Back to Journals » Cancer Management and Research » Volume 10
CircRNA CDR1as/miR-7 signals promote tumor growth of osteosarcoma with a potential therapeutic and diagnostic value
Authors Xu B , Yang T, Wang Z, Zhang Y, Liu S, Shen M
Received 25 June 2018
Accepted for publication 11 August 2018
Published 23 October 2018 Volume 2018:10 Pages 4871—4880
DOI https://doi.org/10.2147/CMAR.S178213
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Harikrishna Nakshatri
Bo Xu,1,* Tieyi Yang,1 Zhi Wang,1,* Yan Zhang,1 Shuyi Liu,1 Mingquan Shen2
1Department of Orthopaedics, Gongli Hospital of Pudong New Area, Shanghai, 200135, China; 2Department of Orthopaedics, Pudong New Area People’s Hospital affiliated to Shanghai University of Medicine and Health Sciences, Shanghai, 201200, China
*These authors contributed equally to this work
Background: The circular RNA (circRNA) antisense to the cerebellar degeneration-related protein 1 transcript (CDR1as)/micro RNA-7(miR-7) signal axis has been investigated in many diseases via regulation of the target genes of miR-7, which participates in the carcinogenesis and metastasis. However, the clinical role and function of CDR1as/miR-7 pathway in osteosarcoma (OS) remain to be identified.
Materials and methods: Noncancerous bone tissues (n=18) and OS tissues (n=38) were used to determine the expressions and roles of CDR1as and miR-7. We knocked down the expression of CDR1as via siRNAs in OS cell lines to analyze its function in vitro and in vivo.
Results: CDR1as was upregulated in OS tissues with significant diagnostic value (cutoff value: 1.613). OS patients with high tumor size, Enneking stage, and distant metastasis have high CDR1as levels, but the miR-7 as tumor suppressor negatively correlated with CDR1as. Inhibition of CDR1as in OS cell lines U2OS and MG63 with high CDR1as levels, leading to de-repressed miR-7 levels, impaired cell vitality and increased apoptosis and G1/S arrest in parallel with reduced ability of cell migration, which, however, could be restored by miR-7 inhibitor. Mechanistically, knockdown of CDR1as could restore the availability of miR-7 and inhibit the target genes of miR-7 including EGFR, CCNE1, PI3KCD, and RAF1. Moreover, CDR1as also upregulated N-cadherin and inhibited E-cadherin to promote the epithelial–mesenchymal transition via miR-7 for cell migration. CDR1as inhibition in vivo also induced tumor regression with decreased PCNA levels, and miR-7 inhibitor could reverse these effects via upregulation of EGFR, CCNE1, PI3KCD, and RAF1. The expressions of these genes were confirmed to be higher in CDR1as-high OS samples than in CDR1as-low OS samples.
Conclusion: These findings suggested that the CDR1as/miR-7 signal axis could be the molecular target for the treatment of OS.
Keywords: CDR1as, miR-7, osteosarcoma, apoptosis, EMT
Corrigendum for this paper has been published
Introduction
As the most common primary malignant bone tumor, osteosarcoma (OS) is a leading cause of cancer-related death among children and adolescents with an annual estimated incidence of about 4 million worldwide. The peak age of OS patients is predominantly from 10 to 20 years.1 Due to the introduction of combinatorial surgical resection with chemotherapy, the prognosis of OS in younger patients has improved markedly from <20% to around 70% at 5 years for patients who present without known metastases. Unfortunately, there are still at least 40% OS patients who respond poorly to chemotherapy, resulting in eventual death due to the recurrence and metastasis of tumors.2,3 Thus, it is indispensable to identify efficient diagnostic markers and critical molecular mechanisms underlying pathogenesis and progression of this disease.
Noncoding RNAs (ncRNAs) constitute the majority of the human transcribed genome with no protein-coding capacity, play diverse roles in a multitude of cellular processes, and have been implicated in many pathological conditions. The ncRNAs comprise a diverse range of RNA species including micro RNA (miRNA) and long ncRNA.4,5 Circular RNAs (circRNAs) are a new class of lncRNAs which do not have 5¢ or 3¢ ends but are covalently linked to form a closed circular structure and regulate gene expression at the transcriptional or posttranscriptional level by binding to miRNAs or other molecules.6 CircRNAs are tissue and cell-type specific, are developmentally regulated, and exist during various human diseases and life processes, such as diabetes development, tissue development, atherosclerotic vascular disease risk, cardiac hypertrophy, and cancer.7,8
Antisense to the cerebellar degeneration-related protein 1 transcript (CDR1as) is the most investigated circRNA which contains 63 binding sites for miR-7.9 Knockdown of CDR1as or overexpression of miR-7 promotes the degradation of miR-7-target mRNAs. Knockdown of the CDR1as/miR-7 signal from the mouse genome leads to displayed impaired sensorimotor gating, a deficit in the ability to filter out unnecessary information associated with neuropsychiatric disorders.9 CDR1as was found to be reduced in the islets of diabetic db/db mice with impaired insulin secretion and reduced β-cell proliferation and survival.10 The CDR1as/miR-7 signal has also been investigated in cancer, including hepatocellular carcinoma, bladder cancer, colorectal carcinoma (CRC), and esophageal squamous cell carcinoma (ESCC).11 For example, CDR1as was significantly upregulated in CRC tissues compared with matched normal mucosae, and its overexpression was associated with poor patient survival and may serve as a therapeutic target for reducing EGFR-RAF1 activity via miR-7 in CRC patients.12 However, the role of CDR1as/miR-7 signal in OS is unclear.
This study investigated the expression of CDR1as/miR-7 signal in OS tissues and identified its potential diagnostic role and function during the growth, apoptosis, and metastasis of OS cells in vitro and in vivo.
Materials and methods
Tumor samples
Ethical approval for the study was obtained from the Ethics Committee of Gongli Hospital of Pudong New Area. The samples used in this study were de-identified due to the subsequent copy, cryopreservation, and storage time, thus the ethics committee agreed to waive the related informed consent in this retrospective study. The OS tissues (n=38) and adjacent non-tumor tissues (n=18) were obtained from patients who had undergone surgical resection of OS in the Gongli Hospital of Pudong New Area according to its ethical and legal standards. The median age was 18 years (range 8–25 years), the median tumor size was 6 cm, and 17 out of 38 patients were found to have distant metastases. The tumor stages were confirmed according to the Enneking staging system.
RNA extraction and RT-qPCR
Total RNA was extracted to determine the expression of CDR1as, miR-7, EGFR, PIK3CD, CCNE1, and RAF1 by using TRIzol® reagent (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions. A260/A280 nm >1.8 was qualified for quantitative analysis. Reverse transcription reaction was carried out to synthesize cDNA. qPCR was performed on 7500 Real-Time PCR detection system (Thermo Fisher Scientific). The expression levels of genes were estimated via 2−ΔΔCt method and were normalized to GAPDH and U6 for gene expression. The primer of genes used in this study are listed in Table 1.
  | Table 1 The primer of genes |
Cell lines and reagents
Five OS cell lines U2OS, MG63, 143B, HOS, and Saos2 cells and one human osteoblastic cell line hFOB1.19 cells were used in this study and were purchased from the cell bank of the Chinese Academy of Sciences (Shanghai, China), and cultured in DMEM (HyClone; Thermo Fisher Scientific (China) Co., Ltd., Shanghai, China) containing 10% FBS (Thermo Fisher Scientific) in an atmosphere of 5% CO2 at 37°C. Three different siRNAs for CDR1as were conducted by RiboBio (Guangzhou, China). The antibodies used in this study including anti-EGFR, PIK3CD, CCNE1, RAF1, and PCNA were obtained from Cell Signaling Technology (Denver, MA, USA) and Santa Cruz Biotechnology Inc. (Dallas, TX, USA). The oligonucleotide sequences of miR-7 mimics, inhibitors, and negative control were purchased from GenePharma (Shanghai, China). The lentivirus vector of siRNA-CDR1as, miR-7 mimics, inhibitor, and negative control was conducted by GeneChem (Shanghai, China). The Renilla luciferase reporter vector psiCHECK2-CDR1as with wild-type or mutant miR-7 binding site was conducted by GenePharma.
Cell transfection
The U2OS and MG63 cell lines were cultured to about 80% confluence in 12/96-well plates and then using Lipofectamine 2000 (Thermo Fisher Scientific) were transfected with indicated agents for 24, 48, or 72 hours according to the manufacturer’s instructions.
Luciferase reporter assay
The U2OS cells were co-transfected containing psiCHECK2-CDR1as and miR-7 mimics. Luciferase activity was measured using the Dual-Luciferase Reporter Assay System (Promega Corporation, Fitchburg, WI, USA). Firefly luciferase acted as a reporter gene for normalized control. The activities of Firefly and Renilla luciferase were evaluated successively by applying dual-luciferase reporter assay system (Promega Corporation) in 48 hours after transfection. All the assays were repeated for at least three times.
CCK-8 assay
The U2OS and MG63 cell lines were transfected with siRNA-CDR1as, miR-7 mimics, inhibitor, and negative control for 24, 48, or 72 hours. Then the cells were harvested and washed with PBS, and then cell counting kit-8 (Dojindo Molecular Technologies, Kumamoto, Japan) mixed with DMEM was used for cell viability assay according to the manufacturer’s instructions. The absorbance was measured at 450 nm by a microplate reader.
Hoechst assay
The U2OS and MG63 cell lines were transfected with siRNA-CDR1as, miR-7 mimics, inhibitor, and negative control for 72 hours. The cells were harvested and incubated with Hoechst 33342 (5 μg/mL; Sigma-Aldrich Co., St Louis, MO, USA) for 10 minutes at room temperature. Following washing with 0.5% TritonX-100 in PBS, the changes in nuclear morphology were observed under a fluorescence microscope (Olympus Corporation, Tokyo, Japan).
Flow cytometry
The cell apoptosis and cell cycle were analyzed by flow cytometry. Briefly, the cells were harvested and washed with PBS. Two microliters of annexin V mixed with 2 µL of propidium iodide (PI; eBioscience, Waltham, MA, USA) was used to stain cells at 4°C for 30 minutes for apoptosis analysis. In addition, the cells were harvested, washed with PBS, and fixed in 70% ethanol at 4°C overnight. After fixation, the cells were washed with PBS before suspension in RNase A/PI solutions (100 mg/mL RNase A and 5 µg/mL PI) and were incubated at room temperature for an hour. The results were analyzed using a flow cytometry provided with the Cell-Quest software.
Transwell assay
At 24 hours after transfection, 2×104 cells were suspended with 200 µL of serum-free medium and then seeded into the upper chambers (8 mm pore size; Costar [Corning Incorporated, Corning, NY, USA]). Medium containing 10% FBS was added to the bottom chamber as a chemoattractant. The cells were incubated at 37°C for 24 hours for the migration assay. Subsequently, cells at the top chamber were removed with cotton swabs, and those on the lower surface were fixed with methanol, stained by crystal violet, and counted under an inverted microscope.
Western blot
To determine the expression of EGFR, PIK3CD, CCNE1, and RAF1, the whole protein extracts were lysed and separated as previously described.13 The blots were visualized using the ECL-Plus reagent (EMD Millipore, Billerica, MA, USA). GAPDH was used as the loading control in the Western blotting.
Immunofluorescence assays and immunohistochemistry
Immunofluorescence assays were performed to determine the expression of EMT markers E-cadherin and N-cadherin as previously described,5 and the coverslips were mounted onto the glass slides with neutral gum and observed under an FV10i confocal microscope (Olympus Corporation). Immunohistochemistry assays were performed to estimate the expression of PCNA on 2-μm-thick, formalin-fixed, paraffin-embedded specimen sections as previously described.14
Tumor model
To investigate the role of CDR1as/miR-7 signal in OS in vivo, U2OS cells were infected with lentivirus-RNAi-siRNA-CDR1as, miR-7 mimics, inhibitor, and negative control (MOI =10). A total of 2×106 U2OS cells were subcutaneously co-injected in the rear flank of nude mice (six per group). Results are presented as the mean tumor size (mm3) (V (cm3) = width2 (cm2)*length (cm)/2) for every group at various time points until the endpoint of the experiment. The animal study was approved by institutional animal research committee of Gongli Hospital of Pudong New Area, and the animals were cared for following the guidelines of Gongli Hospital of Pudong New Area for use and care of laboratory animals.
Statistical analyses
The Prism statistical software package (Version 5.0; GraphPad Software, Inc., La Jolla, CA, USA) was used in this study to perform the statistical analyses. Unpaired t-test or Mann–Whitney U test was used to compare the two groups, and multiple group comparisons were analyzed with one-way ANOVA. P<0.05 was considered statistically significant. All experiments were performed at least three times.
Results
Participation of upregulated CDR1as in the progression of OS
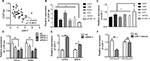
We first investigated the clinical significance of CDR1as in OS. Relative normal bone tissues (n=18) and OS tissues (n=38) were used to estimate the expression of CDR1as, and the results indicated that the CDR1as level was enhanced in OS samples when compared with the noncancerous bone tissues (Figure 1A). The receiver operating characteristic curve analyses showed that the level of CDR1as was a good diagnostic marker to discriminate OS samples from healthy samples, and the cutoff value was 1.613 (AUC =0.857) (Figure 1B). According to the clinicopathological characteristics of OS patients, the CDR1as expression was analyzed. The CDR1as levels were similar in different patients with the age, gender, and tumor localization, but higher CDR1as levels were found in patients with high Enneking stage, tumor size, and pulmonary metastasis (Figure 1C–H). These data indicated that CDR1as was involved in the progression of OS.
MiR-7 as tumor suppressor during the development of OS
Since miR-7 often functions as a tumor suppressor and is inhibited by CDR1as in multi-types of cancer, we investigated the clinical role of miR-7 in OS. The expression pattern of miR-7 was conversed to CDR1as. The miR-7 level was reduced in OS tissues (Figure 2A) and showed a relative poor diagnostic value for OS samples (cutoff value =0.787 and AUC value =0.686) (Figure 2B). The patients’ age, gender, tumor size, and tumor localization showed no relation to the expression of miR-7 (Figure 2C–F). But miR-7 was downregulated in patients with high Enneking stage and metastasis (Figure 2G–H), suggesting the tumor suppressive role of miR-7 in OS.
CDR1as antagonization and inhibition of miR-7 in OS in vitro
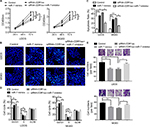
We next aimed to confirm the relationship between CDR1as and miR-7 in OS. The expression of CDR1as negatively correlated with the expression of miR-7 in OS tissues (Figure 3A). In addition, five OS cell lines U2OS, MG63, 143B, HOS, and Saos2 cells and one human osteoblastic cell line hFOB1.19 cells were used to confirm the upregulated CDR1as level and the downregulated miR-7 level in OS cell lines (Figure 3B and C). Knockdown of CDR1as by siRNA-2 in U2OS, MG63 cell lines, which harbored high level of CDR1as, could restore the expression of miR-7 (Figure 3D and E). The luciferase reporter assays were also performed and a luciferase reporter vector of CDR1as with full-length (wild-type or mutant miR-7 binding sites) was co-transfected with miR-7 mimics into U2OS cells (Figure 3F). The results showed that miR-7 mimics could significantly impair the luciferase activity of wild-type reporter, but not the mutant-type. These data indicated that CDR1as could regulate miR-7 in OS.
CDR1as knockdown restricts tumor growth and migration via miR-7
The function of CDR1as/miR-7 signal in OS cells was determined. The U2OS and MG63 cell lines were transfected with miR-7 mimics, siRNA-CDR1as with or without miR-7 inhibitor. The results showed that knockdown of CDR1as and miR-7 mimics could inhibit the cell vitality of OS cells, but the miR-7 inhibitor abrogated the effects of siRNA-CDR1as and restored the cell vitality (Figure 4A). Similarly, the apoptosis and cell cycle were determined by Hoechst assay (Figure 4B) and flow cytometry (Figure 4C and D). The results showed that knockdown of CDR1as and miR-7 mimics promoted apoptosis and G1/S arrest, which could be recovered by the inhibition of miR-7 in two OS cell lines. Moreover, the ability of migration of U2OS and MG63 cell lines was also determined by Transwell assay (Figure 4E). We found that knockdown of CDR1as and miR-7 overexpression significantly impaired the ability of migration, but inhibition of miR-7-inhibited CDR1as knockdown induced migration arrest. These findings demonstrated that CDR1as could promote tumor growth and migration via miR-7 in OS cell lines in vitro.
Upregulation of targeted genes of miR-7 and EMT phenotype by CDR1as
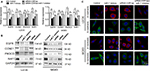
CDR1as has been reported to antagonize the availability of miR-7 via regulation of its target genes. Thus, we here analyzed the miR-7 pathway under the knockdown of CDR1as in two cell lines. The target genes including EGFR, CCNE1, PIK3CD, and RAF1 were involved in cell proliferation, apoptosis, cell cycle, and metastasis. The mRNA (Figure 5A) and protein expressions (Figure 5B) of these target genes were remarkably attenuated by miR-7 mimics and siRNA-CDR1as, but were restored by miR-7 inhibitor in U2OS and MG63 cell lines with CDR1as inhibition, indicating that CDR1as could upregulate these oncogenes via inhibition of miR-7 in OS cells. Moreover, TGF-β-induced EMT phenotype of OS cells was also analyzed (Figure 5C). CDR1as knockdown downregulated epithelial phenotype with reduced E-cadherin and upregulated mesenchymal phenotype with increased N-cadherin, leading to the inhibition of TGF-β-induced EMT. MiR-7 inhibitor could abrogate the CDR1as knockdown-induced restoration of miR-7 and enhanced the EMT phenotype in OS cell lines.
CDR1as inhibition in vivo induces tumor regression via miR-7 pathway
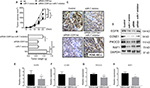
The function of CDR1as/miR-7 signals in vivo during the development of OS was analyzed. The xenograft model of human U2OS cells was established and the nude mice were administered conditional tumor cells with miR-7 overexpression, CDR1as knockdown, or miR-7 inhibition via lentivirus vector. The results showed that mice with CDR1as knockdown and miR-7 mimics had delayed tumor growth (Figure 6A) and lower tumor weight (Figure 6B) than the control mice, but simultaneously inhibition of miR-7 in mice with CDR1as knockdown restored the tumor growth with upregulated expression of proliferation index PCNA (Figure 6C). Meanwhile, the target genes of miR-7 in tumor tissues were also regulated by CDR1as in vivo (Figure 6D).
Furthermore, the expressions of EGFR, CCNE1, PIK3CD, and RAF1 in OS patients were analyzed according to the median expression of CDR1as (>median: CDR1as-High; ≤median: CDR1as-LOW). The results confirmed that patients with high CDR1as levels had high expression of target genes of miR-7 (Figure 6E–H), suggesting that the tumorigenic role of CDR1as/miR-7/target genes pathway in OS.
Discussion
Clinical diagnostic data show that OS is predominantly in the second decade of life and in elderly individuals, and >80% of patients appeared to have pulmonary metastasis with 5-year survival only at 20%. The combination of surgery and chemotherapy for OS treatment by inhibiting cell growth or invasion has been investigated during the last decade. However, the molecular mechanisms involved in OS progress remains to be further elucidated.15,16 In this study, the circRNA CDR1as was significantly upregulated during the development of OS with efficient diagnostic value and promoted the cell growth and migration via antagonizing the tumor suppressor miR-7 to elevate its target genes EGFR, CCNE1, PIK3CD, and RAF1 in OS patients for tumor growth in vitro and in vivo.
Accumulating evidence is now unveiling the importance of circRNA-mediated regulatory mechanisms in cancer pathogenesis. Owing to the lack of free ends and resistance to exonucleolytic degradation, high cellular stability is a common circRNA feature, which underlines the potential of being as diagnostic markers in cancer.11 Circ-CBFB was markedly overexpressed during the progression of chronic lymphocytic leukemia (CLL) and could serve as a diagnostic and prognostic biomarker for CLL patients.17 Circ-ITCH was found to be downregulated in glioma tissues and cell lines and showed a relatively high diagnostic accuracy. Kaplan–Meier assay revealed that decreased circ-ITCH level was associated with poor survival of glioma patients.18 Similarly, we found that the expression of CDR1as was significantly elevated in OS samples with efficient diagnostic value. High expression of CDR1as was found in patients with high tumor size, stage, and metastasis in OS. Although the upregulated CDR1as has been reported in hepatocellular carcinoma, bladder cancer, CRC, and ESCC,11,14 this is the first time we investigated its role in OS.
MiR-7 have been recognized as tumor suppressor to suppress tumor growth. Shi et al reported that miR-7 suppresses cell proliferation and induces apoptosis of breast cancer cells mainly by targeting REGγ.19 miR-7 also attenuated the abilities of invasion and self-renewal of breast cancer stem-like cells to suppress brain metastasis by modulating KLF4.20 In this study, we found that miR-7 was downregulated in OS samples with high tumor stage and metastasis. Recently, the interaction between miR-7 and circRNA CDR1as has been intensively identified. MiR-7 expression was downregulated in hepatocellular carcinoma (HCC) tissues, and knockdown of CDR1as suppressed the HCC cell proliferation and invasion through targeting miR-7 to reduce the expression of CCNE1 and PIK3CD.21 CDR1as could also specifically target miR-7 in non-small-cell lung cancer (NSCLC) to reduce its expressions and increase its target gene RELA expression, implicating that the ciRS-7/miR-7/NF-κB axis could exert pronounced impacts on the proliferation, migration, invasion, and apoptosis of NSCLC cells.22 We here found that the CDR1as/miR-7 signal promoted the cell vitality and inhibited apoptosis and migration via regulation of downstream genes including proliferation, apoptosis, cell cycle, and metastasis-related EGFR, CCNE1, PIK3CD, and RAF1 and suppression of EMT phenotype in OS cells. These studies demonstrated the common tumorigenic role of CDR1as signal in different types of cancer.
Conclusion
We identified that CDR1as was upregulated in OS tissues and predicted a poor clinical outcome. CDR1as was able to promote cell growth and migration, and inhibited miR-7-induced apoptosis and G1/S arrest via restoration of EGFR, CCNE1, PIK3CD, and RAF1 levels and EMT phenotype in OS cells. Targeting the CDR1as/miR-7 signal would be a potential strategy for the treatment of OS.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81772383 to MQS), Key Discipline of Pudong New Area, Shanghai (PWZxk2017-18 to MQS) and Shanghai Municipal Commission of Health and Family Planning (No. 201540396 to ZW).
Disclosure
The authors report no conflicts of interest in this work.
References
Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Cancer Treat Res. 2009;152:3–13. | ||
Moore DD, Luu HH. Osteosarcoma. Cancer Treat Res. 2014;162:5–92. | ||
Bagcchi S. Osteosarcoma survivors’ risk of second cancer. Lancet Oncol. 2014;15(10):e425. | ||
Panwar B, Arora A, Raghava GP. Prediction and classification of ncRNAs using structural information. BMC Genomics. 2014;15:127. | ||
Ding L, Ren J, Zhang D, et al. The TLR3 Agonist Inhibit Drug Efflux and Sequentially Consolidates Low-Dose Cisplatin-Based Chemoimmunotherapy while Reducing Side Effects. Mol Cancer Ther. 2017;16(6):1068–1079. | ||
Chen LL, Yang L. Regulation of circRNA biogenesis. RNA Biol. 2015;12(4):381–388. | ||
Huang C, Shan G. What happens at or after transcription:insights into circRNA biogenesis and function. Transcription. 2015;6(4):61–64. | ||
Yang Z, Xie L, Han L, et al. Circular RNAs: Regulators of Cancer-Related Signaling Pathways and Potential Diagnostic Biomarkers for Human Cancers. Theranostics. 2017;7(12):3106–3117. | ||
Piwecka M, Glažar P, Hernandez-Miranda LR, et al. Loss of a mammalian circular RNA locus causes miRNA deregulation and affects brain function. Science. 2017;357(6357):eaam8526. | ||
Stoll L, Sobel J, Rodriguez-Trejo A, et al. Circular RNAs as novel regulators of β-cell functions in normal and disease conditions. Mol Metab. 2018;9:69–83. | ||
Kristensen LS, Hansen TB, Venø MT, Kjems J. Circular RNAs in cancer: opportunities and challenges in the field. Oncogene. 2018;37(5):555–565. | ||
Weng W, Wei Q, Toden S, et al. Circular RNA ciRS-7-A Promising Prognostic Biomarker and a Potential Therapeutic Target in Colorectal Cancer. Clin Cancer Res. 2017;23(14):3918–3928. | ||
Ding L, Ren J, Zhang D, et al. A novel stromal lncRNA signature reprograms fibroblasts to promote the growth of oral squamous cell carcinoma via LncRNA-CAF/interleukin-33. Carcinogenesis. 2018;39(3):397–406. | ||
Sang M, Meng L, Sang Y, et al. Circular RNA ciRS-7 accelerates ESCC progression through acting as a miR-876-5p sponge to enhance MAGE-A family expression. Cancer Lett. 2018;426:37–46. | ||
Botter SM, Neri D, Fuchs B. Recent advances in osteosarcoma. Curr Opin Pharmacol. 2014;16:15–23. | ||
du X, Yang J, Yang D, Tian W, Zhu Z. The genetic basis for inactivation of Wnt pathway in human osteosarcoma. BMC Cancer. 2014;14:450. | ||
Xia L, Wu L, Bao J, et al. Circular RNA circ-CBFB promotes proliferation and inhibits apoptosis in chronic lymphocytic leukemia through regulating miR-607/FZD3/Wnt/β-catenin pathway. Biochem Biophys Res Commun. 2018;503(1):385–390. | ||
Li F, Ma K, Sun M, Shi S. Identification of the tumor-suppressive function of circular RNA ITCH in glioma cells through sponging miR-214 and promoting linear ITCH expression. Am J Transl Res. 2018;10(5):1373–1386. | ||
Shi Y, Luo X, Li P, et al. miR-7-5p suppresses cell proliferation and induces apoptosis of breast cancer cells mainly by targeting REGγ. Cancer Lett. 2015;358(1):27–36. | ||
Okuda H, Xing F, Pandey PR, et al. miR-7 suppresses brain metastasis of breast cancer stem-like cells by modulating KLF4. Cancer Res. 2013;73(4):1434–1444. | ||
Yu L, Gong X, Sun L, Zhou Q, Lu B, Zhu L. The Circular RNA Cdr1as Act as an Oncogene in Hepatocellular Carcinoma through Targeting miR-7 Expression. PLoS One. 2016;11(7):e0158347. | ||
Su C, Han Y, Zhang H, et al. CiRS-7 targeting miR-7 modulates the progression of non-small cell lung cancer in a manner dependent on NF-κB signalling. J Cell Mol Med. 2018;22(6):3097–3107. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.