Back to Journals » Cancer Management and Research » Volume 14
CircRNA6783 Inhibits Cell Proliferation and Promotes Apoptosis in Lung Adenocarcinoma
Authors Mu Y , Xie F, Yang D, Xu G
Received 28 October 2021
Accepted for publication 11 March 2022
Published 22 March 2022 Volume 2022:14 Pages 1229—1236
DOI https://doi.org/10.2147/CMAR.S346616
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Beicheng Sun
Yinyu Mu,1 Fuyi Xie,1 Dongdong Yang,1 Guodong Xu2
1Department of Clinical Laboratory, Ningbo Medical Centre, Lihuili Hospital, Ningbo University, Ningbo, Zhejiang, 315040, People’s Republic of China; 2Department of Cardiothoracic Surgery, Ningbo Medical Centre, Lihuili Hospital, Ningbo University, Ningbo, Zhejiang, 315040, People’s Republic of China
Correspondence: Yinyu Mu; Fuyi Xie, Department of Clinical Laboratory, Ningbo Medical Centre Lihuili Hospital, Ningbo University, 57 Xingning Road, Ningbo, Zhejiang, 315040, People’s Republic of China, Email [email protected]; [email protected]
Purpose: Circular RNA (circRNA) serves an important role in tumour genesis and development. But, little is known about its role in lung adenocarcinoma (LA). This study aimed to investigate circRNA6783 expression in peripheral whole blood (PWB) of LA and controls and explore its effect on proliferation and apoptosis in human lung adenocarcinoma cells (LAC).
Patients and Methods: The levels of circRNA6783 in LA cell lines and peripheral whole blood (PWB) of 40 patients and 30 controls were detected by real-time quantitative reverse transcription-polymerase chain reaction (RT-PCR). In order to explore the effect of circRNA6783 on LA behavior, we overexpressed circRNA6783 in NCI-H1975 cells. The impact on the proliferation of tumor cells was then examined by Cell Counting Kit 8 (CCK8) assay, and the effects on apoptosis in the cell line were detected using flow cytometry.
Results: The expression levels of circRNA6783 were significantly higher in LA cell lines and PWB (P < 0.05). The diagnostic value of the area under the receiver operating characteristic curve (AUC) was 0.830, with a sensitivity of 60% and specificity of 96.7%. In addition, functional experiments showed that overexpression of circRNA6783 restrained cell proliferation, significantly increased spontaneous apoptosis.
Conclusion: CircRNA6783 was upregulated in LA PWB. In vitro assessment demonstrated that circRNA6783 could act as a potential biomarker for LA diagnosis.
Keywords: lung adenocarcinoma, circular RNA6783, proliferation, apoptosis
Graphical Abstract:
Introduction
Lung cancer is the most common malignant tumor with a high mortality rate in the world.1 Although it can be comprehensively treated by operation, chemotherapy, radiotherapy and targeted treatment, the 5-year survival rate is only about 15%.2 LA is the most common pathological type in lung cancer.3 The prognosis of LA depends on early detection, early diagnosis and early treatment. The gold standard for the diagnosis of LA is biopsy, but this method is invasive and high cost. So, it is an urgent need to find potential non-invasive diagnostic methods for LA. Although some biomarkers such as CEA and CA199 are used to screen LA, which have weak sensitivity or specificity for early LA. Therefore, it is more important to study the pathogenesis and find new molecular targets for diagnosis and treatment for LA.
CircRNA is a novel class of endogenous non-coding RNA molecule. CircRNA is characterized by good expression, preservation and stability in the cytoplasm, and mammalian cells show tissue-specific and development-specific expressions.4–6 Therefore, they are more suitable as biomarkers than other types of RNA. Recently, many studies have reported that circRNAs serve an important role in the development of cancer, acting as therapeutic and prognostic factors for lung cancer.7–10 Other reports have also shown that circRNAs play a major role in LA, involving tumour-related pathways.11–13 Nevertheless, little is known about the circRNA6783 in human LA.
CircRNA6783 has 644 nt in spliced sequence length. The gene is located in chr2: 58084089-58089723, which is produced by the exon of the VRK2 gene. CircRNA6783 was selected for this study, based on the finding of our earlier circRNA study14 where circRNA6783 showed high expression in LA patients compared with control group. The current study aimed to investigate the expression level of circRNA6783 and further studied the biological function of circRNA6783.
Patients and Methods
Clinical Sample Collections
Samples were drawn from 40 lung adenocarcinoma cases and 30 healthy control cases at Ningbo Medical Center Lihuili Hospital from October 1, 2018 to May 31, 2021. The experiments were approved by the Ethics Committee of Ningbo Medical Centre Lihuili Hospital.The specimens were stored at −80°C until use. Table 1 lists the characteristics of study population. Patients complied with the following criteria: (1) a pathologic diagnosis of LA; (2) no any other treatment before surgery; (3) no previous history of cancer and (4) no chemotherapy or radiotherapy. All patients were staged based on the International Association for the Study of Lung Cancer (IASLC) Tumor-Node-Metastasis (TNM) classification, 8th edition.15
 |
Table 1 Pathological Characteristics of Study Population (n=70) |
Cell Culture
Human LA NCI-H1975 cell line (20180612-06, OBIO, Shanghai) and human bronchial epithelial cell (BEAS-2B) were purchased from OBiO Technology (Shanghai) Corp., Ltd. ((Shanghai, China).Shanghai, China). NCI-H1975 cells were cultured in 1640 medium (C11875500BT, Gibco, Thermo Fisher Scientific, Inc.), supplemented with 10% fetal bovine serum (FBS)(10270-106, GIBCO), 1μg/mL puromycin. The cell lines were incubated at 37 °C with 5% CO2. BEAS-2B cultures were maintained in BEGM medium (Lonza, Cobioer). All cells were cultured at 37 °C with 5% CO2.
Transfection
The circRNA6783 cDNA plasmid (pcDNA3.1)(H20123, OBIO, Shanghai) was first developed by OBiO Technology (Shanghai) Corp., Ltd. (Shanghai, China), then the lentiviruses expressing circRNA6783 and negative control were subsequently constructed by OBiO Technology (Shanghai) Corp., Ltd. (Shanghai, China). The NCI-H1975 cells were collected and transplanted into 6-well plates. The seeded cells were incubated with circRNA6783 lentiviruses 24 h later. The multiplicity of infection [MOI] is 10 for NCI-H1975 cells. After 48 h transfection, the NCI-H1975 cells were again cultured with free medium containing 5 μg/mL polybrene. After 14 days, the stably transfected cells were collected for further study.
RNA Isolation
Trizol (15596026, Invitrogen, USA) was used to extract total RNA from LA cell lines and PWB from LA and control groups, according to the manufacturer’s instructions. The concentration of RNA was detected by OD A260/A280, and RNA concentration was measured using Scandrop100 (Analytikjena, Germany). The extracted RNA was stored at −80°C until use.
RT-PCR Assay
RT-PCR was explored by the SYBR Green PCR kit (DBI-2073/2043, DBI, Germany) by applying a QTOWER 2.2 (Analytikjena, Germany). CDNA was obtained by the PrimeScript First Strand cDNA Synthesis kit (PC1802, Aidlab Biotechnologies Co., Ltd, China) according to the manufacturer’s instructions. The PCR primers used in this study were listed in Table 2. Related expression levels of circRNAs were normalized to cel-miR-39-5p. RT-PCR reactions were as following: initial denatured at 95°C for 3 min, followed by 39 circles of denaturation at 95°C for 10 sec, annealing at 58°C for 30 sec, final extension at 79°C for 4 sec. The expression levels of circRNA6783 were calculated by the 2−ΔΔCt method.
 |
Table 2 Primer Sequences |
Cell Proliferation Assay
CCK8 assay was performed to assess cell proliferation (40203ES76, Yusheng, China). The transinfected Lung Adenocarcinoma cells were transferred to 96-well plates with a density of 2000 cells every well, which were stored in DMEM, with 10% fetal bovine serum, and cultivated for 24 h, 48 h and 72 h at 37°C with 5% CO2. Next, cells were incubated with CCK8 solution (10 µL) for 2 h at 37°C. Then, cell proliferation was analyzed at 450nm by automatic enzyme-linked immune detector (Spark 10M, TECAN, Switzerland) after 2 h incubation.The values were the average of six independent experiments.
Cell Apoptosis Assay
The transfected cells were collected and divided into two groups: NCI-1975 GL109 control and NCI-1975 H17806 over expression. The Annexin V-APC/7-AAD apoptosis detection kit (KGA1026, KeyGEN, Nanjing, China) was used to detect apoptotic cells with Annexin V-APC and 7-AAD double staining according to the manufacturer’s instructions. The experiment was explored using a flow cytometer (ACEA NovoCyte, ACEA). The experiment was repeated three times.
Statistical Analysis
All data were presented as mean ± SD. Student’s t-test or χ2 was used in the present study. Two-way analyses of variance were used to compare the differences among multiple groups. The ROC curve was drawn to evaluate the diagnostic efficacy. The data were analyzed with GraphPad Prism 7.0 (GraphPad Software, Inc.) and SPSS version 23.0 software (SPSS Inc., Chicago, IL, USA). P-value less than 0.05 was regarded as statistically significant.
Results
CircRNA6783 Expression Was Increased in LA PWB and Line Cells
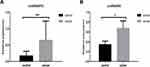
We used RT-PCR to detect circRNA6783 expression level in LA cell lines and PWB. We first detected circRNA6783 expression level in LA PWB. CircRNA6783 expression level was significantly higher in LA PWB than that in control PWB (P<0.05, Figure 1B and Table 3). Next, we found that circRNA6783 expression level was increased in NCI-1975 cells, comparing with that in human lung epithelial BEAS-2B cells (P<0.001, Figure 1A).
 |
Table 3 The Levels of CircRNA6783 in Patients with Lung Adenocarcinoma |
Diagnostic Values of CircRNA6783 in LA
A ROC curve was drawn for differentiating LA from controls to further evaluate the diagnostic value of circRNA6783 in LA. The ROC was to 0.830 (95% confidence interval (CI) = 0.731–0.929, P< 0.001, Figure 2), with the sensitivity of 60% and specificity of 96.7%.
 |
Figure 2 The ROC curve of circRNA6783. The area under the ROC curve (AUC) was to 0.830, 95% confidence interval (CI) = 0.731-0.929, P<0.001. |
CircRNA6783 Inhibits Proliferation in LA Line Cells
The CCK8 assays were performed to study the effect of circRNA6783 on the proliferation of LA line cells. The expression of circRNA6783 was significantly increased in line cells after transfection with circRNA6783 by qRT-PCR (P< 0.001, Figure 3A). Results of the CCK8 assay showed that overexpression circRNA6783 significantly inhibited NCI-1975 cell proliferation, comparing with the two control groups (P< 0.001, Figure 3B).
CircRNA6783 Promotes Apoptosis in LA Line Cells
After overexpression of circRNA6783, the apoptosis rates of NCI-1975 GL109 and NCI-1975 H17806 cells were detected by flow cytometry. As shown in Figure 4, the apoptosis rates were 3.8±0.1% in the NCI-1975 GL109 control groups, and significantly increased to 8.6±0.4% in the NCI-1975 H17806 overexpression groups (P <0.001).
Discussion
CircRNAs are more stable in mammalian cells, comparing to microRNAs and long non-RNAs. This property makes circRNA an ideal biomarker for human diseases.4,16,17 So, it is very attractive for researchers to study circRNA as a biomarker for patients with different diseases.18–22 Our previous studies have also shown that the evidence of circRNA in the diagnosis of LA.14 In this study, we further found levels of circRNA6783 in PWB and cell lines were higher comparing with the controls and had a high sensitivity (60%) and specificity (96.7%) in differentiating patients and controls. This indicated circRNA6783 could be used as a high potential and noninvasive diagnostic biomarker for LA.
Role of circRNAs in tumours development is not very clear. Currently most reports are that they act as miRNA sponges to regulate gene expression.23–27 For example, circSHKBP1 suppresses gastric cancer progression through serving as the sponge of oncogenic miR-582-3p to suppresses HSP90 degradation.28 Circ-AASDH acts as a sponge for miR-140-3p regulates LUAD cells by inhibiting the proliferation and migration].29 However, biological functions of the circRNA6783 have not been reported. In our previous study, we first verified that circRNA6783 could be highly expressed in peripheral blood. Then, we found that circRNA6783 might be targeted with hsa-miR-770-5p, hsa-miR-4712-5p, hsa-miR-4436b-5p, hsa-miR-1286 and hsa-miR-6839-5p by bioinformatics prediction.14 In this study, we confirmed that overexpression of circRNA6783, the cell proliferation ability was significantly inhibited and the proportion of apoptosis was significantly increased. The above results show that circRNA6783 plays a key role in the process of cell proliferation and apoptosis of LA. So, it also suggests that circRNA6783 will be an important marker and a new target for the diagnosis and treatment of LA in the future. Nevertheless, the exact molecular mechanism of circRNA6783 to inhibit cell proliferation and promote cell apoptosis needs to be further investigated.
Of course, our research also has some limitations. Firstly, the number of samples is limited. We will expand the number of copies in future investigations. Secondly, the specific miRNA that interacts with circRNA6783 to regulate the target genes remains unclear, and related binding proteins have not been explored. Therefore, these limitations will be the focus of further studies.
Conclusion
All in all, the results showed that the expression of circRNA6783 in LA was up- regulated and circRNA6783 inhibited the cell proliferation and promoted cell apoptosis. These results suggested that circRNA6783 might be used as a potential biomarker for LA.
Abbreviations
circRNA, circular RNA; PWB, peripheral whole blood; LA, lung adenocarcinoma; ROC, receiver operating characteristic; NSCLC, non-small cell lung cancer; SCLC, small cell lung cancer; RT-PCR, real-time quantitative reverse transcription-polymerase chain reaction; AUC, area under the curve; FBS, fetal bovine serum; CCK8, cell counting Kit 8; BEAS-2B, human bronchial epithelial cell.
Data Sharing Statement
The data used and analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
This study was reviewed and approved by the ethics committee of Ningbo Li Huili Hospital, and informed consent was obtained from all participants. This study was conducted in accordance with the Declaration of Helsinki.
Acknowledgments
This work is supported by Nature Science Foundation of Ningbo City (Grant No. 2019A610230); Zhejiang Province Medical and Health Project (Grant No. 2019KY606); Zhejiang Province Medical and Health Project (Grant No. 2019319634); Basic Public Welfare Project of Ningbo (Grant No. 2019C50041).
Disclosure
The authors declare that they have no competing interests.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. doi:10.3322/caac.21590
2. Siegel RL, Miller KD, Fuchs HE. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. doi:10.3322/caac.21654
3. Li P, Shi JX, Dai LP, et al. Serum anti-MDM2 and anti-c-Myc autoantibodies as biomarkers in the early detection of lung cancer. Oncoimmunology. 2016;5(5):e1138200. doi:10.1080/2162402X.2016.1138200
4. Goodall GJ, Wickramasinghe VO. RNA in cancer. Nat Rev Cancer. 2021;21(1):22–36. doi:10.1038/s41568-020-00306-0
5. Wang SM, Zhang K, Tan SY, et al. Circular RNAs in body fluids as cancer biomarkers: the new frontier of liquid biopsies. Mol Cancer. 2021;20(1):13. doi:10.1186/s12943-020-01298-z
6. Patop IL, Wüst S, Kadener S. Past. present, and future of circRNAs. EMBO J. 2019;38(16):e100836. doi:10.15252/embj.2018100836
7. Fan CM, Qu HK, Xiong F, et al. CircARHGAP12 promotes nasopharyngeal carcinoma migration and invasion via ezrin-mediated cytoskeletal remodeling. Cancer Lett. 2021;496(1):41–56. doi:10.1016/j.canlet.2020.09.006
8. Li BT, Zhu L, Lu CL, et al. circNDUFB2 inhibits non-small cell lung cancer progression via destabilizing IGF2BPs and activating anti-tumor immunity. Nat Commun. 2021;12(1):295. doi:10.1038/s41467-020-20527-z
9. Mao Y, He JX, Zhu M, et al. Circ0001320 inhibits lung cancer cell growth and invasion by regulating TNFAIP1 and TPM1 expression through sponging miR-558. Hum Cell. 2021;34(2):468–477. doi:10.1007/s13577-020-00453-4
10. Liu HL, Lan T, Li H, et al. Circular RNA circDLC1 inhibits MMP1-mediated liver cancer progression via interaction with HuR. Theranostics. 2021;11(3):1396–1411. doi:10.7150/thno.53227
11. Mu Y, Liu WJ, Bie LY, et al. Blocking VRK2 suppresses pulmonary adenocarcinoma progression via ERK1/2/AKT signal pathway by targeting miR-145-5p. Riv Eur Sci Med Farmacol. 2021;25(1):145–153. doi:10.26355/eurrev_202101_24378
12. Lu T, Qiu T, Han B, et al. Circular RNA circCSNK1G3 induces HOXA10 signaling and promotes the growth and metastasis of lung adenocarcinoma cells through hsa-miR-143-3p sponging. Cell Oncol (Dordrecht). 2021;44(2):297–310. doi:10.1007/s13402-020-00565-x
13. Ye GC, Liu YF, Huang L, et al. Key microRNAs and hub genes associated with poor prognosis in lung adenocarcinoma. Aging. 2021;13(3):3742–3762. doi:10.18632/aging.202337
14. Mu YY, Xie FY, Huang YF, et al. Circular RNA expression profile in peripheral whole blood of lung adenocarcinoma by high: throughput sequencing. Medicine. 2019;98(42):e17601. doi:10.1097/MD.0000000000017601
15. Asamura H, Chansky K, Crowley J, et al. The International Association for the Study of Lung Cancer Lung Cancer Staging Project: proposals for the Revision of the N Descriptors in the Forthcoming 8th Edition of the TNM Classification for Lung Cancer.Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. J Thorac Oncol. 2015;10(12):1675–1684. doi:10.1097/JTO.0000000000000678
16. Tang WW, Fu K, Sun HD, et al. CircRNA microarray profiling identifies a novel circulating biomarker for detection of gastric cancer. Mol Cancer. 2018;17(1):137. doi:10.1186/s12943-018-0888-8
17. Li SC, Sun X, Miao SC, et al. Hsa_circ_0000729, a potential prognostic biomarker in lung adenocarcinoma. Thorac Cancer. 2018;9(8):924–930. doi:10.1111/1759-7714.12761
18. Wang JF, Zhao XH, Wang YB, et al. circRNA-002178 act as a ceRNA to promote PDL1/PD1 expression in lung adenocarcinoma. Cell Death Dis. 2020;11:32. doi:10.1038/s41419-020-2230-9
19. Li SQ, Li X, Xue W, et al. Screening for functional circular RNAs using the CRISPR-Cas13 system. Nat Methods. 2021;18(1):51–59. doi:10.1038/s41592-020-01011-4
20. Liu G, Du WY, Xu HX, et al. RNA G-quadruplex regulates microRNA-26a biogenesis and function. J Hepatol. 2020;73(2):371–382. doi:10.1016/j.jhep.2020.02.032
21. Li JY, Wei MM, Liu X, et al. The progress, prospects, and challenges of the use of non-coding RNA for diabetic wounds. Mol Ther Nucleic Acids. 2021;24:554–578. doi:10.1016/j.omtn.2021.03.015
22. Wang XX, Xue BJ, Zhang JY, et al. Up-regulated circBACH2 contributes to cell proliferation, invasion, and migration of triple-negative breast cancer. Cell Death Dis. 2021;12(5):412. doi:10.1038/s41419-021-03684-x
23. Yu FP, Zhang Y, Wang ZZ, et al. Hsa_circ_0030042 regulates abnormal autophagy and protects atherosclerotic plaque stability by targeting eIF4A3. Theranostics. 2021;11(11):5404–5417. doi:10.7150/thno.48389
24. Cen JJ, Liang YP, Huang Y, et al. Circular RNA circSDHC serves as a sponge for miR-127-3p to promote the proliferation and metastasis of renal cell carcinoma via the CDKN3/E2F1 axis. Mol Cancer. 2021;20(1):19. doi:10.1186/s12943-021-01314-w
25. Chen ZZ, Xu WB, Zhang DG, et al. circCAMSAP1 promotes osteosarcoma progression and metastasis by sponging miR-145-5p and regulating FLI1 expression. Mol Ther Nucleic Acids. 2021;23(3):1120–1135. doi:10.1016/j.omtn.2020.12.013
26. Mao YS, Li WF, Hua B, et al. Circular RNA_PDHX promotes the proliferation and invasion of prostate cancer by sponging MiR-378a-3p. Front Cell Dev Biol. 2020;8(6):602707. doi:10.3389/fcell.2020.602707
27. Yao YS, Zhou YJ, Hua QW. CircRNA hsa_circ_0018414 inhibits the progression of LUAD by sponging miR-6807-3p and upregulating DKK1. Mol Ther Nucleic Acids. 2021;23(3):783–796. doi:10.1016/j.omtn.2020.12.031
28. Xie MY, Yu T, Jing XM, et al. Exosomal circSHKBP1 promotes gastric cancer progression via regulating the miR-582-3p/HUR/VEGF axis and suppressing HSP90 degradation. Mol Cancer. 2020;19(1):112. doi:10.1186/s12943-020-01208-3
29. Wang YY, Wo Y, Lu T, et al. Circ-AASDH functions as the progression of early stage lung adenocarcinoma by targeting miR-140-3p to activate E2F7 expression. Transl Lung Cancer Res. 2021;10(1):57–70. doi:10.21037/tlcr-20-1062
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.