Back to Journals » Drug, Healthcare and Patient Safety » Volume 12
Chemotherapy-Related Adverse Drug Reaction and Associated Factors Among Hospitalized Paediatric Cancer Patients at Hospitals in North-West Ethiopia
Authors Workalemahu G, Abdela OA , Yenit MK
Received 26 March 2020
Accepted for publication 11 October 2020
Published 3 November 2020 Volume 2020:12 Pages 195—205
DOI https://doi.org/10.2147/DHPS.S254644
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Siew Siang Chua
Gashaw Workalemahu,1 Ousman Abubeker Abdela,2 Melaku Kindie Yenit3
1Clinical Pharmacy Service Unit, Enat Primary Hospital, Alemketema, Ethiopia; 2Department of Clinical Pharmacy, School of Pharmacy, College of Medicine and Health Sciences and Comprehensive Specialized Hospital, University of Gondar, Gondar, Ethiopia; 3Department of Epidemiology and Biostatistics, Institute of Public Health, College of Medicine and Health Sciences and Comprehensive Specialized Hospital, University of Gondar, Gondar, Ethiopia
Correspondence: Melaku Kindie Yenit
University of Gondar, Gondar, Ethiopia
, Email [email protected]
Background: One of the prevalent treatment modalities for cancer is chemotherapy. Adverse drug reactions, however, are becoming the world’s major public health problem. More than half (54.5 percent) of cancer patients need hospitalization for further management, in addition to the increased health-care costs of treatment. The aim of this study was to evaluate adverse drug reactions associated with chemotherapy and related factors in hospitalized paediatric cancer patients in Ethiopia’s north-west hospitals.
Methods: From July 1, 2017, to August 13, 2019, a cross-sectional study was carried out among 311 paediatric cancer patients at Gondar Comprehensive University, Specialized Hospital and Felegehiwot referral hospital. The data were entered into Epi Info version 7 and exported for further analysis to Statistical Product and Service Solutions (SPSS). To identify associated variables, both the bi-variate and multi-variate logistic regression analyses were computed. Variables with a P-value of less than 0.05 were considered statistically significant in the multivariate logistic regression analysis.
Results: The overall adverse drug reaction in this study was 41.5 percent ((95% CI: 35.8– 47.2%)). Patients who received concomitant medications were at higher risk of experiencing adverse drug reactions (AOR: 2.60 (95% CI: 1.54– 4.40)), according to the multivariate logistic regression analysis. Similarly, there was a risk of developing adverse drug reactions in patients taking four or more chemotherapy agents (AOR: 2.67 (95% CI: 1.52– 4.68)). In addition, regimens based on etoposide (AOR: 1.99 (95% CI: 0.93– 4.27)), mercaptopurine (AOR: 3.91 (95% CI: 1.06– 14.46)) and doxorubicin (AOR: 2.32 (95% CI: 1.30– 4.15)) were at higher risk for adverse drug reactions in patients.
Conclusion: Adverse drug reactions developed in a significant proportion of the study patients (2 out of 5 patients). Therefore, for pediatric cancer patients on concomitant medications and for patients on etoposide, mercaptopurine and doxorubicin drug regimens, efficient prevention and management of adverse drug reactions should be sought.
Keywords: adverse drug reaction, chemotherapy, cancer, paediatric, Ethiopia
Introduction
Adverse Drug Reaction (ADR) has been defined by the World Health Organization (WHO) as the reaction to a drug that is noxious, unintended and occurs at doses used for disease prophylaxis, diagnosis or treatment.1 ADR has become a major concern for the general public, the medical profession, the pharmaceutical industry and regulatory authorities worldwide.2 It affects the recovery rate of patients and decreases the quality of life.3–6 The effect of drug reaction given for treatment varies from a simple inconvenience to permanent disability and death.7 As a result of its health implications, it has become one of the major causes of morbidity associated with higher hospital admission rates and poor outcomes of treatment. About 6.5–10.9% of admissions have been attributed to ADR worldwide.11
Cancer is a group of diseases characterized by the growth and spread of abnormal cells that are uncontrolled.12 Various treatment modalities, including chemotherapy, radiotherapy, surgery, hormonal, immunological and biological therapy, are available for cancer.12,13 One of the most common treatment modalities is chemotherapy, which is extremely useful for the treatment of cancer.14–16 As a result, it controls the spread of cancer cells, relieves tumor-related symptoms, improves the quality of life and prolongs patient survival.14,17
Although cancer treatment options exist, patients become vulnerable to ADR due to various reasons, including poor adherence to drugs and clinical conditions.18 For a number of formerly fatal malignancies, such as lymphomas and leukemias, chemotherapy treatment is curative. Although anti-cancer drugs are well studied and highly beneficial for better treatment outcomes, when compared to other classes of medications, they are associated with various forms of adverse drug reactions (ADRs).9,19,20 In Jordan, anti-neoplastic drugs (37.6%) followed by immune modulators (14.1%), antibiotics (10.3%) and analgesics (6.6%) were the more common classes of drugs involved in ADRs.21 This is because anti-cancer medicines have a narrow therapeutic window and a highly toxic nature; as a consequence, cells such as the bone marrow, gastrointestinal cell lining and hair follicle are damaged.20,22–24 They therefore induce various forms of ADRs, such as suppression of the bone marrow, hair loss, nausea and vomiting, oral mucositis, hepatotoxicity, and nephrotoxicity.23–25
In different regions of the world, different studies have shown that the prevalence of ADRs during hospitalization varies.26–28 In 11.5% of hospitalized patients in Australia, ADRs occurred, and in 1.7–50.9% of patients in Europe.27 The magnitude of hospitalization associated with ADR ranges from 0.2% to 54.5% worldwide.8 In Europe, adverse drug events accounted for 0.5% to 12.8% of hospital admissions.27 There is a shortage of data in Africa showing drug reactions at either regional or national levels.29,30 There are few studies available within the country, but the national ADR figure is not revealed. A study showed that 67% of patients experienced ADRs in South Africa.31 There is a shortage of data in Africa showing drug reactions at either regional or national levels. There are few studies available within the country, but the national ADR figure is not revealed. Although ADR can be affected by various factors, some of the reported risk factors for adverse drug reactions were gender, age, multiple medications, new medications, type of medication, race, alcohol intake, comorbidity, liver and kidney status, anxiety and the perception of patients.32–35 Studies have also shown that, due to developmental changes affecting the pharmacokinetics of many of the medications used in pediatrics, paediatric patients taking chemotherapy are among the most vulnerable to ADRs.4,9
The World Health Organization (WHO), aware of the burden of the problem, has set up a pharmacovigilance system to detect, assess and prevent adverse drug reactions.5,36,37 Thirty-five African countries, including Ethiopia, cumulatively submitted almost 103,499 ADR cases to the global pharmacovigilance database in this system up to 2015, representing only 0.88% of global ADRs.36 The prevalence of chemotherapy-related ADRs and associated risk factors in paediatric cancer patients in Ethiopia, where self-medication and poor adherence to treatment is high, is not well studied. This study, therefore evaluated the magnitude of ADRs associated with chemotherapy and independent factors among patients with hospitalized pediatric cancer, which is critical for understanding the level of the problem.
Materials and Methods
Study Setting and Design
An institutional based cross-sectional study was conducted at the University of Gondar Comprehensive Specialized Hospital (UoGCSH) and Felegehiwot Referral Hospital (FRH), North West, Ethiopia. The study was conducted from August 16 to September 22, 2019. The hospitals selected are among the oldest hospitals in the country with a range of medical specialists and oncology centers in north-western Ethiopia that provide cancer patients with treatment. In the catchment area, each hospital serves more than 5 million individuals and has in-patient and out-patient service units.
Population and Sampling
All paediatric patients diagnosed in the selected hospitals with any type of cancer were included. The study population was those hospitalized paediatric patients diagnosed with cancer that had taken at least one chemotherapy treatment from July 1, 2017 to August 13, 2019. The study excluded patients who were receiving radiotherapy. Finally, this study included a total of 311 hospitalized paediatric cancer patients that were admitted to the two hospitals.
Variables and Measurement
The dependent variable Adverse Drug Reaction (ADR) is defined as any reaction to a noxious and unintended chemotherapy that occurs during treatment in the usual clinical practice of paediatric cancer patients. The causality of the ADRs was evaluated using the World Health Organization-Uppsala Monitoring Centre (WHO-UMC) causality assessment scale. The severity of ADR was also measured using the severity assessment of the Hartwig and Siegel scales (Appendix A and B).
Data Collection and Management
Medical records of paediatric cancer patients in both hospitals (UoGCSH and FRH) were traced from the registration book for oncology wards. Using a patient Medical Record Number (MRN), patient charts were drawn from the card room. Using the structured questionnaire, two trained pharmacists collected the data from the charts. Data such as age, weight, height, sex, residence and body surface area (BSA) were collected from demographics. In addition, type and number of chemotherapy, cycle of treatment, history of previous chemotherapy, type and stage of cancer, laboratory results, co-morbidity, concomitant medications and ADR were also reviewed from the chart.
Data collectors and supervisors were intensively trained on questionnaire content, methods of data collection and ethical concerns. To check its consistency, twenty questionnaires were pre-tested. After pre-testing, the questions were modified. During the data collection period, data quality was ensured by the principal investigator for completeness.
Data Entry, Analysis and Interpretation
The collected data were entered into Epi Info version 7, then exported and analyzed using Statistical Product and Service Solutions (SPSS) version 22. For continuous variables such as age, height and weight, descriptive statistics such as mean and standard deviations were calculated. For variables including age, height, weight, body surface area (BSA), cancer stage, number of chemotherapy, type of chemotherapeutic agents, previous history of chemotherapy, presence of co-morbidity and concomitant medication, the Chi-square assumption was tested.
Those variables satisfied the assumptions were analyzed using bivariate binary logistic regression to identify associations with the outcome variable (ADRs). Variables with a P-value of less than or equal to 0.2 at the bivariate logistic regression analysis were taken for multivariate analysis. Variables with a P-value of less than 0.05 in the multivariate logistic regression were considered as a significant association with ADRs.
Results
Socio-Demographic Characteristics of Paediatric Cancer Patients
A total of 311 patient charts were reviewed and 287 were eligible for analysis, while 24 of those charts were excluded because of incomplete outcome variable records. Almost two-thirds of the participants were males, 176 (61.3 percent). The mean age of patients was 7.06 years with a standard deviation of ± 3.55. Nearly half (47.5%) of the respondents were 6 to 12 years of age. The mean weight was 18.06 Kg (± 6.8961 kg) for the patients. The majority (83.6%) of patients were rural inhabitants (Table 1).
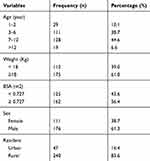 |
Table 1 Socio-Demographic Characteristics of Pediatric Cancer Patients at Northwest Hospitals, Ethiopia, from August 16 to –September 22, 2019 |
Clinical Characteristics of Paediatric Cancer Patients
The most prevalent type of cancer, diagnosed in 67 (23.3%) patients, was acute lymphoblastic leukemia (ALL), followed by Wilms tumor 65 (22.6%) and non-Hodgkin lymphoma (NHL) 34 (11.8%) (Figure 1).
 |
Figure 1 The type of cancer among paediatric patients attending in the University of Gondar comprehensive, specialized hospital and Felegehiwot referral hospital, northwest Ethiopia, 2019. |
A total of 18 types of chemotherapeutic agents were used to treat cancer. Vincristine, which was used by 245 (85.4%) patients, was the most frequently prescribed agent, followed by doxorubicin 177 (61.7%) and cyclophosphamide 166 (57.8%) respectively (Table 2).
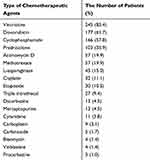 |
Table 2 Type of Prescribed Chemotherapeutic Agents for Paediatric Cancer Patients at UOGCSH and FRH, Amhara Region, Ethiopia, 2019 |
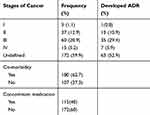
Out of the total patients included in this study (287), a total of 172 (59.2%) had no defined stage of cancer. Moreover, more than half 63 (52.9%) of patients who experienced ADRs had undecided stage of cancer (Table 3).
Magnitude of Adverse Drug Reactions
In this study, a total of 119 paediatric patients were developed Adverse Drug Reactions (ADRs). The prevalence of ADRs was 41.50% (95% CI: 35.8–47.2%). Vomiting, which accounted for 24 (16.3%), was the most common of the reported drug reactions, followed by alopecia 22 (15.0%) and febrile neutropenia 15 (10.2%) (Figure 2).
In addition, more than half of the cases of ADRs reported among males were 69 (58.0%). Nearly half (44.5%) of ADR cases were between the ages of 6–12 and about 42% were between the ages of 2–6. Acute lymphoblastic treatment was diagnosed in a total of 35 (23.8%) patients with ADR.
Co-Morbidity and Concomitant Medication in Paediatric Cancer Patients
There were comorbidities in a total of 180 (62.7%) of cancer patients. The most common co-morbidity was malnutrition (41.7%), followed by anemia (24.4%), infectious disease (18.9%) and other health conditions (15.0%). Nearly half (45.6%) of patients with co-morbidities experienced adverse drug reactions (ADRs) in this study.
Causality and Severity Assessment of Adverse Drug Reaction
According to the World Health Organization-Uppsala monitoring Centre (WHO-UMC) causality assessment scales, 98 (66.7%) were possible and 49 (33.3%) were probable ADR cases. There were no certain and unlikely category of ADRs. In addition, the Hartwig’s and Siegel severity assessment Scale in this study indicated that most of the ADR reactions were a moderate 109 (74.1%), and mild 38 (25.9%) drug reactions.
Factors Associated with Chemotherapy-Related Adverse Drug Reaction
In a bivariate logistic regression analysis, concomitant medicinal products, the number of chemotherapy drugs and chemotherapy agents such as doxorubicin, etoposide and mercaptopurine were found to be adverse drug reaction factors with a p-value of less than 0.2. Consequently, following the multivariate logistic regression analysis of these variables, only one variable (comorbidity) was not found to be statistically significant in relation to adverse drug reactions at a p-value lower than 0.05. The chi-square assumption was not met for variables such as sex, age, weight, residence, BSA, stage of cancer and prior history of chemotherapy, so that regression analysis was not considered.
According to the multivariate logistic regression analysis, the presence of concomitant medication (AOR: 2.60 (1.54–4.40); p<0.000), etoposide (AOR: 3.97 (1.64–9.63); p<0.002), mercaptopurine (AOR: 3.91 (1.06–14.46); p<0.041), doxorubicin (AOR: 2.32 (1.30–4.15); p<0.005) and the administration of four or more types of chemotherapeutic agents (AOR: 2.67 (1.52–4.68) and p<0.001) were significantly associated with the occurrence of ADRs (Table 4).
Discussion
In this study, among 287 paediatric cancer patients, a total of 119 Adverse Drug Reactions (ADRs) was reported. The overall magnitude of chemotherapy-related ADR was 41.5% (95% CI: 35.8–47.2%). This finding was consistent with a study conducted in Tikur Anbessa Specialized Hospital, Ethiopia that reported the prevalence of 45.5%.6 This is due to the similarity between the types of chemotherapy agents (vincristine, doxorubicin and cyclophosphamide) prescribed in the two study settings. On the contrary, our outcome was lower than studies reported from Eastern India (86.53%),19 Mexico City (62.5%),38 and South African (56.5%).39 The difference may be due to variation between study areas in the type of drugs administered to cancer patients.
In our study, the most anti-cancer medications cause for ADRs were vincristine, doxorubicin and cyclophosphamide; but in studies reported from India and Mexico, cisplatin based regimen, cyclophosphamide, 5-Fluorouracil and paclitaxel chemotherapeutic agents were involved in the occurrence of ADRs.19 In addition, the difference in the inclusion of study participants among studies might be the other reason for such discrepancy; where our study included under-fifteen children while the study from India included all age groups including adults.19 Furthermore, the various combinations of medications given to prevent ADRs in Ethiopia can be the reason for the lower ADR compared with studies in India and Mexico. For instance, medications such as Ondansetron, aprepitant and dexamethasone are given to prevent nausea and vomiting associated with moderate and high emetogenic chemotherapy. Mesna is also given for prevention of chemotherapy associated hemorrhagic cystitis such as cyclophosphamide. Filgrastim agent is also used to reduce the duration of neutropenia and incidence of febrile neutropenia (neutropenic fever) in cytotoxic chemotherapy such as cyclophosphamide. Allopurinol is taken as prophylaxis to prevent hyperuricemia.
Chemotherapy induced vomiting was among the major ADR reported in our study that accounted for 16.2% (95% CI: 10.3–22.3%). This is due to the fact that most of the study population in our study took cyclophosphamide, doxorubicin, and vincristine based drug regimen that have a higher risk of emetogenic effect. A consistent finding was also reported from the Kumaun region (17.5%),5 West Rajasthan (10.34%),38 and North Eastern parts of India (15.09%).40
Among the different type of cancer, almost a quarter of ADRs, 23.8% (95% CI: 16.9–30.7%) were reported from patient diagnoses with acute lymphoblastic leukaemia (ALL). This can be explained in both studies by the highest prevalence of patients diagnosed with acute lymphoblastic leukaemia. In addition, when treated with chemotherapy, children diagnosed with acute lymphoblastic leukaemia may have a higher rate of adverse drug reactions.42 The present study finding, on the other hand, was lower compared to studies reported from Mexico (66.9%),43 and Philadelphia (52.1%).44 The variation may be due to the difference in patterns of drug use. The Mexican study showed that the three common anti-cancer drugs caused by ADRs were indicated that cytarabine (29.2%), methotrexate (18.1%), and L-asparaginase (10.8%),43 whereas in our study, vincristine, doxorubicin, and cyclophosphamide were drugs that may have developed ADRs. The high occurrence of ADRs from Philadelphia, contrary to our finding, could be due to co-administered concomitant medications such as acetaminophen and diphenhydramine.44
Out of 115 patients who received concomitant drugs, more than half (54%) of them had ADRs. This is due to the fact that concomitant drugs are a potential source of drug-drug interaction that could alter chemotherapy pharmacokinetics, thus increasing the occurrence of ADRs.45 Vincristine is the most common chemotherapeutic agent used in more than two-thirds (68.0%) of patients who have experienced ADR alone or in combination with other anticancer drugs. This finding is higher than studies reported from china 0.88%,46 and India (5.9%).47 This may be due to the fact that most of the ADR was associated with vincristine-based regimens. Likewise, in 55.8% of patients who developed ADR in our study, Doxorubicin is the second common chemotherapeutic agent used. This result was higher than studies in New Delhi (8%),12 east Indians (13.9%),47 and Iran (24%).41 This could be due to more prescribed anticancer drugs that could be associated with ADRs based on doxorubicin regimens. Cyclophosphamide was the other chemotherapy agent used in 50.3% of patients experiencing ADRs. This outcome was higher than the Indian report (13.5%). The difference in the population between studies might be such variations in chemotherapeutic agents. The study findings, collected from both pediatric and adult populations, were reported from India.47
According to the multivariable logistic regression analysis, paediatric cancer patients who received a doxorubicin-based regimen had a higher chance of receiving adverse drug reactions (ADRs) (AOR: 2.32 (95% CI: 1.30–4.15); p < 0.005) compared to patients who were not on a doxorubicin-based regimen. This finding was consistent with the study conducted in Mexico.38 Similarly, the odds of ADRs among pediatric cancer patients who received mercaptopurine were 3.91 times higher in paediatric cancer patients (AOR: 3.91 (95% CI: 1.06–14.46); p<0.041) than in patients who did not receive mercaptopurine therapeutic agents. This means that mercaptopurine is an important factor for ADRs to occur. In the current study, the chances of receiving etoposide-administered ADRs were 3.97 times higher (AOR: 3.97 (95% CI: 1.64–9.63); p < 0.002) than those not receiving etoposide. This may be due to the effect of etoposide on the induction of various ADRs such as myelosuppression, thrombocytopenia, mucositis, stomatitis, nausea, diarrhea and alopecia.48 In addition, the ADR probability among paediatric cancer patients who received four or more chemotherapeutic agents was 2.67 times higher (AOR: 2.67 (95% CI: 1.52–4.68); p < 0.001) compared to patients who were exposed to less than four tons of chemotherapeutic agents. This implies that the occurrence of ADRs is significantly associated with increasing the number of chemotherapeutic agents. This is also supported by a London study that reported a significant association with ADRs in an increasing number of drugs.49 In addition, our finding also showed that in comparison with patients without concomitant medication, the higher odds of ADR were noted among patients with concomitant medication (AOR: 2.60 (95%: CI 1.54–4.40); p<0.000). This finding was supported by studies conducted in the Netherlands,50 and Japan.51 This is due to the fact that concomitant medication results in potential drug-drug interactions that may alter the pharmacokinetics of chemotherapy agents and thus increase the incidence of ADRs.45
Conclusion
This study found that a significant proportion (2 out of 5) of cancer patients experienced an adverse drug reaction related to chemotherapy. Three-fourth of the patients had moderate adverse drugs, according to the Hartwig and Siegel severity assessment scale. Drugs (such as etoposide, mercaptopurine, and doxorubicin), concomitant medication, and taking at least four chemotherapeutic agents were independent predictors of chemotherapy-related ADRs in the multivariable logistic regression analysis. Therefore, for paediatric patients, early identification and management of ADR is highly recommended.
Abbreviations
ADR, adverse drug reaction; AOR, adjusted odds ratio; CI, confidence interval; COR, crude odds ratio; SPSS, Statistical Package for Social Sciences; UoG, University of Gondar; WHO, World Health Organization.
Data Sharing Statement
Data will be shared upon request from the corresponding author.
Ethical Approval and Consent to Participate
After obtaining ethical approval from the ethics review committee of the Department of Clinical Pharmacy, School of Pharmacy, University of Gondar, the study was conducted. The study was therefore a retrospective review of patient data; the committee took into account the nature of the research and waived the need for consent. Above all, this study was carried out according to the Helsinki Declaration of Ethical Principles for Research. After obtaining permission from the two hospitals, the data collection was started by the medical director and the coordinator of the oncology ward. The patient’s personal identifiers, such as name, were not used in the research report or any other in order to maintain patient confidentiality. Also, the patient’s chart was not removed from the chart space and was not exposed to anyone other than data collectors. They returned to their shelf properly after the graphs were reviewed.
Acknowledgments
The authors are indebted to the University of Gondar for the approval of the ethical clearance, University of Gondar Specialized comprehensive hospital and Felegehiwot referral hospital for giving us permission to collect data. The authors also forward its gratitude to study participants, data collectors and supervisors who participated in the study.
Author Contributions
GW designed the study, supervised data collection, performed data analysis and interpretation, and drafted the manuscript. OA assisted in designing the study, did data analysis and interpretation, and critical review of the manuscript. MKY assisted in designing the study, did data analysis and interpretation, drafted the manuscript, and substantially revised the manuscript. All authors read and approved the final manuscript and agree to be accountable for all the contents of the work in the manuscript.
Funding
There was no specific funding obtained for this study.
Disclosure
Gashaw Workalemahu reports the study was conducted after getting ethical approval from the ethics review committee of the Department of Clinical Pharmacy, School of Pharmacy, and University of Gondar. I have official letter from the department of clinical pharmacy. The authors declare that they have no other potential conflicts of interest for this work.
References
1. Organization WH. International Drug Monitoring: The Role of National Centres, Report of a WHO Meeting [Held in Geneva from 20 to 25 September 1971]. World Health Organization; 1972.
2. Lihite RJ, Lahkar M, Das S, et al. A study on adverse drug reactions in a tertiary care hospital of Northeast India. Alexandria J Med. 2017;53(2):151–156. doi:10.1016/j.ajme.2016.05.007
3. Gaur S, Sinha A, Gunjita B, Bhardwaj R, Renu K, Srivastava B Pattern of adverse drug reactions of various chemotherapeutic agents in cancer patients in Kumaun Region.
4. Schatz S, Weber R. Adverse drug reactions. Pharm Pract (Granada). 2015;1:1.
5. Sunny S, Thampi A, Johnkennedy NS, Babasahib SK, Chacko C. Assessment of adverse effects of most commonly prescribed anticancer drugs in a Tertiary Care Teaching Hospital. Int J Pharm Pract. 2017;10(4):271.
6. Sisay EA, Engidawork E, Yesuf T, Ketema E. Drug related problems in chemotherapy of cancer patients. J Cancer Sci Ther. 2015;7(2):55–59.
7. Joseph SG, Badyal DK. Spontaneous adverse drug reaction monitoring in a tertiary care hospital in Northern India. JK Science. 2016;18(2):103.
8. Angamo M. Adverse Drug Reaction-Related Hospitalisation in Ethiopia. University of Tasmania; 2018.
9. Parande F, Anand A, Khaparde M, Pawar S. Chemotherapy-induced adverse drug reactions in pediatric oncology. J Young Pharm. 2018;10(3):340. doi:10.5530/jyp.2018.10.75
10. Suyagh M, Farah D, Farha RA. Pharmacist’s knowledge, practice and attitudes toward pharmacovigilance and adverse drug reactions reporting process. Saudi Pharm J. 2015;23(2):147–153. doi:10.1016/j.jsps.2014.07.001
11. Angiji A Adverse drug reactions related to mortality and morbidity: drug-drug interactions and overdoses. Xendo. 2010. Available from: http://wwwxendocom/images/pdf/ADRs-by-Majorsystems-II-Final-Reportpdf.
12. Aggarwal M, Chawla S, Singh K, Rana P. Evaluation of anticancer drug utilization and monitoring of adverse drug reaction in the indoor patients receiving cancer chemotherapy in a Tertiary Care Hospital in New Delhi. J Basic Clin Pharmacol. 2018;9:2.
13. Brunton LL. Goodman & Gilman’s the Pharmacological Basis of Therapeutics. Univerza v Ljubljani, Medicinska fakulteta; 2011.
14. Dewan P, Singhal S, Harit D. Management of chemotherapy-induced nausea and vomiting. Indian Pediatr. 2010;47(2):149–155.
15. Ng CY, Chen C-B, Wu M-Y, et al. Anticancer drugs induced severe adverse cutaneous drug reactions: an updated review on the risks associated with anticancer targeted therapy or immunotherapies. J Immunol Res. 2018;2018.
16. Visacri MB, Souza C, Pimentel R, et al. Pharmacovigilance in oncology: pattern of spontaneous notifications, incidence of adverse drug reactions and under-reporting. Braz J Pharm Sci. 2014;50(2):411–422. doi:10.1590/S1984-82502014000200021
17. Baudino AT. Targeted cancer therapy: the next generation of cancer treatment. Curr Drug Discov Technol. 2015;12(1):3–20. doi:10.2174/1570163812666150602144310
18. Rout A, Panda RK, Mishra V, Parida P, Mohanty S. Pharmacovigilance in cancer chemotherapy in regional cancer center of Eastern India: prospective observational study. Int J Basic Clin Pharmacol. 2017;6(8):1910.
19. Prasad A, Datta PP, Bhattacharya J, Pattanayak C, Chauhan AS, Panda P. Pattern of adverse drug reactions due to cancer chemotherapy in a tertiary care teaching hospital in Eastern India. J Pharmacovigil. 2013;1(2):1000107.
20. Behera SK, Kishtapati CR, Gunaseelan V, Dubashi B, Chandrasekaran A, Selvarajan S. Chemotherapy induced adverse drug reactions in cancer patients in a tertiary care hospital in South India. J Young Pharm. 2017;9(4):593. doi:10.5530/jyp.2017.9.113
21. Alsbou M, Abdeen G, Batarseh A, Bawaresh N, Jaber J, Qawasmi G. Analysis of the National Pharmacovigilance Database in Jordan (2010– 2014). Biomed Pharmacol J. 2017;10(1):319–328. doi:10.13005/bpj/1112
22. Åvall Lundqvist E, Fujiwara K, Seoud M. Principles of chemotherapy. Int J Gynaecol Obstet. 2015;131:S146–S149. doi:10.1016/j.ijgo.2015.06.011
23. Chan H-K, Ismail S. Side effects of chemotherapy among cancer patients in a Malaysian General Hospital: experiences, perceptions and informational needs from clinical pharmacists. Asian Pac J Cancer Prev. 2014;15(13):5305–5309. doi:10.7314/APJCP.2014.15.13.5305
24. Zhang Q-Y, Wang F-X, Jia -K-K, Kong L-D. Natural product interventions for chemotherapy and radiotherapy-induced side effects. Front Pharmacol. 2018;9:1253.
25. Poulopoulos A, Papadopoulos P, Andreadis P. Chemotherapy: oral side effects and dental interventions. A review of the literature. Stomatological Dis Sci. 2017;2573–0002:2017.
26. Alhawassi TM, Krass I, Bajorek BV, Pont LG. A systematic review of the prevalence and risk factors for adverse drug reactions in the elderly in the acute care setting. Clin Interv Aging. 2014;9:2079.
27. Bouvy JC, De Bruin ML, Koopmanschap MA. Epidemiology of adverse drug reactions in Europe: a review of recent observational studies. Drug Saf. 2015;38(5):437–453. doi:10.1007/s40264-015-0281-0
28. Miguel A, Azevedo LF, Araújo M, Pereira AC. Frequency of adverse drug reactions in hospitalized patients: a systematic review and meta‐analysis. Pharmacoepidemiol Drug Saf. 2012;21(11):1139–1154. doi:10.1002/pds.3309
29. Angamo MT, Chalmers L, Curtain CM, Bereznicki LR. Adverse-drug-reaction-related hospitalisations in developed and developing countries: a review of prevalence and contributing factors. Drug Saf. 2016;39(9):847–857. doi:10.1007/s40264-016-0444-7
30. Eshetie TC, Hailemeskel B, Mekonnen N, Paulos G, Mekonnen AB, Girma T. Adverse drug events in hospitalized children at Ethiopian University Hospital: a prospective observational study. BMC Pediatr. 2015;15(1):83. doi:10.1186/s12887-015-0401-0
31. Geel JA. Toxicity and survival rates in 75 paediatric patients with germ cell tumours treated over a 28 year period at the Charlotte Maxeke Johannesburg academic hospital.
32. Alomar MJ. Factors affecting the development of adverse drug reactions. Saudi Pharm J. 2014;22(2):83–94. doi:10.1016/j.jsps.2013.02.003
33. Amartya D. Monitoring of suspected adverse drug reactions in oncology unit of an urban multispeciality teaching hospital. Int J Res Pharm Biomed Sci. 2010;1(2):1–32.
34. Bhabhor PH, Patel TK, Vahora R, Patel PB, Desai N. Adverse drug reactions in a tertiary care teaching hospital in India: analysis of spontaneously reported cases. Int J Basic Clin Pharmacol. 2014;3(6):1078–1086. doi:10.5455/2319-2003.ijbcp20141228
35. Ruggiero A, Rizzo D, Catalano M, Coccia P, Triarico S, Attiná G. Acute chemotherapy-induced nausea and vomiting in children with cancer: still waiting for a common consensus on treatment. J Int Med Res. 2018;46(6):2149–2156. doi:10.1177/0300060518765324
36. Ampadu HH, Hoekman J, de Bruin ML, et al. Adverse drug reaction reporting in Africa and a comparison of individual case safety report characteristics between Africa and the rest of the world: analyses of spontaneous reports in VigiBase®. Drug Saf. 2016;39(4):335–345. doi:10.1007/s40264-015-0387-4
37. Goshime A. Assessment of Knowledge, Attitude and Practices on Adverse Drug Reaction Reporting Among Pharmacy Personnel Working at Community Pharmacy, Addis Ababa, Ethiopia. Addis Ababa University; 2015.
38. Castelán-Martínez OD, Rodríguez-Islas F, Vargas-Neri JL, et al. Risk factors for febrile neutropenia in children with solid tumors treated with cisplatin-based chemotherapy. J Pediatr Hematol Oncol. 2016;38(3):191–196. doi:10.1097/MPH.0000000000000515
39. Makiwane M, Decloedt E, Chirehwa M, Rosenkranz B, Kruger M. Adverse drug reactions in paediatric in-patients in a South African tertiary hospital. J Trop Pediatr. 2019;65(4):389–396. doi:10.1093/tropej/fmy067
40. Wahlang JB, Laishram PD, Brahma DK, Sarkar C, Lahon J, Nongkynrih BS. Adverse drug reactions due to cancer chemotherapy in a tertiary care teaching hospital. Ther Adv Drug Saf. 2017;8(2):61–66. doi:10.1177/2042098616672572
41. Vaseghi G, Abed A, Jafari E, Eslami N, Eshraghi A. Assessment of adverse drug reaction due to cancer chemotherapy in a teaching oncology hospital in Isfahan, central of Iran. Rev Recent Clin Trials. 2016;11(3):266–272. doi:10.2174/1574887110666150818112648
42. Özdemir BC, Csajka C, Dotto G-P, Wagner AD. Sex differences in efficacy and toxicity of systemic treatments: an undervalued issue in the era of precision oncology. J Clin Oncol. 2018;36(26):2680. doi:10.1200/JCO.2018.78.3290
43. Rivera-Luna R, Cárdenas-Cardós R, Velasco-Hidalgo L, et al. A profile of the adverse effects of antineoplastic agents for the treatment of children with cancer. J Cancerol. 2015;2.
44. Barrett JS, Patel D, Dombrowsky E, Bajaj G, Skolnik JM. Risk assessment of drug interaction potential and concomitant dosing pattern on targeted toxicities in pediatric cancer patients. AAPS J. 2013;15(3):775–786. doi:10.1208/s12248-013-9489-z
45. Hanigan MH, Dela Cruz BL, Shord SS, Medina PJ, Fazili J, Thompson DM. Optimizing chemotherapy: concomitant medication lists. Clin Pharmacol Ther. 2011;89(1):114–119. doi:10.1038/clpt.2010.253
46. Guo H-J, Ren F, Zhang D, Ji M. Monitoring report on 341 cases of adverse reactions caused by antitumor drugs. Afr J Microbiol Res. 2012;6(16):3774–3777.
47. Chopra D, Rehan HS, Sharma V, Mishra R. Chemotherapy-induced adverse drug reactions in oncology patients: a prospective observational survey. Indian J Med Paediatr Oncol. 2016;37(1):42. doi:10.4103/0971-5851.177015
48. Fujiwara T, Kenmotsu H, Naito T, et al. The incidence and risk factors of febrile neutropenia in chemotherapy-naïve lung cancer patients receiving etoposide plus platinum. Cancer Chemother Pharmacol. 2017;79(6):1229–1237. doi:10.1007/s00280-017-3324-7
49. Zopf Y, Rabe C, Neubert A, Hahn EG, Dormann H. Risk factors associated with adverse drug reactions following hospital admission. Drug Saf. 2008;31(9):789–798. doi:10.2165/00002018-200831090-00007
50. Van Leeuwen R, Brundel D, Neef C, et al. Prevalence of potential drug–drug interactions in cancer patients treated with oral anticancer drugs. Br J Cancer. 2013;108(5):1071. doi:10.1038/bjc.2013.48
51. Sasaki T, Fujita K-I, Sunakawa Y, et al. Concomitant polypharmacy is associated with irinotecan-related adverse drug reactions in patients with cancer. Int J Clin Oncol. 2013;18(4):735–742. doi:10.1007/s10147-012-0425-5
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.