Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 17
Characterization of Patients with Chronic Obstructive Pulmonary Disease Initiating Single-Inhaler Long-Acting Muscarinic Antagonist/Long-Acting β2-Agonist Dual Therapy in a Primary Care Setting in England
Authors Requena G , Banks V , Czira A , Wood R , Tritton T, Wild R , Compton C, Duarte M, Ismaila AS
Received 8 March 2022
Accepted for publication 21 July 2022
Published 10 August 2022 Volume 2022:17 Pages 1781—1795
DOI https://doi.org/10.2147/COPD.S365480
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Richard Russell
Gema Requena,1 Victoria Banks,2 Alexandrosz Czira,1 Robert Wood,2 Theo Tritton,2 Rosie Wild,2 Chris Compton,1 Maria Duarte,1 Afisi S Ismaila3,4
1Value Evidence and Outcomes, Epidemiology, GSK, R&D Global Medical, Brentford, Middlesex, UK; 2Real-world Evidence, Adelphi Real World, Bollington, Cheshire, UK; 3Value Evidence and Outcomes, GSK, Collegeville, PA, USA; 4Department of Health Research Methods, Evidence and Impact, McMaster University, Hamilton, ON, Canada
Correspondence: Gema Requena, Epidemiology, Value Evidence and Outcomes, R&D Global Medical, GSK, Brentford, Middlesex, UK, Tel +44 20 80476893, Email [email protected]
Purpose: Treatment pathways of patients with chronic obstructive pulmonary disease (COPD) receiving single-inhaler dual therapies remain unclear. We aimed to describe characteristics, prescribed treatments, healthcare resource use (HCRU) and costs of patients with COPD who initiated single-inhaler long-acting muscarinic antagonist/long-acting β2-agonist (LAMA/LABA) dual therapy in primary care in England.
Patients and Methods: Retrospective study using linked data from Clinical Practice Research Datalink Aurum and Hospital Episode Statistics datasets. Patients with COPD with ≥ 1 single-inhaler LAMA/LABA prescription between June 2015 and December 2018 (index) were included. Demographic and clinical characteristics, prescribed treatments, HCRU and costs were evaluated in the 12 months pre-index. Data are presented for patients not receiving concomitant inhaled corticosteroids at index (non-triple users).
Results: Of 10,991 patients initiating LAMA/LABA, 9888 were non-triple users, of whom 21.3% (n=2109) received aclidinium bromide/formoterol, 18.1% (n=1785) received indacaterol/glycopyrronium, 12.0% (n=1189) received tiotropium bromide/olodaterol and 48.6% (n=4805) received umeclidinium/vilanterol. Demographic and clinical characteristics were similar across indexed therapies. LAMA monotherapy was the most frequently prescribed respiratory therapy at 12 (18.4– 25.8% of patients) and 3 months (23.9– 33.7% of patients) pre-index across indexed therapies; 42.5– 59.0% of patients were prescribed no respiratory therapy at these time points. COPD-related HCRU during the 12 months pre-index was similar across indexed therapies (general practitioner consultations: 62.0– 68.6% patients; inpatient stays: 19.3– 26.1% patients). Pre-index COPD-related costs were similar across indexed therapies, with inpatient stays representing the highest contribution. Mean total direct annual COPD-related costs ranged from £ 805–£ 1187.
Conclusion: Characteristics of patients newly initiating single-inhaler LAMA/LABA dual therapy were highly consistent across indexed therapies. As half of non-triple users were not receiving respiratory therapy one year prior to LAMA/LABA initiation, there may be an opportunity for early optimization of treatment to relieve clinical burden versus current prescribing patterns in primary care in England.
Keywords: COPD, initial maintenance therapy, primary care setting, patient characteristics, single-inhaler LAMA/LABA dual therapy, treatment patterns
Plain Language Summary
Patients with chronic obstructive pulmonary disease (COPD) may start treatment with long-acting muscarinic antagonist/long-acting β2-agonist (LAMA/LABA) dual therapy, a combination of two bronchodilator medications that work together to open the airways. There are four different LAMA/LABA dual therapies available as single inhalers in England, which are called aclidinium bromide/formoterol fumarate, indacaterol/glycopyrronium, tiotropium bromide/olodaterol, and umeclidinium/vilanterol. It is unclear whether there are differences between the patients treated with each drug. In this study, we evaluated the characteristics of patients with COPD who began treatment with LAMA/LABA dual therapy in a single inhaler at general practitioner (GP) practices in England. We used data from a large database containing records from GPs linked with hospital records to gather information on patients’ demographics, disease characteristics, prescriptions, health service use, and healthcare-related costs in the year before they started treatment with LAMA/LABA dual therapy. We compared these factors across groups of patients who were prescribed any of the four different LAMA/LABA dual therapies. We found that patients who were prescribed different treatments had similar characteristics, used healthcare services in a similar way, and had similar healthcare costs. Our results suggest that groups of patients who are treated with different LAMA/LABA dual therapies in England are similar. However, there may be an opportunity to provide earlier access to appropriate treatments for patients with COPD.
Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by persistent respiratory symptoms and airflow limitation that is usually progressive.1,2 Globally, it is the most prevalent chronic respiratory disease accounting for approximately 55% of all cases of chronic respiratory diseases.3
In the UK, over 1 million people live with diagnosed COPD and the actual number of patients with the disease is thought to be much higher due to underdiagnosis.4,5 The symptomatic burden of COPD is detrimental to patient health-related quality of life (HRQoL), and increasing symptomatic burden is associated with an increased risk of COPD exacerbations that can lead to hospitalization.6–8 Indeed, healthcare resource use (HCRU) among patients with COPD is common, leading to a substantial clinical and economic burden.6,7 In the UK alone, the annual cost of COPD is approximately £1.9 million.9
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) strategy report recommends first-line long-acting muscarinic antagonist (LAMA) or long-acting β2-agonist (LABA) monotherapy for symptomatic patients with infrequent exacerbations, or initial LAMA/LABA dual therapy as an option for patients with more severe breathlessness.10 The UK National Institute for Health and Care Excellence (NICE) guidelines recommend that LAMA/LABA therapy should be offered to patients without features suggesting corticosteroid responsiveness who remain breathless or have exacerbations despite short-acting bronchodilator therapy and optimized non-pharmacological management.11 LAMA/LABA dual therapy can be delivered via a single inhaler (fixed-dose combinations) or separate inhalers for each drug. Four LAMA/LABA single-inhaler combination therapies are licensed for the treatment of COPD in the UK: indacaterol/glycopyrronium (IND/GLY), aclidinium bromide/formoterol fumarate (ACL/FF), tiotropium bromide/olodaterol (TIO/OLO) and umeclidinium/vilanterol (UMEC/VI).12–15 These therapies have all been shown to reduce COPD symptoms and/or the risk of exacerbations compared with placebo or their monocomponents.16–20
Given the numerous treatment options and pathways available for patients with COPD, it is of interest to understand the clinical drivers for switching from LAMA or LABA monotherapy or open combination LAMA+LABA dual therapy to single-inhaler LAMA/LABA dual therapy in clinical practice. However, the extent of variability in treatment pathways for patients with COPD who receive treatment with single-inhaler dual therapies in England remains unclear. The objective of this study was to describe characteristics, treatments prescribed, HCRU and costs in the year prior to initiation of single-inhaler dual LAMA/LABA therapy in a primary care setting in England. This reflects the need for an up-to-date overview of COPD treatment in primary care in England, to understand the treatment pathways and their respective effects on healthcare service burden for patients with COPD prior to single-inhaler dual therapy initiation.
Materials and Methods
Study Design
This was a retrospective, longitudinal cohort study using anonymized, linked primary care electronic medical record data and secondary care administrative data from England from the Clinical Practice Research Datalink (CPRD-Aurum) and Hospital Episode Statistics (HES) datasets, respectively. CPRD-Aurum captures diagnoses, symptoms, prescriptions, referrals and tests for over 19 million patients as of September 2018, while the HES Admitted Patient Care, Outpatient, and Accident & Emergency datasets contain details of all admissions to, or attendances at English National Health Service (NHS) healthcare providers.21
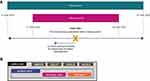
Patients with COPD who were prescribed single-inhaler LAMA/LABA dual therapy in primary care between 1 June 2015 and 31 December 2018 (the indexing period) were identified. The index date was defined as the date of the first/earliest prescription of a LAMA/LABA within the indexing period. The baseline period was defined as the 12-month period prior to the index date. Data from the 12-month follow-up period were analyzed separately and are not reported in this manuscript. All data reported is therefore from the indexing period and prior baseline period (Figure 1A). The study period was selected in part to ensure all therapies were approved and available for use in the UK throughout the baseline period.
Study Population
Eligible patients had a COPD diagnosis (a list of CPRD codes is shown in Supplementary Table 1) at age ≥35 years in the primary care setting and ≥1 prescription of a single-inhaler LAMA/LABA dual therapy within the indexing period. Patients were also required to have a forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) ratio <0.7 at any time prior to and including the index date, primary care data eligible for linkage to HES, and continuous registration with a general practitioner (GP) practice for ≥12 months prior to the index date. Patients with ≥1 diagnostic code of any medical conditions incompatible with a COPD diagnosis (eg, conditions related to lung or bronchial development anomalies, cystic fibrosis, pulmonary fibrosis) at any time in their medical history were excluded.
Patients were categorized into index treatment subgroups (ACL/FF, IND/GLY, TIO/OLO, UMEC/VI; Figure 1B). Within the overall cohort of LAMA/LABA users, the incident user cohort was defined as patients without a record of LAMA/LABA use (single-inhaler or open combination) prior to the index date. Within the incident user cohort, the non-triple user cohort included patients with no concomitant use of an inhaled corticosteroid (ICS)-containing medication on the index date. The initial maintenance therapy (IMT) user cohort included non-triple users with no prescription of any COPD maintenance therapy prior to the index date.
Study Outcomes
Study outcomes included demographics and clinical characteristics (age, sex, region, body mass index [BMI], lung function, smoking status, modified Medical Research Council [mMRC] dyspnea score, comorbidities, moderate-to-severe acute exacerbations of COPD [AECOPD], GOLD 2019 grade and blood eosinophil count) on the index date or during the baseline period, COPD treatment use (ICS only, LABA only, LAMA only, ICS/LABA, short-acting bronchodilators [SABD], other, or no respiratory therapy) at 3, 6, 9, and 12 months prior to the index date, all-cause and COPD-related HCRU (prescriptions, GP consultations, outpatient visits, inpatient stays, accident and emergency [A&E] visits) during the baseline period, cumulative length of all-cause and COPD-related inpatient stays across all admissions per patient during the baseline period, and all-cause and COPD-related costs (total direct and HCRU-specific healthcare costs) during the baseline period. COPD-related HCRU was defined as a COPD-related event (prescription for a respiratory medication, diagnosis or service provider) recorded on the same day as the resource use. COPD-related costs were those associated with COPD-related HCRU.
Moderate-to-severe AECOPDs were defined using a pre-specified algorithm adapted for COPD22 that included any of: a prescription for antibiotics and oral corticosteroids (OCS) for 5–14 days each; the presence of respiratory symptoms and a prescription for antibiotics or OCS (or both) on the same day; a lower respiratory tract infection medical code; or an AECOPD-specific medical code. Severe AECOPDs were defined as those requiring hospital admission, regardless of length of stay in hospital.
GP consultation costs were ascertained using the 2019 Unit Costs of Health and Social Care document compiled by the Personal Social Service Resource Unit (PSSRU).23 Prescription costs were taken from the 2019 NHS Drug Tariff.24 Outpatient, inpatient stay, and A&E visit costs were taken from the 2019/2020 national prices and national tariff workbook compiled by NHS Improvement and NHS England.25 The most recent version of each source at the time of the analysis was used.
Statistical Analysis
This was a descriptive study and no formal sample size calculations were performed. Patient demographics and clinical characteristics were examined during the baseline period, including index, or in the patient’s medical history. Counts, means, and standard deviations (SD) were reported for numeric variables, and relative frequencies and proportions were reported for the nominal variables. Total and HCRU type all-cause and COPD-related costs were reported for resource users, defined as those who had at least one unit of use for the respective healthcare service. Results based on small numbers of patients (n<5) were not reported, in line with standard CPRD reporting policies.
Results
Study Population
Of the 19,141 LAMA/LABA users that met the study eligibility criteria, 10,991 (57.4%) were included in the incident user cohort. The incident user cohort was further categorized into non-mutually exclusive subsets of non-triple users (n=9888) and IMT users (n=2963). This article will focus on findings in the non-triple user cohort as they represent the majority of the incident user cohort.
Of the non-triple user cohort, 21.3% (n=2109) received ACL/FF at index, 18.1% (n=1785) received IND/GLY, 12.0% (n=1189) received TIO/OLO, and 48.6% (n=4805) received UMEC/VI.
Demographics and clinical characteristics by cohort and by indexed therapy are shown in Table 1 and Supplementary Table 2; these were largely similar across cohorts, with little variation between the different indexed therapies. Overall, the majority of non-triple users were in the western regions, with 18.6% of patients located in the North West, 15.9% in the West Midlands and 22.0% in the South West. The mean (SD) FEV1% predicted ranged from 60.3 (17.5) in the ACL/FF subgroup to 61.3 (17.5) in the TIO/OLO subgroup and the proportion of patients with a current asthma diagnosis ranged from 10.9% in the UMEC/VI subgroup to 13.1% in the ACL/FF subgroup. In total, 26.3% (n=2111) and 19.4% (n=1559) of patients met the criteria for GOLD 2019 group B and D, respectively, with the highest proportions generally seen in the IND/GLY group. The IND/GLY group also had the highest proportion of patients experiencing moderate-to-severe AECOPDs in the 12-month baseline period (35.1%).
 |
Table 1 Baseline Demographics and Clinical Characteristics by Cohort and by Index LAMA/LABA Treatment |
Treatment Pathways
Across indexed therapy subgroups, 51.8–59.0% and 42.5–50.4% of patients were not prescribed any respiratory therapy at 12 and 3 months prior to index, respectively (Figure 2). At both 12 and 3 months prior to index, LAMA monotherapy was the most frequently prescribed respiratory medication across all indexed therapy subgroups (18.4–25.8% at 12 months; 23.9–33.7% at 3 months) (Figure 2). Across all indexed therapies, SABD use increased between 12 months (11.5–12.3%) and 3 months (12.5–15.6%) prior to index (Figure 2). Respiratory therapy use at all other pre-index time points showed a similar pattern, with LAMA monotherapy and SABD being the most frequently prescribed therapies and 40.4–57.7% of patients receiving no respiratory therapies (Supplementary Figure 1).
The mean number of prescribed respiratory medications per patient was consistently higher at 3 months prior to index than at 12 months prior to index in patients with and without AECOPDs in the prior year (Figure 3). Notably, at 3 months prior to index, the mean number of prescribed respiratory medications per patient was generally higher among patients with ≥1 versus 0 AECOPDs in the prior year (Figure 3). The mean (SD) number of respiratory therapies per patient across all pre-index time points ranged from 0.58 (0.78) to 0.86 (0.85) (Supplementary Figure 2).
HCRU and Costs
Across indexed therapy subgroups, the proportion of patients with a COPD-related prescription ranged from 61.3% to 72.3% (Table 2). Over the 12-month baseline period, 62.0–68.6% attended COPD-related GP consultations, 13.2–20.2% attended COPD-related outpatient visits, 19.3–26.1% needed COPD-related inpatient stays and 0.9–1.3% required a COPD-related A&E visit; similar trends were seen for all-cause HCRU (Table 2; Supplementary Table 3). Notably, during both the 12-month baseline period and the 1 day to ≤3 months prior to index, the proportion of patients with COPD-related prescriptions, outpatient visits and inpatient stays in the non-triple user cohort was highest in the IND/GLY subgroup (Table 2), as was the proportion of patients with all-cause outpatient visits, inpatient stays and A&E visits (Supplementary Table 3).
 |
Table 2 Proportion of Patients with COPD-Related HCRU During the Baseline Period (Non-Triple Users Cohort) |
Mean COPD-related HCRU per patient was also similar across indexed therapy subgroups. Among patients ≥1 use of the relevant resource, the mean number of GP consultations per patient was 1.99–2.15 in the 12-month baseline period and 1.30–1.38 between 1 day and ≤3 months prior to index, and the mean number of outpatient visits per patient was 2.18–2.74 and 1.50–1.71 in the same time periods (Figure 4). All-cause HCRU was also similar across indexed therapy subgroups. The mean number of GP consultations per patient was 11.78–12.19 in the 12-month baseline period and 3.93–4.02 between 1 day and ≤3 months prior to index, and the mean number of outpatient visits per patient was 5.74–6.67 and 2.45–2.80 in the same time periods (Supplementary Figure 3). The mean cumulative length of COPD-related inpatient stays during the 12-month baseline period ranged from 4.04 days in the ACL/FF subgroup to 6.06 days in the TIO/OLO subgroup (Supplementary Table 4). Between 1 day and ≤3 months prior to index, the cumulative length of COPD-related inpatient stays ranged from 3.79 in the ACL/FF subgroup to 5.53 in the TIO/OLO subgroup (Supplementary Table 4).
Across indexed therapy subgroups, the range of mean costs was £116–£138 for COPD-related prescriptions, £74–£80 for GP consultations, £347–£424 for outpatient visits, and £184–£216 for A&E visits, with costs generally highest in the IND/GLY subgroup and lowest in the UMEC/VI subgroup (Figure 5). Inpatient stays comprised the highest proportion of total costs (£3196–£3666) in all indexed therapy subgroups (Figure 5). A similar pattern was seen for COPD-related costs during the 3 months prior to index and for all-cause costs during both periods (Figure 5; Supplementary Figure 4). Mean total direct COPD-related healthcare costs per patient during the 12-month baseline period ranged from £805 in the ACL/FF subgroup to £1187 in the IND/GLY subgroup, while all-cause total direct healthcare costs ranged from £2116 in the UMEC/VI subgroup to £2617 in the IND/GLY subgroup (Table 3). Similar patterns were seen in the 3 months prior to index (Table 3).
 |
Table 3 Total Direct All-Cause and COPD-Related Healthcare Costs During the Baseline Period (Non-Triple Users Cohort) |
Discussion
This retrospective longitudinal cohort study used primary and secondary care data in England to determine treatment patterns and characteristics among patients with COPD who initiated single-inhaler LAMA/LABA dual therapy in a primary care setting in England. Within the non-triple users cohort, approximately 46% of patients were classified at baseline as GOLD group B or D, for which GOLD recommends that LAMA/LABA can be considered as an initial treatment option in patients with severe symptoms (eg, COPD Assessment Test score ≥20 for group D).10 As such, LAMA/LABA use may differ from guideline recommendations for the remaining 54% of patients. However, GOLD 2019 classification was based on exacerbation frequency in the 12 months prior to index and the most recent mMRC measurement in the 24 months prior to index, so it is possible that GOLD grades may have changed during the baseline period. Furthermore, patients with missing mMRC scores were not included in the GOLD 2019 categorization. Notably, only 15.5% of the overall incident user cohort were IMT users, suggesting that LAMA/LABA dual therapy is not commonly prescribed as IMT in England.
In the non-triple user cohort, there was little difference across indexed therapy subgroups in baseline demographics and clinical characteristics including lung function and the presence of comorbid asthma, although the IND/GLY subgroup had the highest proportion of patients with GOLD 2019 grade D and the highest proportion of patients with moderate-to-severe AECOPDs during the 12-month baseline period. There were differences across indexed therapy subgroups in the number and type of respiratory treatments received in the year prior to LAMA/LABA initiation. Some regional differences in LAMA/LABA prescribing were observed; for example, IND/GLY and TIO/OLO appeared to be the preferred prescribed treatments in the Northern regions, while ACL/FF and UMEC/VI were more commonly prescribed in the Midlands. The data cover a number of Clinical Commissioning Groups which may have different prescribing practices, but it is not possible to identify the reasons underlying the observed regional differences in this database study. Taken together, these results suggest that there are no consistent drivers for selecting one single-inhaler LAMA/LABA over another in England. This is consistent with recent findings of comparable safety and efficacy between dual therapy treatments,26,27 although some differences have been found in specific metrics, for example, a network meta-analysis demonstrated that patients receiving UMEC/VI had lower risk of exacerbations in comparison with other LAMA/LABA therapies.28
In the current study, some differences were observed between indexed therapy subgroups. However, no statistical comparisons were performed to substantiate these observations, so they should be interpreted with caution. Numerically, the IND/GLY therapy subgroup had the highest proportion of patients requiring for both, all-cause and COPD related, prescriptions, outpatient and inpatient stays and highest healthcare costs over the 12-month baseline period among non-triple users. Non-triple users receiving IND/GLY also had the highest mean number of respiratory therapies prescribed at every time point prior to index.
The study highlighted that around half of the patients in the non-triple users cohort did not receive respiratory therapy in the year prior to LAMA/LABA initiation, although this proportion was approximately 10% lower at 3 months prior to LAMA/LABA initiation than at 12 months prior. Importantly, the mean number of respiratory medications prescribed at 12 months and 3 months prior to starting LAMA/LABA treatment was generally higher in patients who had experienced ≥1 AECOPD in the 12 months prior to initiating LAMA/LABA than in those who had not experienced an AECOPD during this time period. This is in line with the NICE guidelines which recommend LAMA/LABA therapy in patients who remain breathless or have exacerbations despite SABD use.11
COPD-related HCRU during the 12-month baseline period was similar across the index therapy subgroups, with 61–72% of patients receiving a prescription, 62–69% attending GP consultations, 13–20% attending outpatient visits, 19–26% needing inpatient stays and ~1% requiring an A&E visit. Outpatient visits had the highest mean number per patient during the baseline period; our estimates were higher than those reported in a retrospective, observational study of patients with COPD in the UK in 2014, where the median number of secondary care visits ranged from 0 to 1.0 depending on disease severity.29 This difference may be due to the lower proportion of patients with very severe COPD (10%) in the 2014 study compared with ~20% GOLD group D patients in the non-triple users cohort in the current study. Notably, the mean number of COPD-related primary care contacts per patient in the present study (1.99–2.15) was similar to the median (2.33) reported in patients with mild-moderate COPD in the 2014 study.29 Total all-cause direct costs during the 12-month baseline period were approximately £2000–£2500, which is similar to the annual total COPD management costs shown in a cohort of patients with COPD in the UK 12 months following their COPD diagnosis.30 Inpatient stays were the greatest contributor to COPD-related costs during the baseline period, in line with a previous study conducted across the UK, USA, Canada, France, Germany, Italy, the Netherlands, and Spain, in which hospitalizations were found to account for 54% of total direct costs among patients with COPD.31 Given that approximately 50% of patients in the non-triple users cohort were not receiving respiratory therapies in the year prior to LAMA/LABA initiation, there appears to be an opportunity to provide early treatment optimization to reduce the unmet clinical and economic burden of disease and HCRU among patients with COPD in England. The study had a number of strengths, including the size of the cohorts and the use of the CPRD Aurum database which adequately captures the majority of patients’ healthcare journeys given that patients with COPD are largely managed in the primary care setting in the UK. As such, the study provided an accurate insight into prescribing patterns among healthcare professionals managing patients with COPD in England. Notably, use of the CPRD Aurum database meant that the study did not include privately insured patients; however, as over 98% of the UK population are registered with a primary care GP and under the NHS,32 the data are likely to be representative of the UK majority in this regard. However, they may not be readily generalizable to other countries where higher proportions of the population may use private healthcare primarily. Further limitations of the study included the possibility that medications may have been prescribed for asthma rather than for COPD in some patients, as well as the potential for misdiagnosis of asthma as COPD and vice versa. The requirement for patients in the study to have a COPD diagnosis helped ensure a focus on medications used to treat COPD. This is an accepted approach to identify patients with COPD, in line with a study showing that the presence of specific COPD codes in CPRD medical records could identify patients with COPD with a high positive predictive value.33 Furthermore, only medications prescribed in the primary care setting were recorded, potentially resulting in a misrepresentation of medication costs. Drugs prescribed in secondary and tertiary care were not included, despite evidence suggesting that these may incur higher costs than those prescribed in primary care in some cases.34 However, most prescriptions are either provided through primary care or managed by a GP after prescription by a specialist, and so would be observable in the data. There are likely to be relatively few medications which are not captured in the data due to being prescribed in secondary care without the involvement of a GP. Notably, approximately 25% of patients were missing mMRC scores, and so GOLD group could not be derived for these patients. Adherence to medication is an important factor in achieving disease control and reducing exacerbation risk; however, adherence was not assessed in this study. Finally, direct costs may have been underestimated, as some tariffs are negotiated locally and not nationally. Nonetheless, this study provides a robust, real-world picture of the characteristics of patients prescribed single-inhaler dual therapy for COPD in England.
Conclusion
This real-world study of patients with COPD in a primary care setting in England demonstrated that the characteristics of patients receiving different single-inhaler LAMA/LABA dual therapies were highly consistent, with no apparent drivers for choosing one therapy over another. Given that approximately half of the non-triple user cohort were not receiving respiratory therapies in the year prior to LAMA/LABA initiation and relatively few patients received LAMA/LABA as initial maintenance therapy, there may be an opportunity for early optimization of treatment to relieve the unmet clinical burden of COPD compared with current prescribing patterns.
Abbreviations
A&E, accident and emergency; ACL, aclidinium; AE, adverse event; AECOPD, acute exacerbations of COPD; BMI, body mass index; COPD, chronic obstructive pulmonary disease; CPRD, Clinical Practice Research Datalink; ED, emergency department; EV, forced expiratory volume in 1 second; FF, formoterol fumarate; FVC, forced vital capacity; GBP, British pound sterling; GERD, gastroesophageal reflux disease; GLY, glycopyrronium; GOLD, Global Initiative for Chronic Obstructive Lung Disease; GP, general practitioner; HCRU, healthcare resource use; HES, Hospital Episode Statistics; HRQoL, health-related quality of life; ICS, inhaled corticosteroid; IMT, initial maintenance therapy; IND, indacaterol; ITT, intent-to-treat; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist; mMRC, modified Medical Research Council; NHS, National Health Service; NICE, National Institute for Health and Care Excellence; NOS, nitric oxide synthase; OCS, oral corticosteroid; OLO, olodaterol; PSSRU, Personal Social Service Resource Unit; RFC, residual functional capacity; SABA, short-acting β2-agonist; SABD, short-acting bronchodilators; SAMA, short-acting muscarinic antagonist; SD, standard deviation; TIO, tiotropium bromide, UMEC, umeclidinium; VI, vilanterol.
Data Sharing Statement
The data analyzed in this publication are derived from the Clinical Practice Research Datalink (www.cprd.com) and Hospital Episode Statistics database (https://digital.nhs.uk/data-and-information/data-tools-and-services/data-services/hospital-episode-statistics). Authors had access to the study data for the purposes of this work only. Data were accessed through an existing GSK license to address pre-specified research questions only. Therefore, the data cannot be broadly disclosed or made publicly available at this time. Access to each database can be requested via the respective websites.
Ethics Approval and Informed Consent
Approval of this study was provided by the GSK Protocol Review Committee and by the Independent Scientific Advisory Committee (ISAC), which reviewed the protocol and approved access to Clinical Practice Research Datalink data (ISAC study no. 20_000145). No personal subject contact or primary collection of individual human data occurred, and anonymized patient-level data were used in this analysis; patient consent was therefore not required.
The study was conducted in accordance with the ethical principles of the Declaration of Helsinki. Informed consent and ethics committee or IRB approval were not required for the study as no direct patient contact or primary collection of patient data occurred. CPRD obtains ethical research approval annually from the UK’s Health Research Authority (HRA) Research Ethics Committee to accumulate and distribute patient data.
Acknowledgments
Editorial support (in the form of writing assistance, including preparation of the draft manuscript under the direction and guidance of the authors, collating and incorporating authors’ comments for each draft, assembling tables and figures, grammatical editing and referencing) was provided by Maria Guillermina Casabona, PhD, of Fishawack Indicia Ltd, UK, part of Fishawack Health, and was funded by GSK.
This study is based in part on data from the Clinical Practice Research Datalink obtained under license from the UK Medicines and Healthcare products Regulatory Agency. The data is provided by patients and collected by the NHS as part of their care and support. The interpretation and conclusions contained in this study are those of the author/s alone.
Copyright © (2022), re-used with the permission of The Health & Social Care Information Centre. All rights reserved.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. AC, VB, GR, RWo, TT, RWi, and ASI were involved in the in the conception and design of the study, the acquisition of data, and data analysis and interpretation. CC and MD were involved in the conception and design of the study, and in data analysis and interpretation.
Funding
This study/analysis was funded by GSK (GSK study 214731). GSK-affiliated authors had a role in study design, data analysis, data interpretation, and writing of the report, and GSK funded the article processing charges and open access fee.
Disclosure
AC, GR, CC, MD and ASI are employees of GSK and hold stock and shares at GSK. ASI is also a part-time member of the McMaster university faculty. VB, TT, RWo and RWi are employees of Adelphi Real World (or were at the time of the study), which received funding from GSK to conduct the study. Adelphi Real World is a business that provides consulting and other research services to pharmaceutical, device, government, and non-government organizations. Adelphi Real World employees work with a variety of companies and organizations and are expressly prohibited from receiving any payment or honoraria directly from these organizations for services rendered. Since concluding the study, VB left employment with Adelphi Real World and started full time employment with Bayer PLC.
References
1. Soriano JB, Polverino F, Cosio BG. What is early COPD and why is it important? Eur Respir J. 2018;52(6):1801448. doi:10.1183/13993003.01448-2018
2. Baraldo S, Turato G, Saetta M. Pathophysiology of the small airways in chronic obstructive pulmonary disease. Respiration. 2012;84(2):89–97. doi:10.1159/000341382
3. G. B. D. Chronic Respiratory Disease Collaborators. Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet Respir Med. 2020;8(6):585–596. doi:10.1016/S2213-2600(20)30105-3
4. British Lung Foundation. Chronic obstructive pulmonary disease (COPD) statistics; 2021. Available from: https://statistics.blf.org.uk/copd.
5. Health and Safety Executive. Work-related chronic obstructive pulmonary disease (COPD) statistics in Great Britain; 2020. Available from: https://www.hse.gov.uk/statistics/causdis/copd.pdf.
6. Iheanacho I, Zhang S, King D, Rizzo M, Ismaila AS. Economic burden of Chronic Obstructive Pulmonary Disease (COPD): a systematic literature review. Int J Chron Obstruct Pulmon Dis. 2020;15:439–460. doi:10.2147/COPD.S234942
7. Ehteshami-Afshar S, FitzGerald JM, Doyle-Waters MM, Sadatsafavi M. The global economic burden of asthma and chronic obstructive pulmonary disease. Int J Tuberc Lung Dis. 2016;20(1):11–23. doi:10.5588/ijtld.15.0472
8. Brien SB, Lewith GT, Thomas M. Patient coping strategies in COPD across disease severity and quality of life: a qualitative study. NPJ Prim Care Respir Med. 2016;26:16051. doi:10.1038/npjpcrm.2016.51
9. Wang Q, Ji W, He K, et al. Genetic analysis of common variants in the ZNF804A gene with schizophrenia and major depressive disorder. Psychiatr Genet. 2018;28(1):1–7. doi:10.1097/ypg.0000000000000185
10. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease; 2021. Available from: https://goldcopd.org/wp-content/uploads/2020/11/GOLD-REPORT-2021-v1.1-25Nov20_WMV.pdf.
11. National Institute for Health and Care Excellence. Chronic obstructive pulmonary disease in over 16s: diagnosis and management; 2018. Available from: https://www.nice.org.uk/guidance/ng115.
12. Novartis Pharmaceuticals UK Ltd. Ultibro Breezhaler SmPC; 2020. Available from: https://www.medicines.org.uk/emc/medicine/29533.
13. AstraZeneca UK Ltd. Duaklir Genuair SmPC; 2021. Available from: https://www.medicines.org.uk/emc/medicine/29652.
14. Boehringer Ingelheim Limited. Spiolto Respimat SmPC; 2020. Available from: https://www.medicines.org.uk/emc/medicine/30495.
15. GlaxoSmithKline UK. Anoro Ellipta SmPC; 2021. Available from: https://www.medicines.org.uk/emc/medicine/28949.
16. Donohue JF, Maleki-Yazdi MR, Kilbride S, Mehta R, Kalberg C, Church A. Efficacy and safety of once-daily umeclidinium/vilanterol 62. 5/25mcg in COPD. Respir Med. 2013;107(10):1538–1546. doi:10.1016/j.rmed.2013.06.001
17. Bateman ED, Chapman KR, Singh D, et al. Aclidinium bromide and formoterol fumarate as a fixed-dose combination in COPD: pooled analysis of symptoms and exacerbations from two six-month, multicentre, randomised studies (ACLIFORM and AUGMENT). Respir Res. 2015;16:92. doi:10.1186/s12931-015-0250-2
18. Buhl R, Maltais F, Abrahams R, et al. Tiotropium and olodaterol fixed-dose combination versus mono-components in COPD (GOLD 2–4). Eur Respir J. 2015;45(4):969–979. doi:10.1183/09031936.00136014
19. Bateman ED, Ferguson GT, Barnes N, et al. Dual bronchodilation with QVA149 versus single bronchodilator therapy: the SHINE study. Eur Respir J. 2013;42(6):1484–1494. doi:10.1183/09031936.00200212
20. Wedzicha JA, Decramer M, Ficker JH, et al. Analysis of chronic obstructive pulmonary disease exacerbations with the dual bronchodilator QVA149 compared with glycopyrronium and tiotropium (SPARK): a randomised, double-blind, parallel-group study. Lancet Respir Med. 2013;1(3):
21. Wolf A, Dedman D, Campbell J, et al. Data resource profile: clinical practice research datalink (CPRD) aurum. Int J Epidemiol. 2019;48(6):1740–1740g. doi:10.1093/ije/dyz034
22. Nissen F, Morales DR, Mullerova H, Smeeth L, Douglas IJ, Quint JK. Validation of asthma recording in the Clinical Practice Research Datalink (CPRD). BMJ Open. 2017;7(8):e017474. doi:10.1136/bmjopen-2017-017474
23. Curtis LA. Burns, Amanda Unit costs of health and social care; 2019.
24. National Health Services. Drug tariff part VIII. Available from: https://www.nhsbsa.nhs.uk/pharmacies-gp-practices-and-appliance-contractors/drug-tariff/drug-tariff-part-viii.
25. National Health Services England. National tariff 2019/20: documents and policies. Available from: https://www.england.nhs.uk/publication/past-national-tariffs-documents-and-policies/.
26. Schlueter M, Gonzalez-Rojas N, Baldwin M, Groenke L, Voss F, Reason T. Comparative efficacy of fixed-dose combinations of long-acting muscarinic antagonists and long-acting β2-agonists: a systematic review and network meta-analysis. Ther Adv Respir Dis. 2016;10(2):89–104. doi:10.1177/1753465815624612
27. Siddiqui MK, Shukla P, Jenkins M, et al. Systematic review and network meta-analysis of the efficacy and safety of glycopyrrolate/formoterol fumarate metered dose inhaler in comparison with other long-acting muscarinic antagonist/long-acting β2-agonist fixed-dose combinations in COPD. Ther Adv Respir Dis. 2019;13:1753466619894502. doi:10.1177/1753466619894502
28. Lee HW, Park J, Jang EJ, Lee C-H. Comparisons of exacerbations and mortality among LAMA/LABA combinations in stable chronic obstructive pulmonary disease: systematic review and Bayesian network meta-analysis. Respir Res. 2020;21(1):1–15. doi:10.1186/s12931-020-01540-8
29. Thomas M, Radwan A, Stonham C, Marshall S. COPD exacerbation frequency, pharmacotherapy and resource use: an observational study in UK primary care. COPD. 2014;11(3):300–309. doi:10.3109/15412555.2013.841671
30. Punekar YS, Wurst K, Shukla A. Resource use and costs up to two years post diagnosis among newly diagnosed COPD patients in the UK primary care setting: a retrospective cohort study. COPD. 2015;12(3):267–275. doi:10.3109/15412555.2014.933953
31. Britton M. The burden of COPD in the UK: results from the confronting COPD survey. Respir Med. 2003;97(Suppl C):S71–79. doi:10.1016/s0954-6111(03)80027-6
32. Herrett E, Gallagher AM, Bhaskaran K, et al. Data resource profile: clinical practice research datalink (CPRD). Int J Epidemiol. 2015;44(3):827–836. doi:10.1093/ije/dyv098
33. Quint JK, Müllerova H, DiSantostefano RL, et al. Validation of chronic obstructive pulmonary disease recording in the Clinical Practice Research Datalink (CPRD-GOLD). BMJ open. 2014;4(7):e005540. doi:10.1136/bmjopen-2014-005540
34. Kim C, Yoo K, Rhee C, et al. Health care use and economic burden of patients with diagnosed chronic obstructive pulmonary disease in Korea. Int J Tuberculosis Lung Dis. 2014;18(6):737–743. doi:10.5588/ijtld.13.0634
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.