Back to Journals » Journal of Inflammation Research » Volume 16
Ciliated Cells Express a Novel Pattern of Brain-Derived Neurotrophic Factor in Allergic Rhinitis
Authors Fang L, Li CH, Zhang Q , Jiang TJ , Liu Y , Shi FP, Yu P, Yu L, Chen AP, Li T , Wan YZ, Shi L
Received 6 March 2023
Accepted for publication 6 June 2023
Published 19 June 2023 Volume 2023:16 Pages 2595—2606
DOI https://doi.org/10.2147/JIR.S407368
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
Li Fang,1,2 Chun-Hao Li,1,3 Qian Zhang,1,3 Tian-Jiao Jiang,1,3 Yuan Liu,1,2 Feng-Po Shi,1,3 Peng Yu,1,3 Liang Yu,1,3 Ai-Ping Chen,1,3 Tao Li,1,3 Yu-Zhu Wan,1,3 Li Shi1,3
1Department of Otolaryngology-Head and Neck Surgery, Shandong Provincial ENT Hospital, Shandong University, Jinan, Shandong, People’s Republic of China; 2Department of Otolaryngology Head & Neck Surgery, The Second People’s Hospital of Shenzhen, Shenzhen, Guangdong, People’s Republic of China; 3Department of Allergy, Shandong Second Provincial General Hospital, Jinan, Shandong, People’s Republic of China
Correspondence: Tao Li; Yu-Zhu Wan, Department of Otolaryngology-Head and Neck Surgery, Shandong Provincial ENT Hospital, Shandong University, Duanxing West Road, Jinan, Shandong, 250033, People’s Republic of China, Tel +86 531 83086279 ; +86 531 83086280, Fax +86 531 87980304, Email [email protected]; [email protected]
Background: Mounting research indicates that brain-derived neurotrophic factor (BDNF), has great potential to increase neuro-hyperresponsiveness and airway resistance in airway allergic disease. The expression level of BDNF has been found to be notably elevated in lung/nasal lavage (NAL) fluid. However, the expression and position of BDNF in ciliated cells with allergic rhinitis remains unclear.
Methods: Nasal mucosal cells were collected from patients with allergic rhinitis (AR) and mice which were performed under different allergen challenge time, then observed the expression and position of BDNF located in ciliated cells through the immunofluorescence staining. Nasal mucosa, serum and NAL fluid were collected also. The expression level of BDNF and IL-4/5/13 were detected by RT-PCR. The expressions of BDNF (in serum and NAL fluid), and total-IgE, ovalbumin sIgE (in serum) were detected by ELISA.
Results: We found that MFI of BDNF in AR group’s ciliated cells was obviously lower than that in the control group, and a negative correlation was discovered between MFI and VAS score. It can be roughly divided into 5 patterns according to its location in the cytoplasm of ciliated cells. In the mouse model, the expressions of BDNF in serum and NAL fluid increased temporarily after allergen stimulation. The MFI of BDNF in ciliated cells displayed an initial increase followed by a subsequent decrease.
Conclusion: Our study shows for the first time that, the expression and localization of BNDF were observed in the human nasal ciliated epithelial cells of allergic rhinitis, and the expression of level was less than the control group under the persistent state of allergy. BDNF expression in ciliated cells was transient increased after allergen stimulation and decreased to normal level after 24h in mouse model of allergic rhinitis. This might be the possible source of the transient increase of BNDF in serum and NAL fluid.
Keywords: allergic rhinitis, BDNF, ciliated cell, NAL fluid
Introduction
It has been reported that allergic rhinitis (AR) affects 20–30% population around the world,1 which disturbs people`s life severely and becomes a major medical burden and disability globally. It`s well known that AR is induced by type 2 helper (Th2) cells, pushing B cells to secret specific immunoglobulin E (IgE) against inhaled allergen.2 With the infiltrate of inflammatory cells, and their secreted inflammatory cytokines like interleukin-4 (IL-4)/interleukin-5 (IL-5)/interleukin-13 (IL-13), the immune microenvironment have changed,3 affecting the normal epithelium growth pattern, including epithelial barrier dysfunction, nasal sensitized, elevation of nasal mucous and also the dysfunction of nasal mucosa cilia cells.3–7 Worldwide researchers have reached a consensus that the function of ciliated cells is disturbed under AR. As found by Liu et al, ciliated cells in the epithelium became sticky integrated, dumping epithelium and even loss in AR patients,8 its mucociliary clearance time (MCCT) was much lower than the normal person,9 this phenomenon may attribute to the development of AR. We observed the changes of ciliated cells in the nasal mucosa, but didn`t detect the deep reason for which factor-induced abnormality of ciliated cells. Some studies indicated that the expressions of FOXJ1/ DNAI1/DNALI1/DNAH9 were dropped in AR patients.10 Neurotrophins in nasal mucosa have an effect on the histopathology of AR, which include both brain-derived neurotrophic factor (BDNF) as well as nerve growth factor (NGF), being secreted by the nasal mucosa.11,12 Upregulated expressions of BDNF and NGF have a positive correlation with nasal symptoms,13 with product in the nasal mucosa, it could release at synapse and influence the sensitivity of nasal nerves.14 In our previous study, we found that the presence of BDNF gene variants elevated the likelihood of developing moderate-to-severe allergic rhinitis.15 Being produced and secreted by eosinophils, BDNF plays several functions in the nasal epithelium, such as affecting the pathogenesis of nasal polyps and influencing the polarization of macrophages in the nasal mucosa. BDNF also can be expressed in endothelial cells, inducing nitric oxide (NO) production, and promoting endothelial cell survival and proliferation.16 In nasal mucosa, BDNF can be expressed in the basal layer of nasal epithelial cells, which may contribute to its differentiation of progenitor/stem cells. Nonetheless, so far few studies focus on BDNF localization in the nasal mucosa, including ciliated cells, goblet cells, and basal cells.
In this study, we try to detect the specific location of BDNF in nasal epithelial cells and explore the expression pattern of BDNF in ciliated cells.
Materials and Methods
Patient Selection
All patient’s data were collected from Shandong Provincial ENT Hospital, China, from 2019-2021.Patients with symptoms of allergic rhinitis (AR) and at least one positive allergy test were included in AR group (n=45), while those without AR symptoms and positive results in all allergy tests were classified as the normal control (NC) group (n=24) (Supplemental Table 1). We collected blood serum, nasal swabs, nasal mucosa, nasal lavage fluid, as well as the visual analogue scale (VAS) of nasal and ocular symptoms, which included six contents: total score, sneezing, runny nose, itchy nose, blockage, and itchy eyes. To ensure accuracy of results, only patients who had not received intranasal steroid or antihistamine treatment for more than two weeks prior to sample collection were incorporated. And ruled out who had acute upper respiratory infection or nasal tumor. We obtained the written consent from all patients and local ethical committee.
Blood Samples
Immuno-CAP (Thermo Fisher Science, Sweden) was used to detected the usual allergen by the same professional physician.a-IgE (NC 19.90 (9.72–48.05); AR 165.00 (50.20–371.00)) had statistically difference in the two group (p<0.0001*). s-IgE included phadiatop: inhaled allergen; d1: house dust mite; d2: Dermatophagoides farina; ex1: animal fur dander; wx5: weed mix etc, under the same standard with the same reagents. Most patients were polysensitive (Supplemental Table 2).
Nasal Swabs and Cytospin
Nasal cytology was studied in eluates from nasal swabs obtained in the middle of inferior turbinate using disposable flocking nasal swabs. Each side of nasal cavity was scraped with a new swab, and two swabs were subsequently processed together. Cell precipitation was obtained after centrifugation, then fixed by 4% paraformaldehyde. Cell suspension was obtained by re-centrifugation. Slides were prepared from cytospin preparations at 800rpm for 5 minutes, then stored at −80°C for further research.
Nasal Lavage Fluid
Nasal lavage fluid were collected by spray method. 2.5 mL sterile saline solutions were slowly sprayed as a fine mist into each nostril using an electronic nasal spray device (AIDEFUER, Guiyang, China), which configured with liquid recovery. The diluted nasal secretions were collected, then centrifuged at 800rpm for 10 min immediately at 4 °C. The supernatant was divided and stored at-80°C for further research.
Nasal Mucosal Tissue
For AR group, samples of nasal mucosal tissue were collected from the middle turbinate of patients undergoing endoscopic sinus surgery for nasal polyps or vidian neurectomy. For NC group, tissue was obtained from patients undergoing endoscopic surgery without nasal symptoms due to trauma, biopsy, or other reasons.
Immunofluorescence
Multiple immunofluorescences staining was performed on cytospin slides. After antigen retrieval and antigen blocking, all slides were co-stained by BDNF (1:400, anti-rabbit BDNF mAb, abcam ab108319), and α-Tubulin (1:500, anti-mouse α-tubulin mAb, abcam ab52866) to check the BDNF localization and expression of the ciliated cells. All primary antibodies were incubated in the dark at 4°C overnight, followed by PBS washing for 3 times at 5 minutes each. Slides were then incubated with fluorescence secondary antibody (1:500, abcam), stained with DAPI (P36931, Invitrogen), and viewed with a fluorescence microscope (Olympus, Japan). To enable quantitative comparison, the gain on the confocal system was maintained constant for all recordings during each experiment. The parameters were as follows: exciting light wavelength was 488nm, and laser intensity was 40mW. Five fields were randomly selected for each sample under a 400x microscope. Nasal ciliated columnar epithelial cells were observed, and cells of each type were counted respectively. Image-Pro Plus 6.0 analysis software (Media Cybernetics, USA) was used to measure the mean fluorescence intensity (MFI) of BDNF in all ciliated epithelial cells of each field.
Allergic Rhinitis Mouse Model
Animals
BALB/c mice (age 8–12 weeks, weight 15–20 g, male/female = 1/1) were purchased from Shandong University Animal Center (Jinan, China) and fed in a specific pathogen-free environment. The animal study was performed after the approval of Shandong Provincial ENT Hospital ethical committee. All animal surgery was conducted under general anesthesia to minimize suffering.
Allergic Sensitization and Airway Allergen Challenges
During the first stage, AR group mice were sensitized by utilizing 3 intraperitoneal injections of ovalbumin (OVA; 25ug per injection; Sigma, Munich, Germany) as well as aluminum hydroxide (250mg per injection; Sigma, Munich, Germany) on days 0, 7, and 14. In the second stage, mice were challenged once daily from day 21 to 30 by intranasal instillation of 20 μL of 1% OVA solution (1% OVA in sterile saline) into both nostrils using a micropipette. NC group mice were given the same operation and volume of sterile saline solution. NC group mice were immediately sacrificed, while those in AR group were sacrificed at 3, 6, 12, and 24 hours after the last challenge on day 30.
Assessment of Nasal Symptoms
Two independent observers counted the number of sneezes, nasal scratching events, and nasal secretion volume during the first 15 minutes following the final intranasal challenge of either OVA or saline.
Sample Collection
After the last challenge, all mice were given an intraperitoneal injection of 10% chloral hydrate sodium at a dose of 0.1g/kg to induce anesthesia. Blood specimens were obtained from the posterior ophthalmic artery of individual mice and centrifuged at 1000×g for 10 min at 4°C. The obtained serum was stored at −80°C freezer for further analysis. Nasal lavage (NAL) fluid was collected as follows: all mice were placed in a supine position and immobilized on an anatomical plate, following which they underwent tracheotomy. A plastic cannula was then inserted through the dissected trachea into the nasopharynx and nasal cavity of the mouse and attached to a syringe. And about 500μL of NAL was drawn from the front nostril by using a miniature aspirator while the nasopharynx was flushed with sterile saline, then was centrifuged at 800rpm for 10 min at 4 °C. Finally, the supernatant was collected and stored at −80°C before analysis. Nasal mucosal tissues were sampled. The mice were decapitated, and their heads were bisected sagittal beside the nasal septum. One section of the nasal mucosal sample was promptly placed in RNAlater for RT-PCR. The remaining samples were lysed with dispase, cell slides were made using a cell centrifuge as described above for further research.
ELISA for BDNF, Mouse Total Immunoglobulin E (aIgE) and Mouse Ovalbumin sIgE
BDNF was measured in NAL fluid of the patients and mice using the Quantikine total BDNF ELISA kit (R&D Systems, Minnesota, USA) according to the manufacturer’s instructions. The detection range was less than 80 pg/mL. Mouse total immunoglobulin E (a-IgE) and Mouse Ovalbumin special immunoglobulin E (s-IgE) were measured in serum of the mice using the Quantikine Mouse IgE ELISA kit (NEOBIOSCIENCE, CHINA) and Mouse Ovalbumin sIgE ELISA kit (MyBioSource, USA) based on the manufacturer’s instructions. The detection limit was also less than 80 pg/mL.
RNA Extraction and Real-Time PCR
In order to determine the mRNA expressions of IL-4/5/13 and BDNF levels, real-time quantitative PCR was conducted. All primers of the study were pre-designed (Shenggong company, Jinan, China), and the primer sequences are listed in Supplementary Table 3. Total RNA was extracted by Trizol (Invitrogen, USA) on the basis of the manufacturer’s instructions. Total RNA 1 µg was reverse transcribed into cDNA through the Prime-Script RT Reagent Reverse Kit (TaKaRa). Subsequently, 1 μg of cDNA was utilized for PCR amplification of IL-4/5/13 and BDNF genes, together with GAPDH as an internal control, following the SYBR Premix Ex TaqII protocol provided by the manufacturer. The comparative 2−ΔΔCt method was used to calculate relative expression levels.
Statistical Analysis
Statistical analysis between the AR and NC groups was conducted using the paired Student’s t-test. ANOVA was performed for comparison among multiple groups of the mouse model.The GraphPad Prism 8.0.0 software was used for graph plotting. All p value of < 0.05 was considered significant and was indicated with asterisks: *Means p< 0.05, **Means p< 0.01,***Means p< 0.001 and ****Means p< 0.0001.
Results
Immunofluorescence Staining of BDNF in Epithelial Ciliated Cells of the Human Nasal Mucosa
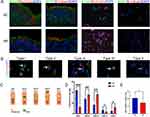
First, the BDNF expression site in the nasal epithelial cells was detected, as shown in Figure 1A. The staining result of nasal brush cytospin slides indicated that BDNF was expressed in ciliated cells mostly. In addition, goblet cells and eosinophils cells also could secret BDNF, among which, eosinophils produced a large amount of BDNF, but goblet cells take a small part in the cytospin slides. Due to the limitation of cytospin slides, we also performed tissue section staining of BDNF in the AR and NC, the results revealed that BDNF could be expressed in the basal and top layer of nasal epithelium. Meanwhile, BDNF-specific staining displayed different localizations in the cytoplasm of ciliated cells, which were divided into five categories (type I-V) (Figure 1B and C). We classified BDNF as mainly expressed in the cytoplasm of ciliated cells at the nucleolar side, presenting a dense band, as type I, accounting for nearly half (46%) of the NC group, but in the AR group, type I only takes part in about 13%, the difference has a statistical significance. Cells with less aggregation area and homogenous staining in the cytoplasm were classified as type II, and homogenous staining in the cytoplasm without aggregation was classified as type III. Type II and type III accounted for 37% and 36% in AR group, but in NC group, these numbers respectively are 27% and 17%. Some cells were mainly located in cilia rather than the cytoplasm (type IV), and the proportion of such cells in NC group (7%) was slightly higher than that in AR group (4%). A part of the cells showed few BDNF expressions (type V), and the proportion of these cells in AR group (10%) was higher than that in NC group (3%) (Figure 1D). In ciliated cells, the fluorescence intensity of BDNF in AR group was significantly lower than that in the NC group. Analyzing the MFI of BDNF in ciliated cells, the difference between the two groups was statistically significant (Figure 1E).
The VAS Scale Was Positively Correlated with NAL BDNF Concentration and Negatively Correlated with MFI of BDNF in Ciliated Cells
NAL was collected and detected by ELISA to evaluate the level of BDNF expression in the nasal cavity. The results revealed that BDNF had a greater concentration in the AR group and statistically differed from NC group (Figure 2A). Additionally, we gathered relevant symptom scores for each individual recruited. And there were statistical differences in the overall scale, sneezing, runny nose, itchy nose, nasal blockage, and itchy eye scales between AR group and NC group (Figure 2B). To explore the relationship between BDNF and nasal symptoms, the correlation analysis was performed, and the result indicated that BDNF concentration in the nasal cavity had a positive correlation with the VAS scale (Figure 2C), but BDNF MFI in ciliated cells had a negative correlation with the VAS scale (Figure 2D). In the mucosa ciliary cells of AR, we found a decreased expression of BDNF, meanwhile, the BDNF concentration in the nasal cavity was upregulated. There was a great chance that ciliated cells secreted BDNF to the nasal cavity contributed to the VAS scale rise.
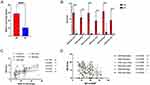
Type 2 Inflammation Cytokines Upregulated in AR and Positively Correlated with BDNF Production
To determine the expression level of type II inflammatory cytokines, Real-time PCR was performed. Shown from Figure 3A–C, the mRNA expressions of IL-4 and IL-13, but not IL-5, were significantly up in AR group. BDNF mRNA level also increased in AR group but have no statistical difference between AR and NC groups (Figure 3D). Correlation between BDNF and type II inflammatory cytokines was also analyzed, as shown in Figure 3E, IL-13 has a positive correlation with BDNF (r= 7128403, p<0.0001). The previous study also indicated that type II inflammatory cytokines could stimulate the expression of BDNF in nasal mucosa,17 the correlation analysis also indicated that with the overexpression of IL-13 or other types II inflammatory cytokines, the BDNF production should be stimulated in nasal epithelial cells, including ciliated cells.
The Expression of BDNF Was Elevated in AR Mouse Model, and Its Expression Pattern is Time Related
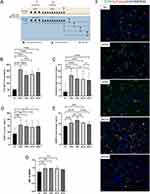
Figure 4A shows the establishment process of AR mouse model. In order to identify the expression pattern of BDNF in allergic rhinitis, we examined its expression level in AR mouse model. IgE levels in serum exert a direct impact on the occurrence of type I allergy, therefore, after 30 day’s provocation, aIgE and ovalbumin sIgE were detected first, and the result revealed that aIgE and ovalbumin sIgE were increased in AR mouse model, their secretion manifested time-related phenomenon. Figure 4B and C demonstrates that following OVA’s provocation, the expression level of aIgE and sIgE swiftly rose, the concentration reaching a peak at 3 hours after allergen stimulation, and then marginally declined at 6 hours, 12 hours, and 24 hours. Compared to NC group, the difference has a statistical significance (P<0.01), the AR mouse model was effective, as evidenced by the increased production of aIgE and sIgE. Then BDNF concentrations were determined at the same time-point, and similarly, BDNF expression levels in the serum of AR mouse models also demonstrated time-related changes, reaching a peak concentration at 3 hours after allergen stimulation and gradually declining over the following 6 hours, 12 hours, and 24 hours, but remaining significantly higher than the NC group (Figure 4D). Additionally, the NAL BDNF concentration of mouse models displayed identical alterations, with the exception that there was no difference between it and the NC group 24 hours after allergen stimulation (Figure 4E). To explore if the AR mouse model has the same expression pattern as nasal ciliated cells in humans, we did immunofluorescence staining, The results showed that the fluorescence intensity of BDNF increased significantly at 3h, 6h, and 12h after allergen excitation, but as time passed, the intensity decreased slightly and approached that of the NC group at 24h (Figure 4F). When the MFI of BDNF of ciliary cells was analyzed, the differences between the two groups were statistically significant (Figure 4G).
Type 2 Inflammation Cytokines Upregulated in the AR Mouse Model and Exhibited a Time-Related Expression Pattern
To analyze type II inflammation cytokines expression level, Real-time PCR was also performed in the mouse model. In mice with allergic rhinitis, we also collected nasal mucosal tissues and detected mRNA levels, correspondingly. Figure 5A shows that IL-4 mRNA levels increased in the AR mouse model, peaked at 6 hours, and then decreased to the same level as the NC group at 24 hours. The mRNA level of IL-5 increased after allergen irritation, with the peak at 3 hours and gradually decreasing with time. At 24 hours, the expression level of IL-5 in AR group had no statistical significance compared to NC group (Figure 5B). IL-13 was significantly increased in AR group, but the expression level decreased heavily at 6 hours then raised again at 12 hours and 24 hours (Figure 5C). As for the mRNA levels of BDNF, they significantly increased at 3 h, 6h, and 12h after allergen stimulation, the peak was at 3 hours and dropped with time, and there was no statistical significance at 24 hours (Figure 5D).
Discussion
As a member of neurotrophic family, BDNF can regulate the immune response of body and affect several cell functions.18,19 Clinical and experimental studies have shown that neurosensory mechanisms are not only active in response to allergic stimuli but also play a critical role in controlling and regulating the allergic response by the release of proinflammatory cytokines and soluble mediators. In allergic disease, BDNF generally are overexpressed in eosinophils (EOS), exerting an anti-apoptosis effect and promoting EOS survival.17 Besides, it can boost the expression levels of proinflammatory cytokines,20 as well as promote tissue remodeling through stimulating the proliferation of airway smooth muscle cells and inducing activation of matrix metalloproteinases.21 In macrophages, BNDF could stimulate M2 macrophage polarization.22 Subsequently, M2 macrophages could secrete IL-10 and TGF-β, which are able to suppress inflammation and improve tissue repair, remodeling and vasculogenesis.23 But on the other hand, tissue remodeling and vasculogenesis may contribute to the persistence of AR symptoms. With the challenge of allergen in AR patients, the NAL BDNF was increased, and the highest level was in the 6 hours after provocation.24 In contrast, based on our research, we found that BDNF production was highly produced after 6 hour’s stimulation in AR mouse model (Figure 4E), furthermore, the BDNF aberrant localization occurred at 6 hours stimulation mostly. We deduced that BDNF may modulate the pathogenesis of AR. In non-allergic diseases, BDNF could enhance the proliferation of epithelial cells, and endothelial cells.25,26 In allergic rhinitis, the epithelial barrier is structurally and functionally disrupted. We found that the expressions of occludin and ZO-1 were lower in AR patients than those in healthy controls, which was related to disease severity.27 The neurogenic and epithelial endotypes are gaining increasing attention due to the growing understanding of the mechanisms, underlying the nervous system’s contribution to inflammation and the role of epithelial tissue in inflammation.28 Some studies found that the type 2 cytokines IL-4 and IL-13 disrupt the epithelial barrier integrity of AR nasal epithelial cells.4 As a crucial factor in the nervous system, BDNF has been shown to influence mucosal immunity and barrier integrity.29 In addition, BDNF could be expressed in the basal cell of nasal epithelium and accelerate the differentiation of progenitor/stem cells. Some studies have shown that the nasal epithelial barrier integrity of AR patients is compromised by type 2 cytokines IL-4 and IL-13.4 Furthermore, BDNF, a significant factor in the nervous system, has been demonstrated to impact mucosal immunity and barrier integrity.29
We discovered that BDNF was downregulated in ciliated cells in AR patients. To confirm this, immunofluorescence staining of BDNF in ciliated cells was performed, and discovered that BDNF has a different expression pattern in ciliated cells. It could be expressed on the nuclear side, as well as in the middle position between the nuclear and cilia, and it can form clumps or spread throughout the cytoplasm. Thus, BDNF was classified into five expression patterns in cilia (Figure 1B), the difference between AR and NC groups was obvious, Type I accounted for the majority of cases in the healthy control group, but type II and type III accounted for the majority of cases in the AR group. This phenomenon was first described, indicating that the aberrant location of BDNF may influence the function of cilia. The previous study suggested that mucociliary clearance time(MCT) was longer in AR patients,7 and ciliated cells expressed abnormal production and localization of FOXJ1 may influence MCT.10 IL-4 and IL-13 are capable of impairing ciliary function of respiratory epithelium, and leading to impaired mucociliary clearance among asthmatic patients.30 Our findings also have aberrant localization of BDNF in AR patients, this phenomenon was also observed in the AR mouse model, suggesting that BDNF may be a crucial factor in ciliated cell function. Our study has revealed the anomalous localization of BDNF in AR patients, which was also observed in AR mouse model. These results imply that BDNF could play a crucial role in ciliated cell function.
In this study, increased NAL BDNF concentration has a positive correlation with the VAS scale, according to the function of BNDF in synapse,14 when the allergen contact with nasal mucosa, the immune response is quickly stated. BDNF stored in the synapse is released rapidly to stimulate nerve transportation and induce the symptom of sneezing and rhinorrhea. In AR nasal cavity, BDNF receptor TrKB was also upregulated, accelerating type II inflammation immune response. Then we discuss the BDNF in the ciliated cell, most studies verified that the BDNF expression level in ciliated cells is not changed or decreased. But the VAS scale in AR was increased, and the symptom of AR patients may not be detected as correlated with BDNF ciliated cells, but the change of BDNF expression pattern in the ciliated cell may contribute to the dysfunction of MCT. The consistently excite substrated secreted by goblet cells or eosinophils will continuously affect nasal mucosa or nasal nerve, leading to AR symptoms persistence. In nasal mucosa, the increased level of BDNF is mostly located in the basolateral cells,31 we also observed this phenomenon in this study. In the AR mouse model, we detected BDNF expression pattern in ciliated cells as well, interestingly, the BDNF production in the AR mouse model showed a time-related phenomenon, the BDNF was upregulated at 3 hours, 6 hours, and 12 hours, but not at 24 hours. That may explain why some research detected that BDNF has no significant difference between AR patients and healthy control.
Conclusion
We firstly investigated the expression pattern of BDNF in the ciliated cell, located in nasal mucosa, and found that there was significant difference of BDNF pattern between AR patients and normal control group. Furthermore, we discovered that the expression level of BDNF decreased in the ciliated cell, which had a negative correlation with the VAS scale. Additionally, we verified the above results in AR mouse model, which indicated that BDNF could be upregulated in the nasal mucosa of AR mouse model and was time dependent. The decreased production of BDNF in ciliated cells may could influence cilia function, cause the disorder of MCT in AR patients.
Ethics Statement
We briefed all participants in detail on the process of sample collection and all participants provided written informed consent following the declaration of Helsinki. All procedures in this study related to human participants and animals were following the ethical standards of the institutional and/or national research committees’ ethical standards. The approval of this study was obtained from the Shandong Provincial ENT Hospital ethical committee (number:KYLL-2016(GJ)P0007 and KYLL-2016(GJ)P0058).
Acknowledgment
We thank all ENT surgeons in the Department of Otolaryngology-Head & Neck Surgery, Shandong Provincial ENT Hospital, Shandong University.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by the National Natural Science Foundation of China (81670909, 81873692); Key R&D Program of Shandong Province (2018CXGC1214); Shandong Provincial Medical and Science Development Project (202107010697).
Disclosure
The authors declare no conflicts of interest.
References
1. Meltzer EO. Allergic rhinitis: burden of illness, quality of life, comorbidities, and control. Immunol Allergy Clin North Am. 2016;36(2):235–248. doi:10.1016/j.iac.2015.12.002
2. Bousquet J, Anto JM, Bachert C, et al. Allergic rhinitis. Nat Rev Dis Primers. 2020;6(1):95. doi:10.1038/s41572-020-00227-0
3. Dainichi T, Kitoh A, Otsuka A, et al. The epithelial immune microenvironment (EIME) in atopic dermatitis and psoriasis. Nat Immunol. 2018;19(12):1286–1298. doi:10.1038/s41590-018-0256-2
4. Steelant B, Seys SF, Van Gerven L, et al. Histamine and T helper cytokine-driven epithelial barrier dysfunction in allergic rhinitis. J Allergy Clin Immunol. 2018;141(3):951–963.e958. doi:10.1016/j.jaci.2017.08.039
5. Van Cauwenberge PB. Nasal sensitization. Allergy. 1997;52(33):7–9. doi:10.1111/j.1398-9995.1997.tb04797.x
6. Tomazic PV, Birner-Gruenberger R, Leitner A, Darnhofer B, Spoerk S, Lang-Loidolt D. Apolipoproteins have a potential role in nasal mucus of allergic rhinitis patients: a proteomic study. Laryngoscope. 2015;125(3):E91–96. doi:10.1002/lary.25003
7. Mikolajczyk M, Janukowicz K, Majewska E, Baj Z. Impact of allergic rhinitis on nasal mucociliary clearance time in children. Int Arch Allergy Immunol. 2019;179(4):297–303. doi:10.1159/000499740
8. Liu J, Liu Y. Nasal ultrastructure ciliates and symptoms changing in rat model of allergic rhinitis. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2010;24(8):365–368.
9. Kirtsreesakul V, Somjareonwattana P, Ruttanaphol S. The correlation between nasal symptom and mucociliary clearance in allergic rhinitis. Laryngoscope. 2009;119(8):1458–1462. doi:10.1002/lary.20146
10. Peng Y, Chen Z, Guan WJ, et al. Downregulation and aberrant localization of forkhead box J1 in allergic nasal mucosa. Int Arch Allergy Immunol. 2018;176(2):115–123. doi:10.1159/000488014
11. Sanico AM, Koliatsos VE, Stanisz AM, Bienenstock J, Togias A. Neural hyperresponsiveness and nerve growth factor in allergic rhinitis. Int Arch Allergy Immunol. 1999;118:154–158. doi:10.1159/000024054.
12. Raap U, Braunstahl GJ. The role of neurotrophins in the pathophysiology of allergic rhinitis. Curr Opin Allergy Clin Immunol. 2010;10:8–13. doi:10.1097/ACI.0b013e328334f5de
13. Raap U, Fokkens W, Bruder M, Hoogsteden H, Kapp A, Braunstahl GJ. Modulation of neurotrophin and neurotrophin receptor expression in nasal mucosa after nasal allergen provocation in allergic rhinitis. Allergy. 2008;63(4):468–475. doi:10.1111/j.1398-9995.2008.01626.x
14. Song M, Martinowich K, Lee FS. BDNF at the synapse: why location matters. Mol Psychiatry. 2017;22(10):1370–1375. doi:10.1038/mp.2017.144
15. Jin P, Andiappan AK, Quek JM, et al. A functional brain-derived neurotrophic factor (BDNF) gene variant increases the risk of moderate-to-severe allergic rhinitis. J Allergy Clin Immunol. 2015;135(6):1486–1493.e1488. doi:10.1016/j.jaci.2014.12.1870
16. Caporali A, Emanueli C. Cardiovascular actions of neurotrophins. Physiol Rev. 2009;89(1):279–308. doi:10.1152/physrev.00007.2008
17. Hahn C, Islamian AP, Renz H, Nockher WA. Airway epithelial cells produce neurotrophins and promote the survival of eosinophils during allergic airway inflammation. J Allergy Clin Immunol. 2006;117(4):787–794. doi:10.1016/j.jaci.2005.12.1339
18. Salvador AFM, Kipnis J. Immune response after central nervous system injury. Semin Immunol. 2022;59:101629. doi:10.1016/j.smim.2022.101629
19. Guo W, Nagappan G, Lu B. Differential effects of transient and sustained activation of BDNF-TrkB signaling. Dev Neurobiol. 2018;78(7):647–659. doi:10.1002/dneu.22592
20. Prakash Y, Thompson MA, Meuchel L, et al. Neurotrophins in lung health and disease. Expert Rev Respir Med. 2010;4(3):395–411. doi:10.1586/ers.10.29
21. Yang YG, Tian WM, Zhang H, Li M, Shang YX. Nerve growth factor exacerbates allergic lung inflammation and airway remodeling in a rat model of chronic asthma. Exp Ther Med. 2013;6(5):1251–1258. doi:10.3892/etm.2013.1284
22. Bi C, Fu Y, Zhang Z, Li B. Prostaglandin E2 confers protection against diabetic coronary atherosclerosis by stimulating M2 macrophage polarization via the activation of the CREB/BDNF/TrkB signaling pathway. FASEB j. 2020;34(6):7360–7371. doi:10.1096/fj.201902055R
23. Shapouri-Moghaddam A, Mohammadian S, Vazini H, et al. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 2018;233(9):6425–6440. doi:10.1002/jcp.26429
24. Castano R, Welman M, Trudeau C, Castellanos L, Maghni K, Malo JL. Specific inhalation challenge with flour induced release of brain-derived neurotrophic factor in nasal fluid. Int Forum Allergy Rhinol. 2014;4(1):49–55. doi:10.1002/alr.21223
25. Paris AJ, Hayer KE, Oved JH, et al. STAT3-BDNF-TrkB signalling promotes alveolar epithelial regeneration after lung injury. Nat Cell Biol. 2020;22(10):1197–1210. doi:10.1038/s41556-020-0569-x
26. Rao F, Wang Y, Zhang D, et al. Aligned chitosan nanofiber hydrogel grafted with peptides mimicking bioactive brain-derived neurotrophic factor and vascular endothelial growth factor repair long-distance sciatic nerve defects in rats. Theranostics. 2020;10(4):1590–1603. doi:10.7150/thno.36272
27. Steelant B, Farré R, Wawrzyniak P, et al. Impaired barrier function in patients with house dust mite-induced allergic rhinitis is accompanied by decreased occludin and zonula occludens-1 expression. J Allergy Clin Immunol. 2016;137(4):1043–1053.e1045. doi:10.1016/j.jaci.2015.10.050
28. Steelant B. Epithelial dysfunction in chronic respiratory diseases, a shared endotype? Curr Opin Pulm Med. 2020;26(1):20–26. doi:10.1097/MCP.0000000000000638
29. Liu S. Neurotrophic factors in enteric physiology and pathophysiology. Neurogastroenterol Motil. 2018;30(10):e13446. doi:10.1111/nmo.13446
30. Gomperts BN, Kim LJ, Flaherty SA, Hackett BP. IL-13 regulates cilia loss and foxj1 expression in human airway epithelium. Am J Respir Cell Mol Biol. 2007;37(3):339–346. doi:10.1165/rcmb.2006-0400OC
31. Jornot L, Lacroix JS, Rochat T. Neuroendocrine cells of nasal mucosa are a cellular source of brain-derived neurotrophic factor. Eur Respir J. 2008;32(3):769–774. doi:10.1183/09031936.00051608
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.