Back to Journals » Journal of Hepatocellular Carcinoma » Volume 10
Cisplatin or Doxorubicin Reduces Cell Viability via the PTPIVA3-JAK2-STAT3 Cascade in Hepatocellular Carcinoma
Authors Li CJ, Tsai HW, Chen YL, Wang CI, Lin YH, Chu PM, Chi HC, Huang YC, Chen CY
Received 9 August 2022
Accepted for publication 29 November 2022
Published 30 January 2023 Volume 2023:10 Pages 123—138
DOI https://doi.org/10.2147/JHC.S385238
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr David Gerber
Chao-Jen Li,1,* Hung-Wen Tsai,2,* Yi-Li Chen,3 Chun-I Wang,4 Yang-Hsiang Lin,5 Pei-Ming Chu,6,7 Hsiang-Cheng Chi,8,9 Yi-Ching Huang,3 Cheng-Yi Chen3
1Department of General & Gastroenterological Surgery, An Nan Hospital, China Medical University, Tainan, Taiwan; 2Department of Pathology, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan; 3Department of Cell Biology and Anatomy, College of Medicine, National Cheng Kung University, Tainan, Taiwan; 4Molecular Medicine Research Center, Chang Gung University, Taoyuan, Taiwan; 5Liver Research Center, Chang Gung Memorial Hospital, Taoyuan, Taiwan; 6Department of Anatomy, School of Medicine, Chung Shan Medical University, Taichung, Taiwan; 7Department of Medical Education, Chung Shan Medical University Hospital, Taichung, Taiwan; 8Graduate Institute of Integrated Medicine, China Medical University, Taichung, Taiwan; 9Chinese Medicine Research Center, China Medical University, Taichung, Taiwan
*These authors contributed equally to this work
Correspondence: Cheng-Yi Chen, Tel/Fax +886-6-2353535#5329, Email [email protected]
Introduction: Hepatocellular carcinoma (HCC) accounts for 80% of all liver cancers and is the 2nd leading cause of cancer-related death in Taiwan. Various factors, including rapid cell growth, a high recurrence rate and drug resistance, make HCC difficult to cure. Moreover, the survival rate of advanced HCC patients treated with systemic chemotherapy remains unsatisfactory. Hence, the identification of novel molecular targets and the underlying mechanisms of chemoresistance in HCC and the development more effective therapeutic regimens are desperately needed.
Methods: An MTT assay was used to determine the cell viability after cisplatin or doxorubicin treatment. Western blotting, qRT‒PCR and immunohistochemistry were utilized to examine the protein tyrosine phosphatase IVA3 (PTP4A3) level and associated signaling pathways. ELISA was utilized to analyze the levels of the inflammatory cytokine IL-6 influenced by cisplatin, doxorubicin and PTP4A3 silencing.
Results: In this study, we found that PTP4A3 in the cisplatin/doxorubicin-resistant microarray was closely associated with the overall and recurrence-free survival rates of HCC patients. Cisplatin or doxorubicin significantly reduced cell viability and decreased PTP4A3 expression in hepatoma cells. IL-6 secretion increased with cisplatin or doxorubicin treatment and after PTP4A3 silencing. Furthermore, PTP4A3 was highly expressed in tumor tissues versus adjacent normal tissues from HCC patients. In addition, we evaluated the IL-6-associated signaling pathway involving STAT3 and JAK2, and the levels of p-STAT3, p-JAK2, STAT3 and JAK2 were obviously reduced with cisplatin or doxorubicin treatment in HCC cells using Western blotting and were also decreased after silencing PTP4A3. Collectively, we suggest that cisplatin or doxorubicin decreases HCC cell viability via downregulation of PTP4A3 expression through the IL-6R-JAK2-STAT3 cascade.
Discussion: Therefore, emerging evidence provides a deep understanding of the roles of PTP4A3 in HCC cisplatin/doxorubicin chemoresistance, which can be applied to develop early diagnosis strategies and reveal prognostic factors to establish novel targeted therapeutics to specifically treat HCC.
Keywords: hepatocellular carcinoma, chemoresistance, cisplatin, doxorubicin, PTP4A3, IL-6
Introduction
Hepatocellular carcinoma (HCC) accounts for almost 80% of all liver cancer cases and is the sixth most common cancer and the 3rd most common cause of cancer-related death worldwide.1,2 To date, patients with advanced HCC have an average survival time of less than one year since HCC is typically diagnosed in the advanced stage. To date, no satisfactory systemic chemotherapy has been developed for patients with advanced HCC. This could be due to the natural chemoresistance of HCC and the high cytotoxicity of chemotherapy, leading to unfavorable outcomes for these patients.3,4 Furthermore, for most chemotherapeutic agents with a very low response rate, the chemoresistance issue needs to be addressed.3,5 Therefore, even though traditional surgical resection with chemotherapy is potentially curative in some advanced HCC patients with a poor prognosis, chemoresistance is still likely to contribute to rapid HCC growth and metastasis after surgical excision with chemotherapy.6–8
Systemic chemotherapeutics, such as cisplatin, doxorubicin, etoposide, 5-fluorouracil (5-FU) and their combinations, have been applied in advanced patients with HCC for more than 30 years.9 To date, doxorubicin has been widely applied as a chemotherapeutic drug for treating a large number of cancers, including advanced HCC, but has low efficacy and a response rate of approximately 15%.10 In addition, numerous reports have demonstrated the potential of doxorubicin to facilitate malignant tumor progression, with various mechanisms proposed for this phenomenon. From a molecular biology perspective, various genes have been shown to not only be involved in tumorigenesis but also contribute to the chemoresistance of HCC.11,12 Previously, Cox et al reported that doxorubicin could accelerate tumor cell invasion via regulation of P53, forkhead box O3 (FOXO3) and microRNAs or through the phosphatidylinositol 3-kinase (PI3K)/Akt and NF-κB signaling pathways.13 In addition to doxorubicin, cisplatin has been extensively utilized as a chemotherapeutic drug for HCC and has been found to be associated with chemoresistance in HCC. The mechanism of cisplatin resistance was proposed to involve human telomerase reverse transcriptase (hTERT) or topoisomerase 2A (TOP2A) dysregulation.14 Furthermore, the use of systemic chemotherapy with cisplatin or doxorubicin in patients with advanced HCC with vascular invasion or extrahepatic spread exhibited no survival benefit.3
Protein tyrosine phosphatase IVA (PTP4A) is a subfamily of protein tyrosine phosphatases (PTPs), including PTP4A1, PTP4A2 and PTP4A3, with at least 75% amino acid sequence identity.12,15 It can actively coordinate with protein tyrosine kinases to precisely modulate the intracellular phosphorylation levels to participate in many physiological processes.16 PTP4A3 is a potential oncogenic phosphatase that plays a critical role in cancer progression.17 PTP4A3 is overexpressed in numerous cancer types, such as liver, breast and bladder cancers, and is correlated with metastasis risk in uveal melanoma.16,18 Several reports have shown that PTP4A3 participates in cell growth, proliferation, invasion and metastasis in various types of cancers, and its expression is upregulated after interleukin (IL)-6 receptor (R) signaling or signal transducer and activator of transcription (STAT)3 and STAT5 activation.16,19,20 Previously, Brocke-Heidrich et al identified a phosphatase molecule, PTP4A3, which was defined as an IL-6-responsive gene, from the microarray database.21 Moreover, canonical IL-6R signaling has been shown to phosphorylate and activate downstream STAT3, followed by the activation of target genes.20 Additionally, PTP4A3 has been demonstrated to be a critical factor influencing the STAT3 phosphorylation status in multiple myeloma.22,23 Numerous reports have also shown that PTP4A3 can increase the expression of the antiapoptotic factor myeloid cell leukemia (Mcl)-1 and accelerate cell migration via the Akt/mTOR signaling pathway in glioblastoma cells.24,25
Previously, PTP4A3 was demonstrated to be correlated with HCC development and associated with poor differentiation in HCC.26 Vandsemb et al reported that PTP4A3 influences cell migration and plays a role in prostate cancer progression.27 However, the mechanisms underlying the role of PTP4A3 and/or IL-6R in cisplatin/doxorubicin chemoresistance and HCC progression remain unknown. According to the evidence, we propose the potential role of PTP4A3 in the process of cisplatin/doxorubicin chemoresistance in HCC. Cisplatin or doxorubicin chemoresistance in patients with a low response rate has been frequently observed in various cancers. Hence, the identification of HCC-related targets is crucial for understanding the molecular mechanisms and developing HCC-associated targeted therapies to address chemoresistance.
In the present study, we found that PTP4A3 was expressed at higher levels at more advanced stages of HCC, and low expression of PTP4A3 was associated with better patient survival. Additionally, cell viability and PTP4A3 expression were downregulated by cisplatin or doxorubicin. IL-6 cytokine secretion was also induced by cisplatin or doxorubicin, as shown by ELISA. Moreover, these phenomenon regulated by cisplatin or doxorubicin may occur via the Janus kinase (JAK) 2-STAT3 signaling pathway. Therefore, we suggest that PTP4A3 should be defined as a marker involved in cisplatin- and/or doxorubicin-stimulated HCC.
Materials and Methods
Cell Culture and Treatments
The human hepatoma cell lines HepG2, Hep3B, J7 and Huh7, which were purchased from ATCC (Hsinchu, Taiwan), were routinely cultured at 37°C in a humidified atmosphere of 95% air and 5% CO2 in Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (HyClone, Road Logan, UT, USA). These cells were utilized and challenged with various stimulators, such as cisplatin (Pharmachemie BV, Short Hills, NJ, USA) and doxorubicin (Sigma–Aldrich, St. Louis, MO, USA).
MTT Assay
Cell proliferation ability was determined by the 3-[4,5-dimethylthiazol-2-yl]-5-[3-carboxymethoxyphenyl]2-[4-sulfophenyl]-2H-tetrazolium (MTT) assay (Promega Corporation, Madison, WI, USA). Cells (5 × 104) were seeded on 96-well plates overnight. After the end of treatment, 20 μL of MTT solution was added to each well for 3 h at 37°C. The absorbance at 570 nm was measured using a SpectraMax microplate reader (Tecan, Männedorf, Switzerland).
Real-Time PCR
Total RNA was extracted using TRIzol reagent (Life Technologies), and the cDNA template was synthesized using oligodeoxythymidine (Promega Corporation, Madison, Wisconsin, USA) and Superscript II Reverse Transcriptase (Life Technologies, Carlsbad, CA, USA). The real-time PCR mixture contained 500 nM forward and reverse primers and 1× SYBR Green reaction mix (Applied Biosystems, Waltham, MA, USA). SYBR Green fluorescence was determined by the ABI PRISM 7500 detection system (Applied Biosystems, Waltham, MA, USA). The cDNA was amplified via PCR for 35 cycles at 95°C for 5 sec, 62°C, for 30 sec. The primers used were as follows: PTP4A3 Forward-5’GCAGCTCACCTACCTGGAGAAATA3’, Reverse 5’TGCGTGTGTGGGTCTTTGAA3’; 18SrRNA Forward-5’GCAGCTCACCTACCTGGAGAAATA3’, Reverse-5’TGCGTGTGTGGGTCTTTGAA3’. The primer sequences were designed with the Nucleotide section of NCBI (GenBank: AY889443) using Primer Express software (Thermo Fisher Scientific, Waltham, MA, USA).
Western Blotting
Total cell extracts (20 μg of protein) were fractionated by 10–12% SDS–PAGE, transferred to an immobilon polyvinylidene difluoride membrane (Amersham Biosciences, Corston, UK), and probed with one of the specific primary antibodies against PTP4A3 (1:1000, GTX100600, GeneTex, Hsinchu, Taiwan), p-STAT3 (1:1000, GTX104616, GeneTex, Hsinchu, Taiwan), STAT3 (1:1000, GTX11800, GeneTex, Hsinchu, Taiwan), STAT5A (1:1000, GTX113351, GeneTex, Hsinchu, Taiwan), FOXO3A (1:1000, GTX100277, GeneTex, Hsinchu, Taiwan), TERT (1:1500, GTX124242, GeneTex, Hsinchu, Taiwan), TOP2A (1:1500, GTX100689, GeneTex, Hsinchu, Taiwan) p-JAK2 (1:1000, GTX132784, GeneTex, Hsinchu, Taiwan), JAK2 (1:1000, GTX01195, GeneTex, Hsinchu, Taiwan), p-P65 (1:1000, GTX133899, GeneTex, Hsinchu, Taiwan), P65 (1:1000, GTX102090, GeneTex, Hsinchu, Taiwan) and β-actin (1:10,000, #4970, Cell Signaling Technology, Danvers, MA, USA) overnight at 4°C. Subsequently, the membrane was hybridized with appropriate HRP–conjugated secondary antibodies for 1 h at room temperature. Finally, immune complexes were visualized using the chemiluminescence method with an ECL detection kit (Merck Millipore, Temecula, CA, USA) on Fujifilm LAS-3000 (Fujifilm, Tokyo, Japan).
Effect of PTP4A3 Knockdown
Small interfering (si)RNA targeting PTP4A3 was purchased from Invitrogen. Cells were transfected with siRNA using Lipofectamine Reagent (Invitrogen, Carlsbad, CA, USA), and the expression of PTP4A3 was examined using Western blotting.
Clinical HCC Specimens
HCC tissues were obtained from the National Health Research Institute Biobank, and doxorubicin-treated HCC patient specimens were obtained from the human biobank of National Cheng Kung University Hospital. All clinical specimen experiments were performed in accordance with the guidelines of the Institutional Review Board of National Cheng Kung University Hospital (IRB No: A-ER-109-533, A-ER-109-135). Informed consent was waived for the use of the specimens from the National Health Research Institute Biobank and human biobank of National Cheng Kung University Hospital.
Immunohistochemistry
Formalin-fixed and paraffin-embedded clinical HCC specimens were evaluated by immunohistochemistry staining using the avidin-biotin complex method, as described previously.28 Sections were air dried and fixed in ice-cold acetone for 10 min, then rehydrated with PBS for 5 min, blocked with protein block (Dako) for 20 min, and incubated with polyclonal antibody against PTP4A3 (GeneTex, Hsinchu, Taiwan) for 1 h at room temperature. Positively stained cells with PTP4A3 immunoreactivity were dark as visualized using DAB/nickel substrate (Vector Laboratories, Burlingame, CA, USA). We examined the PTP4A3 staining intensity using a microscope (Zeiss) equipped with a cooled charge-coupled device camera (Axiocam HRm; Zeiss, Oberkochen, Germany).
Statistical Analysis
The correlations between PTP4A3 levels (39-ΔCt) and clinicopathological indicators were assessed using the Wilcoxon rank sum test. Data are presented as the mean ± SD. Recurrence-free survival and overall survival were calculated using the Kaplan–Meier method, and the Log rank test was used to assess the differences between groups. A Cox proportional hazards regression model was used to measure the independence of different factors. Cox regression analysis was performed by forward stepwise analysis, and only the prognostic variables that were significant in the univariate analysis were included in the model. P values less than 0.05 were considered to indicate statistical significance.
Results
Selection of Cisplatin/Doxorubicin-Regulated Candidate Genes from the Overlap of Microarray Datasets
Previously, cisplatin or doxorubicin have been demonstrated to promote crucial mechanisms of chemoresistance that can influence the survival of HCC through numerous pathways.29,30 First, we retrieved data from three datasets, including the Roessler Liver microarray dataset from Oncomine31 and the cisplatin/doxorubicin resistance datasets for hepatoma cells established by Ng et al.32 The three microarray datasets were analyzed and compared to identify promising targets. The Roessler Liver microarray dataset was filtered to select candidates by (1) determining the fold change in the expression levels of the lowest 25% (in terms of survival rate) vs the top 25% in HCC tissues, (2) combining the Roessler Liver dataset with the cisplatin/doxorubicin regulation datasets obtained by Ng et al, and (3) identifying suitable cisplatin/doxorubicin resistance genes aberrantly expressed in HCC specimens; the genes identified were selected for further study. We applied >1-fold change as the criterion for selection of cisplatin/doxorubicin-regulated candidates in cisplatin/doxorubicin-resistant cells compared with HCC parental cells and > 1.1-fold as the criterion for selection of oncogenes in the lowest 25% (in terms of survival rate) vs the top 25% in HCC tissues of the Roessler Liver microarray for overlap analysis to identify the possible candidates for further testing. Based on these criteria, 66 initial candidate genes were consistently observed in the three databases (Figure 1A). According to the above search, the selected genes were filtered more stringently to identify highly significant molecules. PTP4A3 was the major gene correlated with cisplatin/doxorubicin chemoresistance in liver cancer but has not been well investigated. Hence, we selected PTP4A3 for further study.
Verification of the Clinical Significance of PTP4A3 in the Roessler Liver Microarray Dataset
PTP4A3 is a member of the phosphatase of regenerating liver (PRL) subgroup of protein tyrosine phosphatases, which are involved in numerous signaling pathways and participate in many fundamental physiological processes.33,34 Previously, PTP4A3 was shown to influence breast cancer cell proliferation and overall patient survival.35 Hence, PTP4A3 may be a promising candidate for further study. The Roessler Liver microarray provides large amounts of clinical information, including pathological stage, tumor-node-metastasis (TNM) stage, alpha-fetoprotein (AFP) level, alanine aminotransferase (ALT) activity, tumor size, overall survival rate, recurrence status, and predicted risk metastasis signature score. Therefore, the correlation between clinical parameters and PTP4A3 expression was analyzed statistically to define the role of cisplatin/doxorubicin-regulated PTP4A3 in HCC progression.
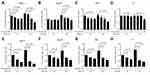
The clinical significance of PTP4A3 expression in the Roessler Liver dataset was analyzed (Figure 1). Patients with low expression of PTP4A3 had a higher overall survival rate (log-rank P<0.05; PTP4A3 high: standard error, 2.368; 95% CI, 40.205–49.486; PTP4A3 low: standard error, 2.190; 95% CI, 46.617–55.203) and recurrence-free survival rate (log-rank P<0.05; PTP4A3 high: standard error, 2.456; 95% CI, 29.913–39.540; PTP4A3 low: standard error, 2.375; 95% CI, 38.433–47.742) (Figure 1B and C). Moreover, PTP4A3 expression was significantly increased in patients with high AFP levels, a predicted risk factor for metastasis (Figure 1D and E), and more advanced pathological stages of HCC (Figure 1F). A high PTP4A3 level (39-ΔCt ≥ 16.1) in HCC was significantly associated with worse overall survival (P=0.010) (Figure 2A) and recurrence-free survival (P=0.032) (Figure 2B). A high PTP4A3 level in HCC was associated with higher AFP levels (P=0.039) (Figure 2C) (Table 1). Univariate analysis showed that cirrhosis (P=0.022), AFP level (P=0.033), tumor size (P=0.043), vascular invasion (P=0.005), American Joint Committee on Cancer (AJCC) stage (P=0.003), and high PTP4A3 level (P=0.035) were significant predictors of worse recurrence-free survival (Table 2). Multivariate analysis showed that AJCC stage (P=0.005, HR=2.049, CI=1.249–3.362) and high PTP4A3 level (P=0.046, HR=1.815, CI=1.010–3.263) were independently associated with recurrence-free survival (Table 2). Regarding overall survival, univariate analysis showed that cirrhosis (P=0.020), AFP level (P=0.012), tumor size (P=0.011), vascular invasion (P<0.001), AJCC stage (P=0.007), and high PTP4A3 level (P=0.013) were significant predictors of worse overall survival (Table 3). Multivariate analysis showed that vascular invasion (P=0.001, HR=2.244, CI=1.407–3.557) and high PTP4A3 levels (P=0.034, HR=2.119, CI=1.060–4.235) were independently associated with overall survival (Table 3). These data indicate that PTP4A3 expression is closely associated with HCC progression and could possibly be selected as a useful prognostic marker for HCC.
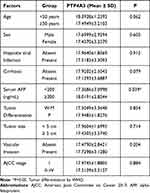 |
Table 1 Association of PTP4A3 Expression with Clinicopathologic Indicators of Hepatocellular Carcinoma |
 |
Table 2 Prognostic Significance of Clinicopathologic Indicators and PTP4A3 for Disease-Free Survival in the Clinical Cohort |
 |
Table 3 Prognostic Significance of Clinicopathologic Indicators and PTP4A3 for Overall Survival in the Clinical Cohort |
Study of the Regulation of PTP4A3 by Cisplatin/Doxorubicin Related to HCC Chemoresistance in vitro
We initially tried to treat hepatoma cells with various doses of cisplatin (Cis, 0–10 μg/mL) for 24 and 48 h to determine HCC cell viability. The data showed that cell viability decreased in a dose-dependent manner after cisplatin treatment in HepG2, Hep3B, Huh7, and J7 cells using the MTT assay (Figure 3A–D). Moreover, we tried to verify whether the phenomenon could be observed for doxorubicin-stimulated HCC. These cells were treated with various doses of doxorubicin (0–1 μg/mL) for 24 and 48 h. Similarly, doxorubicin decreased the viability of HepG2, Hep3B, Huh7, and J7 cells, as shown by the MTT assay (Figure 3E–H). In addition, we treated HepG2, Hep3B, Huh7 and J7 cells with 5 μg/mL cisplatin, 0.3 μg/mL doxorubicin, and 5 μg/mL cisplatin combined with 0.3 μg/mL doxorubicin for 24 h. The MTT assay results indicated that cotreatment with cisplatin and doxorubicin decreased cell viability more obviously than cisplatin or doxorubicin alone in HepG2, Hep3B, Huh7 and J7 cells (Supplementary Figure 1). Taken together, the results show that HCC cell viability could be significantly decreased by cisplatin or doxorubicin chemotherapy.
After retrieving data from the Roessler Liver microarray and cisplatin/doxorubicin resistance datasets,31,32 we found that PTP4A3 expression was highly correlated with numerous clinical parameters and the 5-year survival rate (Figure 1). To further verify the cisplatin/doxorubicin regulation of PTP4A3, Western blotting was performed with cisplatin or doxorubicin treatment. We treated HCC cells with various doses of cisplatin (Cis, 0–10 μg/mL) and doxorubicin (Dox, 0–1 μg/mL) for 24 and 48 h, and the PTP4A3 expression level in HepG2, Hep3B, Huh7, and J7 cells was obviously decreased by cisplatin (Figure 4A–D) or doxorubicin (Figure 4E–H), as determined using Western blotting. Based on the evidence, cisplatin or doxorubicin decreased HCC cell viability through downregulation of PTP4A3 expression.
Assessment of the Effect of PTP4A3 on Cisplatin/Doxorubicin-Regulated Viability in HCC Cell Lines
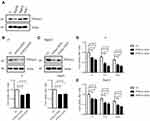
PTP4A3 expression at both the mRNA and protein levels has been shown to be upregulated in numerous cancer types, including liver cancer.16 To determine the roles of PTP4A3, the basal expression levels of PTP4A3 in HCC cell lines, including J7, Hep3B, HepG2 and Huh7 cells, were determined by Western blotting. PTP4A3 was highly expressed in J7, HepG2 and Huh7 cells (Figure 5A); hence, we utilized J7 and HepG2 cells to silence PTP4A3 expression to determine its effect. PTP4A3 expression was successfully silenced with specific PTP4A3 siRNA (KD1, KD2) in J7 (Figure 5B) and HepG2 (Figure 5C) cells, and the vector control (VC) was used for comparison. Previously, PTP4A3 was shown to play a role in cell proliferation in chronic myeloid leukemia (CML) and influence cell proliferation via the C-terminal Src kinase (Csk)-Src cascade in human embryonic kidney 293 (HEK293) cells.36,37 Subsequently, we examined whether PTP4A3 plays a role in HCC progression, and the viability of J7 and HepG2 cells was found to decrease in PTP4A3-silenced cells compared with vector control cells by the MTT assay (Figure 5B and C, lower panel). Moreover, we found that the decrease in cell viability induced by cisplatin (10 μg/mL) or doxorubicin (1 μg/mL) was more obvious in PTP4A3-silenced (PTP4A3 KD) cells than in vector control (VC) J7 and HepG2 cells (Figure 5D and E). The evidence indicated that PTP4A3 plays a role in the cisplatin- and doxorubicin-regulated progression of HCC.
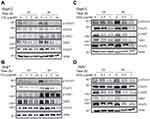
Chong et al reported that IL-6R signaling may be influenced by PTP4A3 to alter the phosphorylation status of downstream STAT3.22,23 It has been shown that the downstream IL-6R signaling JAK/STAT pathway can be phosphorylated to influence their transcriptional activation.38 Therefore, we analyzed whether cisplatin or doxorubicin could regulate the phosphorylation status of STAT3 in HCC. We treated HepG2 and Huh7 cells with various doses of cisplatin (Cis, 0–10 μg/mL) or doxorubicin (Dox, 0–1 μg/mL) for 24 and 48 h, the levels of p-STAT3, STAT3, p-JAK2, JAK2, STAT5 were analyzed, we found these molecules were decreased with cisplatin or doxorubicin treatment at 24 and 48 h in both HepG2 and Huh7 cells. These reductions were dose dependent. The basic levels of p-STAT3 and p-JAK2 were higher at 48 h than 24 h in both HepG2 and Huh7 cells. However, the un-phosphorylated STAT3 and JAK2 of basic levels were similar at both 24 and 48 h in both HepG2 and Huh7 cells (Figure 6). The unphosphorylated forms STAT3 and JAK2 were also analyzed in the same SDS-PAGE, the STAT3 basic levels were similar at 24 and 48 h in both HepG2 and Huh7 cells, in which the levels were higher in Huh7 cells. In addition, the JAK2 levels were a little higher at 48 h in both HepG2 and Huh7 cells, in which the levels were higher in HepG2 cells compared to Huh7 cells (Supplementary Figure 4). The mechanism by which cisplatin or doxorubicin regulate p-STAT3 is similar to the mechanism of JAK2 regulation. Taken together, the results show that cisplatin or doxorubicin regulates PTP4A3 expression through the JAK2-STAT3 cascade. In addition, the expression of FOXO3, Akt and NF-κB was decreased in HepG2 and Huh7 cells treated with 0.5 and 1 μg/mL doxorubicin at 24 and 48 h, as shown by Western blotting. Moreover, telomerase reverse transcriptase (hTERT) or topoisomerase 2A (TOP2A) expression was also reduced with 1–10 μg/mL cisplatin treatment for 24 and 48 h, as shown by Western blotting (Supplementary Figures 2 and 3). These reductions were dose dependent. Therefore, we suggest that HCC cells treated briefly with cisplatin or doxorubicin exhibited reduced expression of resistance targets, including FOXO3, Akt, NF-κB, hTERT and TOP2A.
Previously, the IL-6 pathway was demonstrated to alter the phosphorylation status of STAT proteins via the activation of the JAK-STAT pathway in multiple myeloma.39 Additionally, Brocke-Heidrich et al found that both STAT3 and PTP4A3 were regulated by IL-6 using microarray analysis.21 Therefore, we determined whether IL-6 could be regulated with cisplatin or doxorubicin treatment in HCC. J7 cells were stimulated with various concentrations of cisplatin (Cis, 0–10 μg/mL) and doxorubicin (Dox, 0–1 μg/mL) for 24 and 48 h, and IL-6 expression was significantly induced in a dose-dependent manner (Figure 7A and B). Moreover, we analyzed whether PTP4A3 plays a role in cisplatin- and doxorubicin-regulated IL-6 secretion. IL-6 secretion increased in PTP4A3-silenced (PTP4A3 KD) cells compared to vector control (CTL) cells according to ELISA (Figure 7C). Additionally, the STAT3 and JAK2 levels were decreased after transfection of PTP4A3 silencing compared with the vector control into J7 cells according to Western blotting; however, the IL-6R level was not altered (Figure 7D). Collectively, we suggest that cisplatin- or doxorubicin-induced IL-6 secretion may occur via decreased PTP4A3 expression, and the dysregulation is independent of IL-6R regulation.
Furthermore, we analyzed the role of the IL-6 receptor (R) in HCC, and the expression of IL-6R was correlated with clinical parameters and survival rate. We found that low IL-6R expression was associated with a higher overall survival rate and recurrence-free survival rate, and a high predicted risk metastasis signature score was associated with significantly higher IL-6R expression (Figure 7E–G). Taken together, the results show that IL-6R signaling plays a critical role in HCC progression and is implicated in the survival rate and outcome. Based on the evidence, we conclude that cisplatin or doxorubicin reduces HCC cell viability through the regulation of PTP4A3 via the IL-6R-JAK2-STAT3 cascade.
Moreover, we analyzed the levels of PTP4A3 in clinical HCC specimens, and liver tissues of doxorubicin-treated patients were collected. We examined 10 pairs of liver tissues from doxorubicin-treated HCC patients, and PTP4A3 expression was almost higher in tumor tissues than in normal control tissues using qRT‒PCR (Figure 8A). Immunochemistry was also utilized to detect PTP4A3 levels, and we found that PTP4A3 expression was obviously higher in tumor tissues (Figure 8B, b) than in normal tissues from HCC patients (Figure 8B, a). Collectively, we found that PTP4A3 was highly expressed in tumor tissues compared with normal tissues, implying that PTP4A3 plays an oncogenic role in HCC progression, and which was supported by the analyses of overall and recurrence-free survival rates from both our cohort and the Roessler liver microarray cohort.
Discussion
In the present study, we selected a critical factor, PTP4A3, which was highly correlated with overall and recurrence-free survival rates and might be involved in cisplatin- and doxorubicin-regulated cell viability. Cisplatin or doxorubicin significantly reduced cell viability and decreased PTP4A3 expression in hepatoma cells. IL-6 secretion increased with cisplatin or doxorubicin treatment and after PTP4A3 silencing in J7 cells. Cisplatin or doxorubicin also decreased the p-STAT3 and JAK2 levels, and IL-6R was highly correlated with the survival rate and predicted risk metastasis signature score. We found that cisplatin or doxorubicin reduced the expression of PTP4A3 and p-STAT3 according to Western blotting (Figures 4 and 6). Moreover, the inflammatory cytokine IL-6 was also induced by cisplatin or doxorubicin in J7 cells, as shown by ELISA (Figure 7A and B). Moreover, the IL-6 levels were significantly increased after PTP4A3 silencing (Figure 7C). In the present study, we provided indirect evidence to interpret the relationship of PTP4A3, IL-6 and p-STAT3. Previously, the IL-6 pathway was demonstrated to alter the phosphorylation status of STAT proteins via activation of the JAK-STAT pathway in multiple myeloma.39 Therefore, we suggest that cisplatin or doxorubicin decreases HCC cell viability via the downregulation of PTP4A3 expression through the IL-6R-JAK2-STAT3 cascade.
PTP4A3 possesses a unique prenylation motif at the C-terminal end. Nonprenylated PTP4A3 is inactive and is usually located in the nucleus, and prenylated PTP4A3 is active and can be found in membranes and intracellular structures.40 Previously, PTP4A3 has been demonstrated to induce cell migration and invasion via activation of the Rho GTPase and PI3K/Akt cascades.41,42 In the current study, we found that cisplatin or doxorubicin reduced cell viability through the downregulation of PTP4A3 via JAK2-STAT3 signaling. The effect of PTP4A3 on cell migration in the presence of cisplatin or doxorubicin still needs to be clarified. Furthermore, whether PTP4A3 can regulate HCC cell apoptosis or autophagy still needs to be elucidated in the future.
Recently, emerging evidence has implicated dysregulation of protein tyrosine phosphatase activity in a variety of cancers.43 The PTP4A3 gene encodes a 22-kDa tyrosine phosphatase that is involved in tumorigenesis and metastasis.15 Moreover, PTP4A3 has been implicated in drug resistance and cancer progression.24 Protein phosphorylation is mediated by protein kinases via posttranslational modification that leads to the activation of numerous signaling pathways implicated in various cellular biological processes, including cell growth, proliferation, and death. Protein dephosphorylation is mediated by protein tyrosine phosphatases (PTPs) that lead to the termination of signal transduction, which results in the inhibition of cell proliferation and growth.35 PTPs have been demonstrated to have oncogenic roles and are promising candidates for the development of targeted therapies for numerous cancers. Phosphatases are defined as crucial growth-regulatory factors, and the cell growth of numerous cancer types is suppressed with silencing of oncogenic phosphatases.44 However, effective and safe methods for targeting cancer-stimulated molecules need to be discovered to cure various life-threatening cancer types.
Previously, PTP4A3 gene expression was demonstrated to be altered with IL-6R signaling activation and upregulated after STAT3 and STAT5 activation.16,20 It has been shown that the IL-6R signaling downstream molecule JAK/STAT can be phosphorylated to influence their transcriptional activation.38 Wang et al also reported that PTP4A3 could influence cell proliferation, migration and invasion via the Akt/mTOR signaling pathway in glioblastoma cells.25 Recently, PTP4A3 was demonstrated to be an oncogene that influences cancer cell progression, including tumor formation, growth, survival and metastasis. Numerous pathways are altered by PTP4A3; these include the platelet-derived growth factor (PDGF), integrin, EphrinA, PI3K-Akt, extracellular signal–regulated kinase (ERK)1/2, STAT3 and IL-6R pathways, which are involved in tumor development in several cancer types.22,35,44–46 Additionally, several signaling pathways, such as the epidermal growth factor receptor, mitogen-activated protein kinase, PI3K/Akt and Wnt pathways, are associated with chemoresistance in cancer.47 Previously, Guo et al reported that the phosphatase activity of PTP4A3 is important and correlated with tumor-associated angiogenesis and cancer metastasis.48 However, whether cisplatin- and doxorubicin-regulated cell migration and related phenotypes could be rescued by PTP4A3 needs to be elucidated in detail with experiments involving overexpression of PTP4A3, simultaneous stimulation with the PTP4A3 inhibitor BR-149 or silencing of PTP4A3 in the presence of cisplatin and/or doxorubicin in the future. PTP4A3 has been defined as a p53-inducible gene and plays a role in both inducing and blocking cell cycle progression.12 Notably, high levels of PTP4A3 have been shown to predict poor survival in multiple patients with acute myeloid leukemia.50 STAT proteins are critical for signal transduction processes ranging from the extracellular to intracellular response, which is essential for numerous cellular functions, including cell proliferation, growth and apoptosis.51
Oncogenic tyrosine kinases, such as Jak2 and Src, play a role in the aberrant activation of STAT3 in cancers.52 Furthermore, both autocrine and paracrine IL6 signaling promote constitutive activation of STAT3 via Jak2 signaling in multiple myeloma.52 Canonical IL-6 signaling has been reported to be modulated by repressors, such as Src homology containing protein 1 (SHP-1) and SHP-2 tyrosine phosphatases, that interact with suppressor of cytokine signaling 1 (SOCS1). Disruption of this interaction results in hyperactivation of the JAK/STAT pathway. Indeed, the expression of SHP1, SHP2, and SOCS1 is silenced in malignant plasma cells, and this phenomenon is correlated with enhanced JAK/STAT3 activity.53,54 In the present study, we found that the expression of JAK2 and p-STAT3 was reduced by cisplatin and doxorubicin. However, whether PTP4A3 can alter the levels of JAK2 and p-STAT3 in the presence of cisplatin or doxorubicin needs to be investigated in more detail.
In the present study, we selected an attractive candidate, PTP4A3, which was highly correlated with survival rate and related to numerous clinical parameters, from the Roessler Liver microarray and cisplatin and doxorubicin resistance datasets. Cell viability decreased with PTP4A3 silencing; hence, PTP4A3 has an oncogenic role in HCC. We found that cisplatin or doxorubicin decreased HCC cell viability through dysregulation of PTP4A3 via the IL-6-JAK2-STAT3 cascade. In the future, we suggest that PTP4A3 may be a useful marker and therapeutic target to overcome the chemotherapy resistance and improve the survival of HCC patients.
Data Sharing Statement
All data generated or analyzed during this study are included in this published article.
Ethics Approval and Consent to Participate
The National Health Research Institute Biobank and its associated institutions or providing the biological specimen and related clinical data (all of which have been deidentified) for our research. The doxorubicin-treated HCC patient specimens were obtained from the human biobank of National Cheng Kung University Hospital.
Consent for Publication
The authors agree to publish.
Acknowledgments
We would like to thank the National Health Research Institute Biobank and its associated institutions for providing the biological specimen and related clinical data (all of which have been deidentified) for our research. The National Health Research Institute Biobank is supported by grants from the Ministry of Health and Welfare, Ministry of Science and Technology and National Health Research Institutes, Taiwan. We are grateful for the support from the Human Biobank, Research Center of Clinical Medicine and Cancer Data Bank of National Cheng Kung University Hospital. This work was supported by a grant from the Ministry of Science and Technology of the Republic of China (MOST 109-2320-B-006-067 to C.-Y.C.) and a grant from National Cheng Kung University Hospital (NCKUH-11102040), An Nan Hospital, China Medical University (ANHRF111-11). We would like to thank Ching-Chuan Hsieh and Pin-Jia Huang for performing experiments, acquisition of data, analysis and interpretation for manuscript revision.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by a grant from the Ministry of Science and Technology of the Republic of China (MOST 109-2320-B-006-067, MOST 111-2320-B-006-024, MOST 111-2634-F-006-012 to C.-Y.C.) and a grant from National Cheng Kung University Hospital (NCKUH-11102040), An Nan Hospital, China Medical University (ANHRF111-11).
Disclosure
Chao-Jen Li and Hung-Wen Tsai are co-first authors for this study. The authors declare no conflicts of interest in this work.
References
1. Zhu RX, Seto WK, Lai CL, et al. Epidemiology of hepatocellular carcinoma in the Asia-pacific region. Gut Liver. 2016;10:332–339.
2. Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90.
3. Worns MA, Weinmann A, Schuchmann M, et al. Systemic therapies in hepatocellular carcinoma. Digestive Dis. 2009;27:175–188.
4. Yau T, Chan P, Epstein R, et al. Evolution of systemic therapy of advanced hepatocellular carcinoma. World J Gastroenterol. 2008;14:6437–6441.
5. Yau T, Chan P, Epstein R, et al. Management of advanced hepatocellular carcinoma in the era of targeted therapy. Liver Int. 2009;29:10–17.
6. Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–1255.
7. Ho CM, Hu RH, Lee PH, et al. Long-term survival in patients with t2 hepatocellular carcinoma after primary curative resection can be further stratified by tumor size. Medicine. 2014;93:e203.
8. European Association For The Study Of The L, European Organisation For R, Treatment Of C. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943.
9. Cao H, Phan H, Yang LX. Improved chemotherapy for hepatocellular carcinoma. Anticancer Res. 2012;32:1379–1386.
10. Park KW, Park JW, Choi JI, et al. Survival analysis of 904 patients with hepatocellular carcinoma in a hepatitis b virus-endemic area. J Gastroenterol Hepatol. 2008;23:467–473.
11. Rhodes DR, Kalyana-Sundaram S, Mahavisno V, et al. Oncomine 3.0: genes, pathways, and networks in a collection of 18,000 cancer gene expression profiles. Neoplasia. 2007;9:166–180.
12. Basak S, Jacobs SB, Krieg AJ, et al. The metastasis-associated gene prl-3 is a p53 target involved in cell-cycle regulation. Mol Cell. 2008;30:303–314.
13. Cox J, Weinman S. Mechanisms of doxorubicin resistance in hepatocellular carcinoma. Hepatic Oncol. 2016;3:57–59.
14. Lohitesh K, Chowdhury R, Mukherjee S. Resistance a major hindrance to chemotherapy in hepatocellular carcinoma: an insight. Cancer Cell Int. 2018;18:44.
15. Bessette DC, Wong PC, Pallen CJ. Prl-3: a metastasis-associated phosphatase in search of a function. Cells Tissues Organs. 2007;185:232–236.
16. Hardy S, Kostantin E, Hatzihristidis T, et al. Physiological and oncogenic roles of the prl phosphatases. FEBS J. 2018;285:3886–3908.
17. Al-Aidaroos AQ, Zeng Q. Prl-3 phosphatase and cancer metastasis. J Cell Biochem. 2010;111:1087–1098.
18. Laurent C, Valet F, Planque N, et al. High ptp4a3 phosphatase expression correlates with metastatic risk in uveal melanoma patients. Cancer Res. 2011;71:666–674.
19. Zeng Q, Dong JM, Guo K, et al. Prl-3 and prl-1 promote cell migration, invasion, and metastasis. Cancer Res. 2003;63:2716–2722.
20. Zhou J, Chong PS, Lu X, et al. Phosphatase of regenerating liver-3 is regulated by signal transducer and activator of transcription 3 in acute myeloid leukemia. Exp Hematol. 2014;42(1041–1052):e1041–e1042.
21. Brocke-Heidrich K, Kretzschmar AK, Pfeifer G, et al. Interleukin-6-dependent gene expression profiles in multiple myeloma ina-6 cells reveal a bcl-2 family-independent survival pathway closely associated with stat3 activation. Blood. 2004;103:242–251.
22. Chong PSY, Chng WJ, de Mel S. Stat3: a promising therapeutic target in multiple myeloma. Cancers. 2019;11:731.
23. Chong PSY, Zhou J, Lim JSL, et al. Il6 promotes a stat3-prl3 feedforward loop via shp2 repression in multiple myeloma. Cancer Res. 2019;79:4679–4688.
24. Zhou J, Bi C, Chng WJ, et al. Prl-3, a metastasis associated tyrosine phosphatase, is involved in flt3-itd signaling and implicated in anti-aml therapy. PLoS One. 2011;6:e19798.
25. Wang L, Liu J, Zhong Z, et al. Ptp4a3 is a target for inhibition of cell proliferatin, migration and invasion through akt/mtor signaling pathway in glioblastoma under the regulation of mir-137. Brain Res. 2016;1646:441–450.
26. Mayinuer A, Yasen M, Mogushi K, et al. Upregulation of protein tyrosine phosphatase type iva member 3 (ptp4a3/prl-3) is associated with tumor differentiation and a poor prognosis in human hepatocellular carcinoma. Ann Surg Oncol. 2013;20:305–317.
27. Vandsemb EN, Bertilsson H, Abdollahi P, et al. Phosphatase of regenerating liver 3 (prl-3) is overexpressed in human prostate cancer tissue and promotes growth and migration. J Transl Med. 2016;14:71.
28. Chen CY, Chung IH, Tsai MM, et al. Thyroid hormone enhanced human hepatoma cell motility involves brain-specific serine protease 4 activation via erk signaling. Mol Cancer. 2014;13:162.
29. Chow EK, Fan LL, Chen X, et al. Oncogene-specific formation of chemoresistant murine hepatic cancer stem cells. Hepatology. 2012;56:1331–1341.
30. Ma S, Lee TK, Zheng BJ, et al. Cd133+ HCC cancer stem cells confer chemoresistance by preferential expression of the akt/pkb survival pathway. Oncogene. 2008;27:1749–1758.
31. Roessler S, Jia HL, Budhu A, et al. A unique metastasis gene signature enables prediction of tumor relapse in early-stage hepatocellular carcinoma patients. Cancer Res. 2010;70:10202–10212.
32. Ng KT, Lo CM, Guo DY, et al. Identification of transmembrane protein 98 as a novel chemoresistance-conferring gene in hepatocellular carcinoma. Mol Cancer Ther. 2014;13:1285–1297.
33. Burridge K, Sastry SK, Sallee JL. Regulation of cell adhesion by protein-tyrosine phosphatases. I. Cell-matrix adhesion. J Biol Chem. 2006;281:15593–15596.
34. Werner SR, Lee PA, DeCamp MW, et al. Enhanced cell cycle progression and down regulation of p21(cip1/waf1) by prl tyrosine phosphatases. Cancer Lett. 2003;202:201–211.
35. den Hollander P, Rawls K, Tsimelzon A, et al. Phosphatase ptp4a3 promotes triple-negative breast cancer growth and predicts poor patient survival. Cancer Res. 2016;76:1942–1953.
36. Zhou J, Cheong LL, Liu SC, et al. The pro-metastasis tyrosine phosphatase, prl-3 (ptp4a3), is a novel mediator of oncogenic function of bcr-abl in human chronic myeloid leukemia. Mol Cancer. 2012;11:72.
37. Liang F, Liang J, Wang WQ, et al. Prl3 promotes cell invasion and proliferation by down-regulation of csk leading to src activation. J Biol Chem. 2007;282:5413–5419.
38. Mertens C, Darnell JE. Snapshot: jak-stat signaling. Cell. 2007;131:612.
39. Johnson DE, O’Keefe RA, Grandis JR. Targeting the il-6/jak/stat3 signalling axis in cancer. Nat Rev Clin Oncol. 2018;15:234–248.
40. Zeng Q, Si X, Horstmann H, et al. Prenylation-dependent association of protein-tyrosine phosphatases prl-1, −2, and −3 with the plasma membrane and the early endosome. J Biol Chem. 2000;275:21444–21452.
41. Fiordalisi JJ, Keller PJ, Cox AD. Prl tyrosine phosphatases regulate rho family GTPases to promote invasion and motility. Cancer Res. 2006;66:3153–3161.
42. Wang H, Quah SY, Dong JM, et al. Prl-3 down-regulates PTEN expression and signals through pi3k to promote epithelial-mesenchymal transition. Cancer Res. 2007;67:2922–2926.
43. Ostman A, Hellberg C, Bohmer FD. Protein-tyrosine phosphatases and cancer. Nat Rev Cancer. 2006;6:307–320.
44. Bollu LR, Mazumdar A, Savage MI, et al. Molecular pathways: targeting protein tyrosine phosphatases in cancer. Clin Cancer Res. 2017;23:2136–2142.
45. Fiordalisi JJ, Dewar BJ, Graves LM, et al. Src-mediated phosphorylation of the tyrosine phosphatase prl-3 is required for prl-3 promotion of rho activation, motility and invasion. PLoS One. 2013;8:e64309.
46. Walls CD, Iliuk A, Bai Y, et al. Phosphatase of regenerating liver 3 (prl3) provokes a tyrosine phosphoproteome to drive prometastatic signal transduction. Mol Cell Proteomics. 2013;12:3759–3777.
47. Hu T, Li C. Convergence between wnt-beta-catenin and egfr signaling in cancer. Mol Cancer. 2010;9:236.
48. Guo K, Li J, Tang JP, et al. Catalytic domain of prl-3 plays an essential role in tumor metastasis: formation of prl-3 tumors inside the blood vessels. Cancer Biol Ther. 2004;3:945–951.
49. Ahn JH, Kim SJ, Park WS, et al. Synthesis and biological evaluation of rhodanine derivatives as prl-3 inhibitors. Bioorg Med Chem Lett. 2006;16:2996–2999.
50. Beekman R, Valkhof M, Erkeland SJ, et al. Retroviral integration mutagenesis in mice and comparative analysis in human aml identify reduced ptp4a3 expression as a prognostic indicator. PLoS One. 2011;6:e26537.
51. Souissi I, Ladam P, Cognet JA, et al. A stat3-inhibitory hairpin decoy oligodeoxynucleotide discriminates between stat1 and stat3 and induces death in a human colon carcinoma cell line. Mol Cancer. 2012;11:12.
52. Al Zaid Siddiquee K, Turkson J. Stat3 as a target for inducing apoptosis in solid and hematological tumors. Cell Res. 2008;18:254–267.
53. Beldi-Ferchiou A, Skouri N, Ben Ali C, et al. Abnormal repression of shp-1, shp-2 and socs-1 transcription sustains the activation of the jak/stat3 pathway and the progression of the disease in multiple myeloma. PLoS One. 2017;12:e0174835.
54. Galm O, Yoshikawa H, Esteller M, et al. Socs-1, a negative regulator of cytokine signaling, is frequently silenced by methylation in multiple myeloma. Blood. 2003;101:2784–2788.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.