Back to Journals » ImmunoTargets and Therapy » Volume 13
Characterizing CD8+ TEMRA Cells in CP/CPPS Patients: Insights from Targeted Single-Cell Transcriptomic and Functional Investigations
Authors Zhang F , Ge Q, Meng J , Chen J, Liang C, Zhang M
Received 22 November 2023
Accepted for publication 17 February 2024
Published 26 February 2024 Volume 2024:13 Pages 111—121
DOI https://doi.org/10.2147/ITT.S451199
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Michael Shurin
Fei Zhang,* Qintao Ge,* Jialin Meng, Jia Chen, Chaozhao Liang, Meng Zhang
Department of Urology, The First Affiliated Hospital of Anhui Medical University; Institute of Urology, Anhui Medical University; Anhui Province Key Laboratory of Urological and Andrological Diseases Research and Medical Transformation, Anhui Medical University, Hefei, 230022, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Chaozhao Liang; Meng Zhang, Department of Urology, The First Affiliated Hospital of Anhui Medical University, Jixi Road 218, Shushan District, Hefei City, Anhui Province, 230022, People’s Republic of China, Tel/Fax +86 55162922034, Email [email protected]; [email protected]
Background: The specific involvement of the CD8+ T effector memory RA (TEMRA) subset in patients with chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) has largely not been explored in the literature.
Methods: Targeted single-cell RNA sequencing (scRNA-seq) profiles were generated from peripheral blood mononuclear cells (PBMCs) obtained from two CP/CPPS patients and two healthy controls (HCs) in our recent study. Pseudotime series algorithms were used to reveal the differentiation trajectory, CellChat analysis was used to explore the communication between individual cells, and the SCENIC program was used to identify potential transcription factors (TFs). Based on the cosine similarity, clusters of differentially expressed genes (DEGs) were considered to be further enriched in different pathways. To confirm the functional role of the critical clusters, flow cytometry was employed.
Results: The results revealed the molecular landscape of these clusters, with TEMRA cells exhibiting pronounced cytokine-mediated signaling pathway enrichment. Pseudotime trajectory analysis further mapped the evolution from naïve T cells to that of TEMRA cells, elucidating the developmental pathways involved in the immune context. A significant finding from CellChat analysis was the differential expression of ligands and receptors, with CD8+ TEMRA cells showing enhanced signaling, particularly in the CP/CPPS context, compared to HCs. Flow cytometry confirmed these results, revealing a heightened proinflammatory cytokine profile in patients with chronic prostatitis-like symptoms (CP-LS), suggesting that TEMRA cells play a significant role in disease pathogenesis. TF profiling across the T-cell clusters identified key regulators of cellular identity, identifying novel therapeutic targets. Elevated TNF signaling activity in CD8+ TEMRA cells underscored the involvement of these cells in disease mechanisms.
Conclusion: This study elucidates the pivotal role of the CD8+ TEMRA cell subset in CP/CPPS, which is characterized by increased TNF signaling and proinflammatory factor expression, highlighting potential biomarkers and opening new avenues for therapeutic intervention.
Keywords: chronic prostatitis/chronic pelvic pain syndrome, targeted single-cell RNA sequencing, CD8+ TEMRA, periblood memory T-cell
Background
Chronic prostatitis is an inflammatory disease that occurs in prostate organs and is caused by multiple factors. These factors were assigned to two categories: infectious and noninfectious.1,2 It was proposed by the World Health Organization that prostatitis can be divided into four main types based on its distinct etiology and disease course; chronic prostatitis has attracted increased amounts of attention.3 There are two types of chronic prostatitis: one is caused by pathogens, such as Escherichia coli and Corynebacterium spp., and the other is related to intricate factors. In terms of treatment, antibiotics are the first-line therapy for the former, and combination with nutraceutical products, such as Flogofilm@, significantly improves therapeutic efficacy.4 However, the latter, also known as chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS), is characterized by a combination of pain and urinary and sexual symptoms and has a poorly understood pathogenesis,5,6 often leading to inadequate treatment outcomes. Recent progress in the fields of immunology and molecular biology has opened new avenues for understanding the underlying mechanisms of this condition. Among these, the role of the immune system, particularly in T-cell-mediated responses, has garnered significant interest.
Memory T cells, key components of the adaptive immune system, play crucial roles in the human body’s defense against pathogens and in the development of autoimmune and inflammatory diseases.7–9 These cells are known for their ability to remember past infections and respond more rapidly and effectively upon re-exposure to the same antigen.10,11 However, their role in noninfectious chronic inflammatory conditions such as CP/CPPS is largely unclear and warrants detailed exploration.
Building upon the insights gained from our recent study, which suggested that effector T cells might be the causes of CP/CPPS,12 the present study represents a continuation of our investigation. In this phase, we reanalyzed the extensive dataset generated from targeted single-cell sequencing, focusing specifically on periblood memory T cells and their subtypes, such as T central memory cells (TCMs), T effector memory cells (TEMs), and T effector memory RA cells (TERMAs). Our objective was to uncover the intricate functional variations within these T-cell subsets that could elucidate their contributions to the complex pathogenesis of chronic prostatitis.
Materials and Methods
Row Data Collection and Processing
PBMCs were isolated from two patients with CP/CPPS and two healthy controls (HCs) attending our hospital, as detailed in our previous research.12 Briefly, the volunteers chosen for our study underwent thorough physical exams within one year by themselves without showing any major health concerns. We carefully screened out anyone with significant medical conditions such as HIV/AIDS, autoimmune diseases, or severe cardiac issues. Those who recently used drugs that could alter immune function, had undergone major surgeries, or had serious health problems were also excluded. Additionally, we did not include individuals with recent severe infections or those who had recently been vaccinated. Single-cell sequencing data were analyzed using the “Seurat” package,13 with the resolution parameter set at 0.6, resulting in the classification of all cells (n = 21,759) into 11 distinct clusters. Drawing on the literature,14 these clusters were manually annotated and subsequently consolidated into eight clusters based on signature gene expression profiles (Table S1). The refined clusters were subsequently visualized using uniform manifold approximation and projection (UMAP) for dimensional reduction.
Pathway Enrichment Analysis
To explore the various biological processes involved, we identified the top 100 highly expressed genes within each cell population by employing the cosine similarity-based gene identification (COSG) algorithm. This method, grounded in cosine similarity, enhances the scalability of marker gene identification.15 Subsequently, the identified genes were annotated using Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses. For visualization purposes, we utilized the “ClusterGVis” R package.16 Additionally, we leveraged the integrated FindMarkers() function to uncover differentially expressed genes (DEGs) between the case and control cohorts.
Pseudotime Series Analysis
To reveal the differentiation trajectories of defined cells, the R package “monocle2” was used.17 The genes used for trajectory inference were filtered via the dispersionTable() function, which was further used to describe how the variance in the expression of each gene varied with respect to the average. The data dimensionality was reduced via the “DDRTree” algorithm, and the variable differentiation state was estimated by the reduceDimension() function. The built-in plot_cell_trajectory function was employed for visualization. The starting point for differentiation trajectories was identified according to the relevant biological background, where T naïve cells are the potential starting point. After establishing the pseudotime trajectory, we also performed branch expression analysis modeling (BEAM analysis) to detect branch fate-degerming genes.
Exploration of Cell Communication
The interaction between cells depends on the interaction between ligands and receptors, but it is difficult for us to obtain a relatively perfect explanation of complex ligands and receptor types. The CellChatDB database integrates a variety of ligand and receptor types, including multimeric ligands and receptors and cofactor types. Compared with other known algorithms, we can obtain more accurate conclusions from this approach. Therefore, we employed the “CellChat” package to perform intercellular communication-related analysis, aiming to explore intercellular communication interactions at single-cell resolution and determine the mechanisms of communication molecules.18 In addition, the signature genes of the TNF signaling pathway were obtained from the Molecular Signatures Database (https://www.gsea-msigdb.org/gsea/msigdb). For individual cells, the AUCell algorithm was used to estimate the activation status of the TNF signaling pathway.19
SCENIC Program for Identifying Potential Transcript Regulons
To identify potential transcription factors (TFs) for each cell population, SCENIC was performed via Python (version 3.7). Gene-regulatory networks (GRNs) were first constructed to filter stable cell states based on cis-regulatory networks. TF gene coexpression modules were then re-established using GENIE3, which was further trimmed via RcisTarget algorithms. The SENIC website provided a detailed description of the workflow (https://github.com/aertslab/SCENIC). For the enrolled regulons, we subsequently employed AUCell algorithms to estimate the corresponding activity among single cells, representing a binary regular activity matrix. We also examined the different TFs in the case and control groups.
Flow Cytometry Validation
The Ethics Committee of Anhui Medical University’s First Affiliated Hospital granted approval for this research. Patient participants were sourced from the hospital’s outpatient clinic, while control group members were selected from healthy volunteers. All participants provided informed consent before performing the experiment. Blood samples were harvested from patients with chronic prostatitis-like symptoms (CP-LS) (n=16) and HCs (n=16). The clinical characteristics of the patients and HCs are summarized in Table S2.
Venous blood samples treated with lithium-heparin were obtained from patients with CP-LS and HCs. PBMCs were purified using Percoll (Cytiva). Next, the cells were plated in 24-well cell culture plates (1×106 cells/well) and cultured in Roswell Park Memorial Institute (RPMI) 1640 (Gibco™, Inc., USA) medium supplemented with 10% fetal bovine serum (Gibco™, Inc.) and eBioscienceTM Cell Stimulation Cocktail (plus protein transport inhibitors) (Invitrogen) for 16 h at 37°C with 5% CO2. As a negative control, one sample was kept without stimulation.
After stimulation, the cells were stained with PerCP-Cy™5.5-conjugated anti-CD3 (BD Pharmingen, 560,835), BV605-conjugated anti-CD4 (BD Horizon, 562,658), APC-Cy™7-conjugated anti-CD8 (BD Pharmingen, 557,834), FITC-conjugated anti-CD45RA (BD Pharmingen, 555,488), and APC-conjugated anti-CCR7 (BioLegend, 353,214) for 30 min at room temperature. The cells were fixed with 100 μL of fixation reagent A (Fix/Perm medium A; Thermo Fisher Scientific) for 15 min. After washing, the cells were resuspended in 100 μL of permeabilization reagent B (Fix/Perm medium B; Thermo Fisher Scientific) and labeled with BV421-conjugated anti-perforin (BD Horizon, 563,393), BV510-conjugated anti-Granzyme B (BD Horizon, 563,388), BV786-conjugated anti-IFN-γ (BD Horizon, 563,731), and PE-conjugated anti-TNF-α (BD Horizon, 502,909) for 30 min at room temperature in the dark. Finally, the samples were analyzed using a BD Fortessa flow cytometer and analyzed with FlowJo software (Tree Star). The antibodies used in the present study are listed in Table S3.
Statistical Analysis
All the statistical analyses were performed with R software (version 4.2.2) and Python (version 3.7). The categorical data were analyzed via Fisher’s exact test and the rank-sum test. A t-test was used for comparisons between two groups, while ANOVA was used for pairwise comparisons among multiple groups. A two-tailed p value < 0.05 was considered to indicate statistical significance.
Results
Characterizing Memory T-Cell Clusters in CP/CPPS Patients: Insights from UMAP, COSG, and Pseudotime Techniques
In this phase, the analysis led to the identification of 11 distinct memory T-cell clusters (Figure 1A), which, based on the expression of relevant markers, were subsequently merged into eight principal clusters based on functional similarities (Figures 1B, S1 and Table S1). Utilizing the UMAP algorithm, we visually demonstrated the presence of these marker genes in each cluster via a UMAP plot (Figure 1C) and a bubble plot (Figure 1D). Additionally, the DEGs between CP/CPPS patients and HCs and the corresponding GO pathway enrichment plot are shown in Figure S2. The three most enriched pathways identified were involved in the regulation of T-cell activation, carbohydrate binding, and signaling on the external side of the plasma membrane.
To delineate the boundaries that define the key distinct features and their biological importance, we employed the COSG method in combination with GO analysis (Figure 1E). C1 had the highest concentration of genes in the Doublet-TEMRA cluster among the six layers; these genes involved cytokine−mediated signaling pathways, T-cell activation regulation, etc.; and chemokine signaling pathway participation. For the CD8+ TEMRA cluster, the C3 module had the highest concentration of genes, including EOMES, CX3CR1, GZMB, and GZMA, involved in lymphocyte-mediated immunity and leukocyte-mediated cytotoxicity and involved in cytokine−cytokine receptor interactions and Th1 and Th2 cell differentiation. The C2 module included Doublet-Naïve T cells and CD8+ naïve T cells; the C3 module included Doublet-TEMRA cells, CD8+ TEM cells, and Doublet-Naïve T cells; and the C4 module included Doublet-TEMRA cells, CD8+ TEM cells, and CD4+ TEM cells (see Figure 1E). Additionally, to investigate the relationships between these diverse states, we applied pseudotime analysis20 and observed that the cells formed a continuous progression starting with CD8+ naïve T cells to TCMs and TEMs and gradually progressed toward TEMRA cells (Figures 1F and S3), a result consistent with previous findings.21,22
Deciphering the Role of TEMRA Cells in Chronic Prostatitis: A Comprehensive Analysis of Signaling Pathways and Transcriptional Regulators
Employing manifold learning and quantitative analysis, CellChat effectively discerned the differential overexpression of ligands and receptors within various cell cohorts. Figure 2A and B illustrates the pronounced dominance of CD8+ TEM cell signaling pathways compared to that of other immune variants in terms of both quantity and intensity. Moreover, a more in-depth examination of the right panel of Figure 2A and B reveals that interactions, in terms of number and potency, were markedly intensified in the CP/CPPS patients vs the HCs, particularly between CD8+ TEMRA/Doublet-TEMRA cells and other memory T-cell variants. This insight helps to elucidate the probable influence of TEMRA cells on chronic prostatitis onset.
The following stage involved a thorough investigation of the signaling dynamics present in our cellular network, focusing on the relevant molecules, receptors, and pathways. Figure 2C shows the comparative power of deduced entities such as SELPLG, CLEC, LCK, ICAM, TNF, ITGB2, CXCL, etc. Delving into CD8+ TEMRA cells, a fascinating finding was that most signaling pathways bore a striking resemblance in their contribution across both the CP/CPPS group and the HCs, barring TNF signaling, which was noticeably robust in both directions. Validating this, the R package AUCell confirmed that TNF signaling vigor was indeed superior in the CP/CPPS cohort than in the HC cohort (Figure 2D). These results accentuate that TEMRA cells exhibit enhanced interactions in the context of chronic prostatitis, with the TNF signaling pathway emerging as a pivotal factor.
To identify key regulators for each cell type, we assessed the activities of each regulon in relation to all eight cell clusters.23,24 The five regulators related to the maintenance of cell identity are shown in Figure 2E. For Doublet-TEMRA cells, the five TFs were IKZF2, STAT5A, IRF8, JUN, and JUNB, while for CD8+ TEMRA cells, they were TBX21, EOMES, STAT6, IRF8, and JUN. Figure 2F shows a heatmap that highlights the characteristic TFs for each memory T-cell cluster. Subsequently, we compared the expression of these critical TFs in the CD8+ TEMRA cluster between the CP/CPPS patients and HCs and found that genes, such as STAT6, EOMES, JUN, and FOXO1, were positively regulated, while IKZF2 and BCL6 were negatively regulated (Figure 2G). The upregulation of certain TFs in the TEMRA clusters was observed, suggesting that these TFs play a significant role in the pathology or manifestation of this disease.
Investigating CD8+ TEMRA Cells in Chronic Prostatitis: Transcriptomic Variations and Proinflammatory Cytokine Expression
To evaluate the transcriptomic differences in the inflammatory signature in CD8+ TEMRA cells, as indicated in Figures 3A and S4, high expression of tropic chemokine markers (CCL4, CXCR4, JUN, and ITGA4) and cytotoxicity markers (GZMB, IFNG, and NKG7) was observed in CP/CPPS patients compared with HCs. In addition, IFNG and GZMB are also considered markers of exhausted T cells and are commonly downregulated in these cells. These results confirmed the activating role of CD8+ TEMRA cells in CP/CPPS pathogenesis.
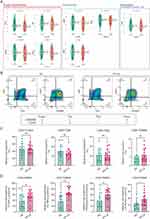
To ascertain the role of CD8+ TEMRA and Doublet-TEMRA cells in chronic prostatitis, we conducted flow cytometry to compare the proportions of these cells in the peripheral blood of CP-LS patients and HCs. The baseline parameter comparisons between the CP-LS and control groups are listed in Table S2, and the antibodies used for flow cytometry are listed in Table S3. Our findings indicated no notable differences in the frequency of these subsets between the groups (refer to Figures 3B and C and S5). Given the potential of TEMRA cells to secrete proinflammatory cytokines implicated in chronic prostatitis pathogenesis—such as TNF-α, IFN-γ, Perforin, Granzyme B25,26 we proceeded to profile these cytokines in CD8+ TEMRA/Doublet-TEMRA cells from both groups. As shown in Figure 3D, our analysis revealed significantly elevated expression of these four proinflammatory cytokines in CD8+ TEMRA cells from CP-LS patients compared to HCs but failed to obtain any positive results between the correlation of these features and clinical parameters due to the limited sample size (Figure S6). However, the intracellular level of the proinflammatory cytokine TNF-α in Doublet-TEMRA cells was substantially greater in the CP-LS group than in the HC group (Figure S5). Collectively, these findings highlight the enhanced proinflammatory profile of CD8+ TEMRA cells in individuals with chronic prostatitis.
Discussion
The identification of 11 distinct memory T-cell clusters, which were subsequently merged into eight principal clusters based on functional similarities, underscores the heterogeneity and complexity of the immune response in CP/CPPS. By utilizing techniques such as UMAP for visual representation, COSG for gene identification, and flow cytometry for proinflammatory cytokine validation, we were able to delineate the unique expression profiles and functional attributes of these clusters. Particularly noteworthy is the role of CD8+ TEMRA cells, which have emerged as a significant subset of cells in the context of CP/CPPS pathogenesis.
TEMRA cells are a unique subset of memory T cells, and CD8+ TEMRA cells are of profound interest due to their remarkable transcriptional and functional attributes.27,28 These cells, as our results delineate, undergo significant transcriptional shifts in CP/CPPS patients vs HCs. Such shifts could be a result of the dynamic environmental interactions they engage in, perhaps as a defense mechanism against specific pathogens or in response to systemic immunological disturbances.29 The pronounced role of CD8+ TEMRA cells, as illustrated in our study, requires further probing of their potential as therapeutic targets, given their importance in the pathogenesis of chronic prostatitis.
One of the most compelling outcomes of our research is the conspicuous role of TNF signaling in chronic prostatitis. Specifically, the enhanced vigor of TNF-beta in CP/CPPS patients, as juxtaposed with that in healthy controls, is indeed telling. TNF-beta, a cytokine involved in cell-mediated immunity, has been associated with inflammatory responses,30–32 suggesting that its role is crucial in diseases such as CP/CPPS, which have an inflammatory underpinning. Its intensified signaling in TEMRA cells, especially in the context of CP/CPPS, corroborates the hypothesis that modulating this pathway could offer therapeutic potential. It is conceivable that TNF-beta, in association with other molecules and pathways, orchestrates a cascade of events that drive the inflammatory processes in CP/CPPS. The next logical step would be to ascertain the exact modulatory mechanisms and therapeutic interventions to target TNF-beta signaling in CP/CPPS.
The identification of key TFs, such as TBX21, EOMES, STAT6, IRF8, and JUN, in CD8+ TEMRA cells underscores the complex regulatory mechanisms governing these cell types.22,33 The differential expression of these TFs between CP/CPPS patients and HCs, particularly the positive regulation of STAT6, EOMES, JUN, and FOXO1 and the negative regulation of IKZF2 and BCL6, suggested a distinct transcriptional landscape in CP/CPPS. This altered transcriptional regulation may contribute to the pathophysiological characteristics of the disease. Furthermore, transcriptomic variations in CD8+ TEMRA cells, particularly the high expression of tropic chemokine markers and cytotoxicity markers in CP/CPPS patients, provide insights into the inflammatory nature of these cells in the context of disease.34–36 The elevated expression of proinflammatory cytokines, including TNF-α, IFN-γ, Perforin, and Granzyme B in CD8+ TEMRA cells from CP-LS patients compared to HCs further supports their role in driving the inflammatory response in CP/CPPS.21,34,37,38
While our study introduces novel insights into the role of CD8+ TEMRA cells in chronic prostatitis, it is imperative to acknowledge that these findings are derived from a very small sample size involving just two patients and two healthy controls. This limitation significantly impacts the generalizability of our results and must be taken into account when interpreting our findings. Although innovative and strengthened by methodologies such as flow cytometry, our approach remains preliminary. Future research with a larger and more diverse sample size is crucial to validate our initial observations and to fully understand the implications of our findings. These additional studies will be vital for confirming the potential therapeutic targets we have identified and for more accurately delineating the role of CD8+ TEMRA cells in the pathogenesis of CP/CPPS.
Conclusion
Taken together, the results of this study elucidate the role of memory T cells, especially CD8+ TEMRA cells, in the etiology of chronic prostatitis. Elevated TNF signaling activity was significantly detected in CP/CPPS patients, implicating its involvement in disease pathogenesis. Additionally, the present research identified upregulated genes, confirming the proinflammatory capacity of CD8+ TEMRA cells and suggesting promising directions for therapeutic exploration.
Abbreviations
TEMRA, T effector memory RA; TCM, T central memory cell; TEM, T effector memory cell; CP/CPPS, chronic prostatitis/chronic pelvic pain syndrome; CP-LS, chronic prostatitis-like symptoms; PBMCs, peripheral blood mononuclear cells; HCs, healthy controls; UMAP, uniform manifold approximation and projection; TFs, transcription factors.
Data Sharing Statement
The data of this study are available from the corresponding author, Meng Zhang (Email: [email protected]), upon reasonable request.
Ethical Approval and Informed Consent
All procedures involving human participants were conducted in accordance with the ethical standards of The Committee on Medical Ethics of The First Affiliated Hospital of Anhui Medical University (Reg. No. PJ-2022-08-34 and NO. PJ-2023-14-46) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all the subjects when they were enrolled.
Consent for Publication
All the authors have read and approved the manuscript for publication.
Acknowledgments
We greatly appreciate the patients and investigators who participated in the corresponding medical project for providing the data and thank Shanghai Biotechnology Corporation for their bioinformatics support.
Author Contributions
All the authors have played a substantial role in this study, from conceptualization, study formulation, implementation, and data gathering to analysis and interpretation, or in all these areas; took part in the initial drafting, subsequent revisions, or in-depth critique of the manuscript; endorsed the final version set for publication; agreed on the choice of the journal for submission; and collectively assumed responsibility for every element of the work.
Funding
This study is supported by the National Natural Science Foundation of China (82170787, 82200860, 82370776), the Natural Science Foundation of Anhui Province (2308085MH247), the Clinical Medical Research Translational Project of Anhui Province (211013672036), the Key Project of Provincial Natural Science Research Project of Anhui Colleges (2022AH051147), the Research Fund of Anhui Institute of Translational Medicine (2017ZHYX02, 2021zhyx-B08, and 2022zhyx-B13), the Scientific Research Funding of Anhui Medical University (2020xkj157), the Postgraduate Innovation and Practice Program of Anhui Medical University (YJS20230025), and the Natural Science Research in Colleges and Universities of Anhui Province (Grant Number: 2022AH051133).
Disclosure
All the authors declare no competing interests in this work.
References
1. Sun D, Xing D, Wang D, et al. The protective effects of bushen daozhuo granule on chronic non-bacterial prostatitis. Front Pharmacol. 2023;14:1281002. doi:10.3389/fphar.2023.1281002
2. Crocetto F, Barone B, De Luca L, Creta M. Granulomatous prostatitis: a challenging differential diagnosis to take into consideration. Future Oncol. 2020;16(13):805–806. doi:10.2217/fon-2020-0185
3. Cyril AC, Jan RK, Radhakrishnan R. Pain in chronic prostatitis and the role of ion channels: a brief overview. Br J Pain. 2022;16(1):50–59. doi:10.1177/20494637211015265
4. Barone B, Mirto BF, Falcone A, et al. The efficacy of flogofilm(®) in the treatment of chronic bacterial prostatitis as an adjuvant to antibiotic therapy: a randomized prospective trial. J Clin Med. 2023;12(8). doi:10.3390/jcm12082784
5. Cai T, Alidjanov J, Palagin I, Medina-Polo J, Nickel JC, Wagenlehner FME. Chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS): look to the future. Prostate Cancer Prostatic Dis. 2023. doi:10.1038/s41391-023-00645-7
6. Liu SJ, Gao QH, Deng YJ, Zen Y, Zhao M, Guo J. Knowledge domain and emerging trends in chronic prostatitis/chronic pelvic pain syndrome from 1970 to 2020: a scientometric analysis based on VOSviewer and CiteSpace. Ann Palliat Med. 2022;11(5):1714–1724. doi:10.21037/apm-21-3068
7. Raeber ME, Zurbuchen Y, Impellizzieri D, Boyman O. The role of cytokines in T-cell memory in health and disease. Immunol Rev. 2018;283(1):176–193. doi:10.1111/imr.12644
8. Jiang P, Jia H, Qian X, et al. Single-cell RNA sequencing reveals the immunoregulatory roles of PegIFN-α in patients with chronic hepatitis B. Hepatology. 2023;79:167–182. doi:10.1097/hep.0000000000000524
9. He A, Sarwar A, Thole LML, et al. Renal inflamm-aging provokes intra-graft inflammation following experimental kidney transplantation. Am J Transplant. 2022;22(11):2529–2547. doi:10.1111/ajt.17154
10. Lanier LL, Sun JC. Do the terms innate and adaptive immunity create conceptual barriers? Nat Rev Immunol. 2009;9(5):302–303. doi:10.1038/nri2547
11. Ratajczak W, Niedźwiedzka-Rystwej P, Tokarz-Deptuła B, Deptuła W. Immunological memory cells. Cent Eur J Immunol. 2018;43(2):194–203. doi:10.5114/ceji.2018.77390
12. Zhang M, Liu Y, Chen J, et al. Single-cell multi-omics analysis presents the landscape of peripheral blood T-cell subsets in human chronic prostatitis/chronic pelvic pain syndrome. J Cell Mol Med. 2020;24(23):14099–14109. doi:10.1111/jcmm.16021
13. Hao Y, Hao S, Andersen-Nissen E, et al. Integrated analysis of multimodal single-cell data. Cell. 2021;184(13):3573–3587.e29. doi:10.1016/j.cell.2021.04.048
14. Larbi A, Fulop T. From “truly naïve” to “exhausted senescent” T cells: when markers predict functionality. Cytometry A. 2014;85(1):25–35. doi:10.1002/cyto.a.22351
15. Dai M, Pei X, Wang XJ. Accurate and fast cell marker gene identification with COSG. Brief Bioinform. 2022;23(2). doi:10.1093/bib/bbab579
16. Zhang J. ClusterGVis: one-step to cluster and visualize gene expression matrix. Available from: https://github.com/junjunlab/ClusterGVis.
17. Qiu X, Hill A, Packer J, Lin D, Ma YA, Trapnell C. Single-cell mRNA quantification and differential analysis with Census. Nat Methods. 2017;14(3):309–315. doi:10.1038/nmeth.4150
18. Jin S, Guerrero-Juarez CF, Zhang L, et al. Inference and analysis of cell-cell communication using cellchat. Nat Commun. 2021;12(1):1088. doi:10.1038/s41467-021-21246-9
19. Aibar S, González-Blas CB, Moerman T, et al. SCENIC: single-cell regulatory network inference and clustering. Nat Methods. 2017;14(11):1083–1086. doi:10.1038/nmeth.4463
20. Qiu X, Mao Q, Tang Y, et al. Reversed graph embedding resolves complex single-cell trajectories. Nat Methods. 2017;14(10):979–982. doi:10.1038/nmeth.4402
21. Xiong H, Cui M, Kong N, et al. Cytotoxic CD161(-)CD8(+) T(EMRA) cells contribute to the pathogenesis of systemic lupus erythematosus. EBioMedicine. 2023;90:104507. doi:10.1016/j.ebiom.2023.104507
22. Shi Z, Wang X, Wang J, et al. Granzyme B + CD8 + T cells with terminal differentiated effector signature determine multiple sclerosis progression. J Neuroinflammation. 2023;20(1):138. doi:10.1186/s12974-023-02810-0
23. Ma A, Wang C, Chang Y, et al. IRIS3: integrated cell-type-specific regulon inference server from single-cell RNA-Seq. Nucleic Acids Res. 2020;48(W1):W275–w286. doi:10.1093/nar/gkaa394
24. Suo S, Zhu Q, Saadatpour A, Fei L, Guo G, Yuan GC. Revealing the critical regulators of cell identity in the mouse cell atlas. Cell Rep. 2018;25(6):1436–1445.e3. doi:10.1016/j.celrep.2018.10.045
25. Gate D, Saligrama N, Leventhal O, et al. Clonally expanded CD8 T cells patrol the cerebrospinal fluid in Alzheimer’s disease. Nature. 2020;577(7790):399–404. doi:10.1038/s41586-019-1895-7
26. Della-Torre E, Bozzalla-Cassione E, Sciorati C, et al. A CD8α- Subset of CD4+SLAMF7+ Cytotoxic T cells is expanded in patients with IgG4-Related Disease and decreases following glucocorticoid treatment. Arthritis Rheumatol. 2018;70(7):1133–1143. doi:10.1002/art.40469
27. Perl A, Morel L. Expanding scope of TEMRA in autoimmunity. EBioMedicine. 2023;90:104520. doi:10.1016/j.ebiom.2023.104520
28. Salumets A, Tserel L, Rumm AP, et al. Epigenetic quantification of immunosenescent CD8(+) TEMRA cells in human blood. Aging Cell. 2022;21(5):e13607. doi:10.1111/acel.13607
29. Tian Y, Babor M, Lane J, et al. Dengue-specific CD8+ T cell subsets display specialized transcriptomic and TCR profiles. J Clin Invest. 2019;129(4):1727–1741. doi:10.1172/jci123726
30. Zhao J, Duan L, Wang R, Liu Y, Jiang J. Roflumilast prevents lymphotoxin α (TNF-β)-induced inflammation activation and degradation of type 2 collagen in chondrocytes. J Inflamm Res. 2020;69(12):1191–1199. doi:10.1007/s00011-020-01404-3
31. Cho SMJ, Lee H, Shim JS, Sex- KHC. Age-, and metabolic disorder-dependent distributions of selected inflammatory biomarkers among community-dwelling adults. Diabet Metabol J. 2020;44(5):711–725. doi:10.4093/dmj.2019.0119
32. Buhrmann C, Popper B, Aggarwal BB, Shakibaei M. Resveratrol downregulates inflammatory pathway activated by lymphotoxin α (TNF-β) in articular chondrocytes: comparison with TNF-α. PLoS One. 2017;12(11):e0186993. doi:10.1371/journal.pone.0186993
33. Willinger T, Freeman T, Hasegawa H, McMichael AJ, Callan MF. Molecular signatures distinguish human central memory from effector memory CD8 T cell subsets. J Immunol. 2005;175(9):5895–5903. doi:10.4049/jimmunol.175.9.5895
34. Kantauskaite M, Vonend O, Yakoub M, et al. The effect of Renal Denervation on T Cells in patients with resistant hypertension. Int J Mol Sci. 2023;24(3):2493. doi:10.3390/ijms24032493
35. Doan Ngoc TM, Tilly G, Danger R, et al. Effector memory-expressing CD45RA (TEMRA) CD8(+) T Cells from kidney transplant recipients exhibit enhanced purinergic P2X4 receptor-dependent proinflammatory and migratory responses. J Am Soc Nephrol. 2022;33(12):2211–2231. doi:10.1681/asn.2022030286
36. Song A, Liu Y, Cao Z, et al. Clinical features and T Cell immune characteristics of postpartum hepatitis flare in pregnant women with HBeAg-positive chronic HBV infection. Front Immunol. 2022;13:881321. doi:10.3389/fimmu.2022.881321
37. Lee SW, Choi HY, Lee GW, et al. CD8(+) TILs in NSCLC differentiate into TEMRA via a bifurcated trajectory: deciphering immunogenicity of tumor antigens. J Immuno Ther Cancer. 2021;9(9). doi:10.1136/jitc-2021-002709
38. Henson SM, Lanna A, Riddell NE, et al. p38 signaling inhibits mTORC1-independent autophagy in senescent human CD8+ T cells. J Clin Invest. 2014;124(9):4004–4016. doi:10.1172/jci75051
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.