streptococcal glomerulonephritis and guttate psoriasis. In extreme instances, toxic shock syndrome or necrotizing fasciitis may ensue. Acute rheumatic fever is now uncommon in most developed countries, but it continues to be a significant cause of acquired heart disease in children in many middle- and low-income countries.3
The second-generation macrolide clarithromycin, which offers significant improvements over its predecessor, erythromycin, with an expanded spectrum of activity and enhanced tolerability,4 is regarded as a viable treatment option for patients with acute bacterial pharyngitis when penicillin V or amoxicillin is unavailable or for any reason inappropriate.1,5
A meta-review in 2012 reported favorably on the comparative efficacy and safety of clarithromycin in pediatric patients with URTIs,6 and a Cochrane systematic review in 2016 reported close equivalence of macrolides and penicillin for the resolution of GABHS pharyngitis in adults.7 The number of patients specifically treated with clarithromycin in that analysis was relatively small (N=499). We sought to augment the evidence base for clarithromycin in patients with URTIs by conducting a meta-analysis of comparative studies in GABHS pharyngitis.
Materials And Methods
Abbott’s internal database was searched for all clinical trials with clarithromycin for the treatment of streptococcal pharyngitis. This database contains all trials conducted by Abbott Laboratories with clarithromycin after obtaining the international rights in 1985. Study quality was evaluated using the Jadad score scale, in which the range of possible scores is 0 (poor/weak) to 5 (good) (see Appendix 1).8
Five trials were identified. All five were randomized, multi-center outpatient trials, in which very similar protocols and case report forms were used. Three studies were double-blind, one study was single-blind and one study was an open-label study. Blindedness was maintained by producing study drugs and placebos in identical appearance and by having all patients comply with the same dosing regimen. All studies had appropriate endorsement from local ethics committees.
Patients who presented with a primary complaint of pharyngeal pain and an associated sign of streptococcal pharyngitis provided two throat swabs. One swab was used to test for the presence of GABHS antigen using the rapid enzyme immunoassay Abbott Testpack™ Strep A (Abbott Laboratories, North Chicago, IL, USA), documented as having ≥90% sensitivity and 97.4% specificity in the detection of Group A Streptococci.9,10 The second was used for culturing to confirm the diagnosis of GABHS pharyngitis. To that end, swabs were plated on two 5% sheep blood agar plates. The primary inoculum was cultured to permit isolation of beta-hemolytic organisms. The blood agar plates were stabbed in several areas to detect the organism’s ability to produce streptolysin 0. One blood agar plate was incubated aerobically and the other incubated anaerobically, both at 35–37°C. All beta-hemolytic colonies were quantified, isolated and identified by Gram stain, bacitracin sensitivity and catalase reaction. Presumptive identification was confirmed using specific antisera. All tests were performed according to the provisions of the Clinical & Laboratory Standards Institute.11
In each of the trials, four study visits were planned. At Visit 1 (day 1) informed consent was obtained, a medical history was recorded, a physical examination was performed, vital signs were checked and a pharyngeal swab was obtained for culturing.
Principal common inclusion criteria in these five studies comprised:
- age ≥12 years
- a complaint of sore throat accompanied by at least one sign of streptococcal pharyngitis
- infection with Group A Streptococcus, as indicated by a positive rapid immunoassay test or as determined clinically by the investigator. Patients were admitted to the studies at the time of rapid antigen testing, before culture test results were available.
- otherwise good health
- written informed consent (from the patient or his/her legal guardian).
Female patients were required to have been post-menopausal for ≥1 year or to have undergone a hysterectomy or tubal ligation. Use of contraceptive medication or a contraceptive device did not qualify a patient for enrolment in these studies.
Patients were randomized to study drug medication using schedules generated by the statistical department of the study sponsor. A separate randomization schedule was prepared for each investigator, generated in randomly allocated blocks of either two, four or six. Patient numbers were assigned to the patients in order of enrolment. Further visits were scheduled at treatment days 5–7 (Visit 2) and at post-treatment days 4–5 (Visit 3) and 19–25 (Visit 4) for patients who completed the study drug treatment. At each visit, pharyngeal swabs were collected, adverse events were recorded and compliance with the study drug treatment was checked (not at Visit 4). Clinical response was scored by the responsible investigator at Visits 3 and 4.
The planned duration of study drug treatment was 10 days in all these trials.
The following assessment scales were used.
Clinical response at the end of the study drug treatment was scored using the following scale:
- Clinical cure: signs and symptoms of the infection resolved
- Clinical improvement: signs and symptoms of the infection improved but did not resolve
- Clinical failure: signs and symptoms of the infection did not improve, or worsened
- Recurrence: pre-treatment signs and symptoms of the infection resolved post-treatment but reappeared during follow-up (only in studies of streptococcal pharyngitis)
- Not evaluable: otherwise.
Clinical success at the end of the study drug treatment was defined as clinical cure or clinical improvement at that time.
Bacteriologic cure at the end of study drug treatment was scored using the following scale:
- Eradication: the initial pathogen(s) was/were eradicated at the end of study drug treatment and for the duration of follow-up
- Recurrence: eradication of the initial pathogen(s) at the end of study drug treatment with recurrence of the same pathogen(s) at any time during follow-up
- Re-infection: eradication of the pre-treatment pathogen at the end of study drug treatment with appearance of a new pathogen at any time during the follow-up period
- Persistence: otherwise.
Results
A series of five studies were identified, all of which were conducted between 1985 and 1988. Summary particulars of all five studies are provided in Table 1. Three studies used double-blind methodology, one was single-blind (investigator-blind) and one was open-label. Study MK86-008 has been published in the peer-reviewed literature.12
 |
Table 1 Indication, Dosage And Format Details For The Analyzed Trials |
In all, 1184 patients with signs and symptoms of streptococcal pharyngitis were enrolled in the five studies, of whom 600 were randomized to treatment with clarithromycin 250 mg twice daily and 584 to treatment with penicillin VK (250 mg three or four times daily; n=412) or erythromycin (500 mg twice daily; n=172).
Exclusions from the statistical analysis, detailed in Table 2, arose primarily either because no pre-treatment pathogen was isolated (n=137) or because no streptococcal pathogen was isolated (n=36). A total of 960 streptococcal pharyngitis-causing pathogens were isolated pre-treatment, overwhelmingly comprising S. pyogenes (98.8% of isolates, n=996, including 507/514 evaluable patients treated with clarithromycin).
 |
Table 2 Reasons For Exclusion From Statistical Analysis In Trials Of Streptococcal Pharyngitis. Values Shown Are Numbers Of Patients |
Median age was 30 years in both the clarithromycin group and the pooled control groups, with age ranges of 12–83 years for clarithromycin and 11–73 years in the pooled control groups. Most patients in both groups were aged ≥18 years (573/600 in the clarithromycin group; 555/584 in comparator groups) and two-thirds were male (n=394 for clarithromycin, n=381 for pooled comparators).
In all three treatment groups, the median total dose and the median duration of study drug treatment corresponded to the targeted total dose and treatment duration, respectively. Median compliance approached 100% in all three treatment groups.
Clinical and bacteriological outcome data at study Visit 3 (4–6 days after termination of study drug treatment) are summarized in Table 3 and Figure 1, respectively. Rates of clinical cure and clinical success (i.e., cured or improved) were very similar in all treatment assignments (Table 3) as well as in the individual trials (data not shown.) However, the rates of bacteriological cure were higher with clarithromycin than with either comparator agent (Figure 1).
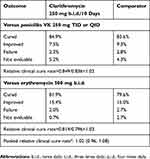 |
Table 3 Clinical Outcomes In Streptococcal Pharyngitis Studies At Visit 3 (4–6 Days Post-Treatment) |
Safety Data
In all, 287 of the 1184 patients reported a total of 457 treatment-emergent adverse events. Fifty-one of the 216 events (23.6%) recorded with clarithromycin 500 mg twice daily were considered probably related to the use of study medication, compared with 27/34 (79.4%) with erythromycin 500 mg twice daily, 9/64 (14%) with penicillin 250 mg four times a day and 7/117 (6%) with penicillin 250 mg three times daily.
Adverse events occurring with a frequency of ≥2% in the patients assigned to the various study medications are summarized in Table 4.
 |
Table 4 Adverse Events Recorded In ≥2% Of Patients |
A total of 23 treatment-emergent adverse events were classified as severe (clarithromycin: nine cases, 1.5%; erythromycin: seven cases, 4.1%; penicillin 250 mg four times a day: five cases, 2.7%; penicillin 250 mg three times daily: two cases, 0.9%). The seriousness of events was not documented. No deaths occurred in any of these studies.
Discussion
The results of this analysis indicate that clarithromycin, while currently not identified as a first-line therapy for URTI in most guidelines, is nevertheless a valid, effective and largely well-tolerated treatment option for GABHS pharyngitis patients who, for whatever reason, cannot benefit from other agents.
The number of patients accrued in the pharyngitis contingent was substantial: we believe that our cohort of >1000 patients (across both treatment assignments) is the largest such population reported for clarithromycin in that indication. These factors lend a degree of resilience to our central conclusion. Most of the patients enrolled were adults (96.0%) and, to that extent, our findings of high rates of clinical and, in particular, bacteriological cure with clarithromycin may be seen as extending to adult URTI patients the findings of the meta-analyses of Gutiérrez-Castrellón et al6 and the earlier results of Abad-Santos et al,13 who concluded that clarithromycin is an effective alternative for the treatment of URTIs in pediatric patients. Our own data also corroborate the views of those authors as to the effectiveness of clarithromycin in pediatric patients but, with limited numbers (n=57), our findings should be regarded as supplementary, not definitive.
The numerical findings for bacteriological cure are notably in favor of clarithromycin. In the largest of the studies in our dataset (M86-030), the intergroup difference in bacteriologic cure rate in pharyngitis patients was determined a priori and significantly favored clarithromycin over penicillin VK (95% vs 87%; p=0.009). The bacteriological superiority of clarithromycin over erythromycin may reside in part in the drug’s pharmacokinetics and metabolism, including tissue penetration and the formation of a bacteriologically relevant 14-hydroxy metabolite, and findings from pharmacodynamic modeling, which suggest that clarithromycin has a lower potential for encouraging the emergence of unrecognized resistant strains within an overall susceptible isolate.13–18
While a large proportion of pharyngitis cases may resolve without treatment, antibiotic use is necessary for pathogen eradication to prevent spread and re-infection and to minimize potential sequelae of the index infection. Complications of pharyngitis are rare in many of the high-income countries where clinical trials are often conducted but different considerations may apply in lower-income areas of the world,19 where poverty in its broad sense plus particular environmental proxies for poverty, such as overcrowding and shared housing, can promote the spread of infection and cycles of re-infection. Effective use of clarithromycin in that context may have a positive and long-lasting impact on the health of both URTI patients and their contacts.
Evidence accrued from a range of clinical studies and in experimental research indicates that clarithromycin exerts wide-ranging anti-inflammatory and immunomodulatory effects that may be pertinent in this context. Mechanisms involved may include inhibiting the production of microbial toxins and other virulence factors, so attenuating the pro-inflammatory host response; suppression of immune cell activity; and modulation of the cytokine profile toward a less pro-inflammatory/more anti-inflammatory balance.20–23
Limitations of our research must be acknowledged. Ours should be construed as a convenience sample: it is possible that either the patient populations or the response of those populations to treatment is not fully representative of the wider population that might be candidates for clarithromycin therapy for GABHS pharyngitis. The dosages of penicillin V were somewhat lower than might be prescribed today. Jadad scores ranged from 2 to 5, with three of the five studies registering scores of 4 or 5 (see Appendix 1). The range of scores nevertheless indicates methodological variability in the conduct of these studies and highlights the desirability of corroborating our central conclusions with additional studies that apply consistent contemporary standards of randomization and blinding.
Women of child-bearing age and potential were excluded from the studies in our dataset. This reflected the application of the contemporary ethical balance of advantage versus risk in the treatment of URTIs with antibiotics. Similar considerations still prevail and the clarithromycin SPC cautions that use in pregnancy requires a careful assessment of risk versus benefit.24 The outcomes of such deliberations may be different in high-income countries and low- or middle-income territories where rheumatic heart disease remains a substantial contributor to maternal death rates, and where there are also hazards from toxic shock syndrome and necrotizing fasciitis.25,26 There is no a priori reason to imagine that clarithromycin would have been anymore or less effective in this sector of the general population and there are no data from later experience to indicate such a difference in response. However, our data can offer no first-hand proof of that supposition.
Adverse events data indicated that clarithromycin was generally well tolerated in these studies, with a relatively low incidence of adverse events and few severe incidents. These findings should be contextualized by the acknowledgement that macrolide therapy has been associated increased rates of gastrointestinal adverse events (vs placebo) and with some increase in the risk of hearing loss.27 A Cochrane review of 2016 reported that the odds ratio for adverse events with “macrolide” was higher than with “penicillin” (OR 2.33, 95% CI 1.06 to 5.15) but that conclusion was based on a single study that compared azithromycin with penicillin V28 and it is not clear whether and to what extent that finding is an accurate refection of a whole-of-class effect of macrolides. A more recent Cochrane assessment found no evidence that macrolides caused more cardiac disorders, hepatobiliary disorders or changes in liver enzymes nor any indications that less serious events such as dizziness, headache, itching or rashes were reported more often with macrolides than with placebo. The overall standard of evidence underpinning these conclusions was, however, deemed weak.27
These considerations notwithstanding, our findings indicate that clarithromycin can be an effective option for patients with GABHS pharyngitis who are candidates for antibiotic therapy. In reaching these conclusions it must be acknowledged that the studies in our dataset can offer no insights into longitudinal trends in antibiotic resistance. Any substantial assessment of global trajectories of antibiotic resistance is beyond the scope of this report but the existence of such trends is indisputable and some of those trends are not favorable to the use of macrolides in URTIs. Over-prescription or inappropriate use of antibiotics is one factor contributing to this state of affairs29–35 and we would firmly endorse the views of Mohan et al35 (writing from a low- to middle-income perspective) on the need to restrict inappropriate antibiotic use in URTIs. Additional recent perspectives on this issue have been provided in a series of reports from the Survey of Antibiotic Resistance program,36–38 the CARTIPS Antimicrobial Surveillance Program in People’s Republic of China39 and other initiatives.30 Antibiotic resistance is not a problem confined to a single agent or class, however, and the extent that effective use of clarithromycin adds to the variety of effective treatment options for URTIs, we regard our findings as evidence that clarithromycin can indeed play a useful and effective role in the treatment of GABHS pharyngitis in pediatric and adult patients.
Data Access
The accessed data is not freely available.
Ethics Approval
Ethics approval for all five studies was obtained by the investigators.
Acknowledgments
The authors thank Dr Wilhelm Sauermann, DATAMAP GmbH, Freiburg for his contribution to data extraction and analysis, undertaken in the context a commercial contract. Abbott Products Operations AG, Allschwil, Switzerland retained Hughes associates, Oxford, UK, to provide editorial assistance in the preparation of this report.
Disclosure
Dr Nauta is a salaried full-time employee of Abbott Healthcare Products B.V., Weesp, The Netherlands, and owns company stock. Dr Hoban has in the past received grant support from Abbott and currently sits on Abbott Advisory boards and lectures for Abbott. This research was supported financially by Abbott Products Operations AG, Allschwil, Switzerland. The authors report no other conflicts of interest in this work.
References
1. Shulman ST, Bisno AL, Clegg HW, et al. Clinical practice guideline for the diagnosis and management of group A streptococcal pharyngitis: 2012 update by the Infectious Diseases Society of America. Clin Infect Dis. 2012;55:e86–102. doi:10.1093/cid/cis629
2. Spinks A, Glasziou PP, Del Mar CB. Antibiotics for sore throat. Cochrane Database Syst Rev. 2013;(11):CD000023.
3. Carapetis JR, Steer AC, Mulholland EK, Weber M. The global burden of group a streptococcal diseases. Lancet Infect Dis. 2005;5:685–694. doi:10.1016/S1473-3099(05)70267-X
4. Peters DH, Clissold SP. Clarithromycin: a review of its antimicrobial activity, pharmacokinetic properties and therapeutic potential. Drugs. 1992;44:117–164. doi:10.2165/00003495-199244010-00009
5. Chiappini E, Regoli M, Bonsignori F, et al. Analysis of different recommendations from international guidelines for the management of acute pharyngitis in adults and children. Clin Ther. 2011;33:48–58. doi:10.1016/j.clinthera.2011.02.001
6. Gutiérrez-Castrellón P, Mayorga-Buitron JL, Bosch-Canto V, Solomon-Santibañez G, de Colsa-Ranero A. Efficacy and safety of clarithromycin in pediatric patients with upper respiratory infections: a systematic review with meta-analysis. Rev Invest Clin. 2012;64:126–135.
7. van Driel ML, De Sutter A, Habraken H, Thorning S, Christiaens T. Different antibiotic treatments for group A streptococcal pharyngitis. Cochrane Database Syst Rev. 2016;9:CD004406.
8. Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12.
9. Schwabe LD, Small MT, Randall EL. Comparison of TestPack Strep A test kit with culture technique for detection of group A streptococci. J Clin Microbiol. 1987;25(2):309–311.
10. Tenjarla G, Kumar A, Dyke JW. TestPack Strep A kit for the rapid detection of group A streptococci on 11,088 throat swabs in a clinical pathology laboratory. Am J Clin Pathol. 1991;96(6):759–761. doi:10.1093/ajcp/96.6.759
11. CLSI.org [homepage on the Internet]. Wayne, PA: CLSI; 2018. Available from: https://clsi.org/.
12. Bachand RT
13. Abad-Santos F, Gálvez-Múgica MA, Espinosa de Los Monteros MJ, Gallego-Sandín S, Novalbos J. [Meta-analysis of clarithromycin compared with other antimicrobial drugs in the treatment of upper respiratory tract infections]. Rev Esp Quimioter. 2003;16(3):313–324. Spanish.
14. Blondeau JM, Shebelski SD, Hesje CK. Killing of Streptococcus pneumoniae by azithromycin, clarithromycin, erythromycin, telithromycin and gemifloxacin using drug minimum inhibitory concentrations and mutant prevention concentrations. Int J Antimicrob Agents. 2015;45:594–599. doi:10.1016/j.ijantimicag.2014.08.014
15. Noreddin AM, Roberts D, Nichol K, Wierzbowski A, Hoban DJ, Zhanel GG. Pharmacodynamic modeling of clarithromycin against macrolide-resistant [PCR-positive mef(A) or erm(B)] Streptococcus pneumoniae simulating clinically achievable serum and epithelial lining fluid free-drug concentrations. Antimicrob Agents Chemother. 2002;46:4029–4034. doi:10.1128/AAC.46.12.4029-4034.2002
16. Kastner U, Guggenbichler JP. Influence of macrolide antibiotics on promotion of resistance in the oral flora of children. Infection. 2001;29:251–256. doi:10.1007/s15010-001-1072-3
17. Metzler K, Drlica K, Blondeau JM. Minimal inhibitory and mutant prevention concentrations of azithromycin, clarithromycin and erythromycin for clinical isolates of Streptococcus pneumoniae. J Antimicrob Chemother. 2013;68:631–635. doi:10.1093/jac/dks461
18. Hoffman HL, Klepser ME, Ernst EJ, Petzold CR, Sa’adah LM, Doern GV. Influence of macrolide susceptibility on efficacies of clarithromycin and azithromycin against Streptococcus pneumoniae in a murine lung infection model. Antimicrob Agents Chemother. 2003;47:739–746. doi:10.1128/AAC.47.2.739-746.2003
19. Karthikeyan G, Guilherme L. Acute rheumatic fever. Lancet. 2018;392:161–174. doi:10.1016/S0140-6736(18)30999-1
20. Basyigit I, Yildiz F, Ozkara SK, Yildirim E, Boyaci H, Ilgazli A. The effect of clarithromycin on inflammatory markers in chronic obstructive pulmonary disease: preliminary data. Ann Pharmacother. 2004;38:1400–1405. doi:10.1345/aph.1D295
21. Steel HC, Theron AJ, Cockeran R, et al. Pathogen- and host-directed anti-inflammatory activities of macrolide antibiotics. Mediators Inflamm. 2012;2012:584262. doi:10.1155/2012/584262
22. Spyridaki A, Raftogiannis M, Antonopoulou A, et al. Effect of clarithromycin in inflammatory markers of patients with ventilator-associated pneumonia and sepsis caused by Gram-negative bacteria: results from a randomized clinical study. Antimicrob Agents Chemother. 2012;56:3819–3825. doi:10.1128/AAC.06446-11
23. Yamaya M, Shinya K, Hatachi Y, et al. Clarithromycin inhibits type a seasonal influenza virus infection in human airway epithelial cells. J Pharmacol Exp Ther. 2010;333:81–90. doi:10.1124/jpet.109.162149
24. medicines.org.uk [homepage on the internet]. Clarithromycin 500mg Tablets; 2019 [updated February 23, 2019]. Available from: https://www.medicines.org.uk/emc/product/6094/smpc.
25. Gustafson LW, Blaakær J, Helmig RB. Group A streptococci infection. A systematic clinical review exemplified by cases from an obstetric department. Eur J Obstet Gynecol Reprod Biol. 2017;215:33–40. doi:10.1016/j.ejogrb.2017.05.020
26. Carapetis JR, Beaton A, Cunningham MW, et al. Acute rheumatic fever and rheumatic heart disease. Nat Rev Dis Primers. 2016;2:15084. doi:10.1038/nrdp.2015.84
27. Hansen MP, Scott AM, McCullough A, et al. Adverse events in people taking macrolide antibiotics versus placebo for any indication. Cochrane Database Syst Rev. 2019;1:CD011825. doi:10.1002/14651858.CD005619.pub3
28. O’Doherty B. Azithromycin versus penicillin V in the treatment of paediatric patients with acute streptococcal pharyngitis/tonsillitis. Paediatric Azithromycin Study Group. Eur J Clin Microbiol Infect Dis. 1996;15:718–724. doi:10.1007/bf01691958
29. Dhanda V, Chaudhary P, Toor D, Kumar R, Chakraborti A. Antimicrobial susceptibility pattern of β-haemolytic group A, C and G streptococci isolated from North India. J Med Microbiol. 2013;62(Pt 3):386–393. doi:10.1099/jmm.0.046672-0
30. Mykhalko YO, Duhovych TV, Kish PP. Susceptibility of streptococcus pneumoniae to fluoroquinolones and macrolides in upper respiratory tract infections. Wiad Lek. 2017;70(2):224–226.
31. Liu X, Shen X, Chang H, et al. High macrolide resistance in Streptococcus pyogenes strains isolated from children with pharyngitis in China. Pediatr Pulmonol. 2009;44(5):436–441. doi:10.1002/ppul.20976
32. Funahashi K, Nakane K, Yasuda N, et al. T serotypes and antimicrobial susceptibilities of group A streptococcus isolates from pediatric pharyngotonsillitis. Jpn J Infect Dis. 2008;61(6):454–456.
33. Sayyahfar S, Fahimzad A, Naddaf A, Tavassoli S. Antibiotic susceptibility evaluation of Group A Streptococcus isolated from children with pharyngitis: a study from Iran. Infect Chemother. 2015;47(4):225–230. doi:10.3947/ic.2015.47.4.225
34. Sauermann R, Gattringer R, Graninger W, Buxbaum A, Georgopoulos A. Phenotypes of macrolide resistance of group A streptococci isolated from outpatients in Bavaria and susceptibility to 16 antibiotics. J Antimicrob Chemother. 2003;51(1):53–57. doi:10.1093/jac/dkg097
35. Mohan S, Dharamraj K, Dindial R, et al. Physician behaviour for antimicrobial prescribing for paediatric upper respiratory tract infections: a survey in general practice in Trinidad, West Indies. Ann Clin Microbiol Antimicrob. 2004;3:11. doi:10.1186/1476-0711-3-11
36. Torumkuney D, Zemlickova H, Maruscak M, Morrissey I. Results from the Survey of Antibiotic Resistance (SOAR) 2014-16 in the Czech Republic. J Antimicrob Chemother. 2018;73(Suppl 5):v22–v27. doi:10.1093/jac/dky067
37. Torumkuney D, Papaparaskevas J, Morrissey I. Results from the Survey of Antibiotic Resistance (SOAR) 2014-16 in Greece. J Antimicrob Chemother. 2018;73(Suppl 5):v36–v42. doi:10.1093/jac/dky068
38. Torumkuney D, Mayanskiy N, Edelstein M, Sidorenko S, Kozhevin R, Morrissey I. Results from the Survey of Antibiotic Resistance (SOAR) 2014-16 in Russia. J Antimicrob Chemother. 2018;73(Suppl 5):v14–v21. doi:10.1093/jac/dky065
39. Zhang Y, Zhang F, Wang H, et al. Antimicrobial susceptibility of Streptococcus pneumoniae, Haemophilus influenzae and Moraxella catarrhalis isolated from community-acquired respiratory tract infections in China: results from the CARTIPS Antimicrobial Surveillance Program. J Glob Antimicrob Resist. 2016;5:36–41. doi:10.1016/j.jgar.2016.03.002
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

