Back to Journals » Journal of Pain Research » Volume 17
Characteristic Behaviors of Pain During Movement in the Older Individuals with Dementia
Authors Nakada K , Shimo K , Ohga S , Matsubara T
Received 7 June 2023
Accepted for publication 5 February 2024
Published 5 March 2024 Volume 2024:17 Pages 865—871
DOI https://doi.org/10.2147/JPR.S424889
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Kushang V Patel
Kenta Nakada,1,2 Kazuhiro Shimo,3 Satoshi Ohga,3 Takako Matsubara1,3
1Faculty of Rehabilitation, Kobe Gakuin University Graduate School, Kobe, Japan; 2Department of Rehabilitation, Ikeda Rehabilitation Hospital, Kurobe, Japan; 3Department of Physical Therapy, Faculty of Rehabilitation, Kobe Gakuin University, Kobe, Japan
Correspondence: Kazuhiro Shimo, Department of Physical Therapy, Faculty of Rehabilitation, Kobe Gakuin University, 518 Arise, Ikawadani-cho, Nishi-ku, Kobe, Hyogo, 651-2180, Japan, Tel/Fax +81-78-974-2461, Email [email protected]
Purpose: This study assessed the pain associated with movement and exercise in older individuals with cognitive decline, using the Abbey Pain Scale (APS) to identify the sub-items that effectively reflect pain during such activities.
Patients and Methods: A cross-sectional study was conducted in 225 older patients with musculoskeletal disorders and cognitive decline at the Ikeda Rehabilitation Hospital in Toyama, Japan. Pain during walking or transferring was assessed using the verbal rating scale (VRS) and the APS. Item response theory (IRT) was used to identify the APS sub-items that most accurately reflected the presence and degree of pain.
Results: Pain associated with movement scored 1.3 ± 1.1 on the VRS and 2.5 ± 2.6 on the APS. The IRT analysis extracted “vocalization”, “facial expression”, and “change in body language” as the most reliable indicators of pain. These extracted items showed good internal consistency (Cronbach’s α = 0.72), were significantly positively related to changes in the VRS (rs = 0.370, p < 0.001), and showed significant differences between patients with and without subjective pain.
Conclusion: Our study suggests that the APS sub-items “vocalization”, “facial expression”, and “change in body language” may be the most effective indicators of pain during movement and exercise in older individuals with cognitive decline. This approach may enhance the reliability of pain assessments and management during exercise therapy.
Keywords: behavior observation assessments, pain assessment, pain behavior, item response theory
Introduction
The number of older individuals is increasing in nearly all nations, and the dementia incidence is also increasing rapidly as the population ages.1 Older individuals with cognitive decline frequently experience pain resulting from chronic tissue degeneration, disease, and surgical procedures related to these conditions.2 The prevalence of pain in older individuals with dementia is estimated to range from 32% to 64%,3–6 and the need for treatment and care for these individuals in a clinical setting is escalating. Musculoskeletal pain is common among older individuals, and exercise therapy is typically the first-line treatment. However, pain can act as a barrier to exercise therapy, and it is essential to evaluate pain, particularly in relation to movement and activity, and to prescribe appropriate exercise loads and modalities for exercise therapy.
Patient-reported outcomes (PROs), such as numerical and verbal rating scales (VRS) are widely used worldwide as the gold standard for assessing the presence and severity of pain. However, the validity of PROs and low response rates can be problematic among older patients with cognitive decline, as it can be difficult for them to understand numerical values and language.7 For this reason, the International Association for the Study of Pain recommends conducting a pain behavior observation evaluation in addition to PROs for pain assessment in older patients with cognitive decline.8 Pain behavior observation evaluation is a method of objectively evaluating pain by observing the subject’s daily life and behavior. The Abbey Pain Scale (APS), DOLOPLUS-2, and Pain Assessment Checklist for Seniors With Limited Ability to Communicate are examples of behavioral observation assessments, particularly for older patients with cognitive decline.9–11 The APS is a highly convenient assessment method in clinical practice because it can determine the presence or absence and degree of a subject’s pain in a short time. The APS is a behavioral observational assessment developed to aid various healthcare professionals and caregivers in efficiently assessing pain in older individuals with dementia.9 The APS consists of six items and observes pain-related behaviors, such as vocalization, facial expression, and change in body language, as well as behavioral, physiological, and physical changes. The nature of musculoskeletal pain is dynamic as it changes with activities such as loading or postural shifts, thereby necessitating a tool that can monitor these fluctuations during exercise therapy. Because APS can quickly assess pain, it seems apt for this purpose. However, identifying which APS sub-items are most indicative of movement- and exercise-related pain in older adults with cognitive impairment is essential. Recognizing specific pain-related behaviors during movement can provide insights into individualized therapeutic interventions.
We utilized the APS to identify which sub-items accurately reflect pain during movement. We focused on the unique pain-related behaviors associated with movement in older adults with cognitive impairment to enhance our understanding and subsequently the quality of care.
Materials and Methods
Ethics Approval and Informed Consent
This study was approved by the Ethical Review Committees of Ikeda Rehabilitation Hospital (approval number: Reha0003) and Kobe Gakuin University (approval number: 20–33) and conducted in accordance with the 1964 Helsinki Declaration and its later amendments. Consent was obtained from 151 participants and 74 family members scoring below 15 on the Mini-Mental State Examination (MMSE, range: 0–30 points). The research protocol clearly stated that for participants with MMSE scores of 15 or below, consent from their families was required. This protocol was reviewed and approved by the ethics committee before the study was conducted.
Subjects
This study included 225 older patients with musculoskeletal disorders and cognitive decline who were admitted to the Ikeda Rehabilitation Hospital in Toyama, Japan. The inclusion criteria were age 65 years or older, suspected cognitive decline based on a score of 27 points or less on MMSE, and ability to provide a PRO-based pain assessment. The exclusion criteria were severe cardiovascular, respiratory, or metabolic diseases, such as heart failure, cerebrovascular disease, cancer, or Parkinson’s disease; impaired alertness; and communication difficulties due to aphasia or severe hearing loss. The sample size should be 10 times the number of items; therefore, since the APS has six items, at least 60 persons were required. In addition, the higher the number of participants, the higher the measurement accuracy of the item response theory (IRT).12
Protocol
This cross-sectional study recorded demographic data and pain intensity using the VRS and APS.
Demographic Data
Demographic and clinical data (age, sex, MMSE score, disease, operative procedure, postoperative days, and use of analgesics) were collected from the medical records.
Pain Intensity
We assessed pain during walking for participants who could walk, and pain during transfers for participants with difficulty walking. Pain was assessed using the VRS as the PRO and the APS as the observational pain assessment during walking or transfer. We used a five-point VRS with the words “no pain”, “slight pain”, “moderate pain”, “severe pain”, and “unbearable pain”. The assessor asked the patient to respond to the VRS regarding pain associated with movement immediately after walking or transferring. The assessor also observed the patient walking or during transferring and assessed the patient’s pain using the APS. We used the Japanese-translated version of the APS,13 which was translated by Takai et al and verified for reliability and validity. A fully experienced physical therapist conducted all assessments from 1:00 pm to 3:00 pm in the rehabilitation rooms at the hospital.
Statistical Analysis
Data are presented as the mean and standard deviation. As most of our data were not normally distributed, we used less sensitive (although more robust) nonparametric tests for all statistical analyses. The IRT14 was used to examine which items of the APS better reflected the presence or absence and degree of pain. IRT methods can provide item and test information, which can help discriminate items that are more attributable to the entire scale. This is beneficial because researchers can remove ineffective items to shorten the scale and improve its efficiency. In the IRT, the two parameters of interest are difficulty and discrimination. Difficulty signifies the average ability required to answer an item correctly. In this study, an item with a higher value implied that it was more readily responded to by those experiencing more intense pain, and less efficiently responded to by those experiencing less pain. Discrimination reflects an item’s sensitivity to varying degrees of pain. Discrimination was represented by item characteristic curves (ICCs), which graphically illustrate the relationship between latent traits and item endorsement, a unique feature of IRT. Based on a previous study, the following guidelines15 for identification scores were adopted: 0.01–0.34 = very low, 0.35–0.64 = low, 0.65–1.34 = moderate, 1.35–1.69 = high, and > 1.70 = very high. The reliability of pain assessment using items extracted by the IRT was examined using Cronbach’s alpha coefficient. The validity of pain assessment by items extracted by the IRT was examined using Spearman’s rank test for correlation with the VRS. The Mann–Whitney U-test was used to determine whether there was a difference in pain assessment based on items extracted by the IRT with and without subjective pain. Statistical analyses were performed using R software (version 4.1.1; R Foundation for Statistical Computing, Vienna, Austria). The significance level was set at p < 0.05.
Results
Demographic Data
A total of 225 participants were included in this study. The participants’ characteristics are presented in Table 1.
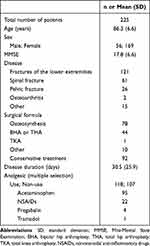 |
Table 1 Characteristics of Study Population |
Pain Intensity
Pain associated with movement scored 1.3 ± 1.1 on the VRS and 2.5 ± 2.6 on the APS. The APS sub-scores were 0.2 ± 0.5 for “vocalization”, 0.6 ± 0.8 for “facial expression”, 0.7 ± 0.9 for “change in body language”, 0.2 ± 0.5 for “behavior change”, 0.1 ± 0.3 for “physiological change”, and 0.7 ± 0.8 for “physical changes”. Sixty-one patients reported no subjective pain (VRS score = 0).
Item Response Theory
Difficulty and discrimination were calculated from the ICCs for each item of the APS using the IRT (Table 2). Based on previous studies, those that met both difficulty (0–3) and discrimination (1.35 or higher) criteria were extracted as “vocalization”, “facial expression”, and ”change in body language”.
 |
Table 2 Difficulty and Discrimination of APS Sub-Items |
Reliability
Cronbach’s alpha was calculated to assess internal consistency. It has been suggested that alpha levels should be above 0.70 to indicate good reliability.16 The internal consistency of pain assessment by items extracted by the IRT was 0.72.
Validity
To test construct validity, we correlated the items extracted by the IRT with the APS. The pain assessment using items extracted by the IRT was significantly and positively related to the APS (rs = 0.909, p < 0.001). The pain assessment by items extracted by IRT differed significantly between patients with and without subjective pain (with pain, 1.9 ± 1.9; without pain, 0.6 ± 1.1) (Figure 1).
Discussion
This study explored the characteristics of pain behavior in older individuals with cognitive decline and examined their responses to movement-associated pain. We assessed the pain during walking or transferring activities using the APS. IRT analysis revealed that three items, “vocalization”, “facial expression”, and “change in body language”, contributed significantly to the total APS score. This score represents the pain level observable from behavioral indicators. Although IRT has been predominantly used in educational settings, it has recently gained traction in the medical field, augmenting classical test theory.17 Our study utilized IRT to compute two parameters: difficulty and discrimination. Higher difficulty values indicated more severe perceived pain, whereas higher discrimination values suggested that the item more accurately identified the presence or absence of pain.
If vocalization, facial expression, or body language changes are observed during painful movements, the total APS score increases, implying a higher likelihood of pain. Of the APS sub-scores, “vocalization”, “facial expression”, and “change in body language” reflect immediate pain responses. Conversely, “behavioral change”, “physiological change”, and “physical changes” signify alterations in a patient’s condition resulting from pain.9
Exercise-induced pain results from the nociceptive stimuli applied through loading and joint movement. Thus, “vocalization” and “facial expression”, immediate pain responses, and “change in body language” are believed to mirror exercise-associated pain more accurately. It is well established that pain causes characteristic facial expressions.18 These include eye-closing, eyebrow lowering, and contraction of the lip-raising muscles. Previous reports have identified changes in body language as pain indicators.19 In our sample, rubbing painful areas, leaning to avoid loads, and physical aggression were body language indicators of pain. Systematic reviews suggest that, while vocalizations relate to pain, they should not serve as standalone indicators.20 Language impairment in patients with dementia can diminish speech and hinder pain communication.21 Hence, combining vocalization with facial expressions and body language may yield a more reliable pain assessment.
The Cronbach’s coefficient for pain assessment during locomotion, using the three items “vocalization”, “facial expression”, and “change in body language”, exceeded 0.7, suggesting excellent internal consistency. When the participants were divided based on the presence of subjective pain according to the VRS, we observed differences in scores between the two groups. Although categorizing the presence of pain based on subjective complaints may not always be suitable, particularly in cognitively impaired individuals, the observed group differences may attest to some validity. Pain management during exercise therapy is essential and requires healthcare providers to prescribe appropriate exercises while considering the subject’s pain complaints. In cognitively impaired individuals, pain assessment based on PROs is often challenging, requiring healthcare providers to determine the presence of pain through behavioral observation. Although we have been empirically estimating the presence of pain in subjects for exercise therapy, focusing on ”vocalization”, ”facial expression”, and “change in body language” may enhance the reliability of pain assessment. Therefore, we believe the results of our study have potential for developing pain assessment methods essential for prescribing exercise for improved pain management in older individuals with cognitive decline.
This study had several limitations that must be acknowledged. First, the pain levels of the participants were relatively low. The study included patients with musculoskeletal pain approximately 1-month post-injury, potentially representing a more progressive recovery stage. While our findings may be applicable to patients requiring exercise therapy during this period, further studies should explore pain during exercise in older patients with dementia and more severe pain immediately after injury or surgery. Second, the participants in our study exhibited mild-to-moderate cognitive decline. We included participants capable of pain assessment using the VRS, a PRO, to examine the correlation between APS and subjective pain. Therefore, patients with severe dementia were excluded. Assessing pain in older patients with severe dementia using behavioral observation assessments presents validity concerns.22 APS scores are known to increase with cognitive function decline,13 and it is expected that behavioral and psychological symptoms of dementia may vary depending on dementia severity. Hence, it should be noted that pain behavior characteristics in patients with severe dementia may differ from those observed in this study.
Conclusion
We investigated the characteristics of pain behaviors associated with movement-related pain in older patients with mild-to-moderate cognitive decline. Pain during walking or transfer was assessed using APS. Our findings suggested that changes in vocalizations, facial expressions, and body language may reflect movement-related pain in older adults experiencing cognitive decline.
Abbreviations
APS, Abbey Pain Scale; ICCs, item characteristic curves; IRT, item response theory; MMSE, Mini-Mental State Examination; PRO, patient-reported outcome; VRS, visual rating scale.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors thank all the subjects examined in this study and the staff at the Department of Rehabilitation, Ikeda Rehabilitation Hospital for their support with data collection. We would like to thank Editage for English language editing. We also thank the first author’s current affiliation, Department of Rehabilitation, Toyama University Hospital, Toyama, Japan. No funding was received for this study.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. World Health Organization. Dementia. Available from: https://www.who.int/news-room/fact-sheets/detail/dementia.
2. International Association for the Study of Pain. Pain in Older Adults. Available from: https://www.iasp-pain.org/resources/fact-sheets/pain-in-older-adults/.
3. Ferrell BA, Ferrell BR, Rivera L. Pain in cognitively impaired nursing home patients. J Pain Symptom Manage. 1995;10(8):591–598. PMID: 8594119. doi:10.1016/0885-3924(95)00121-2
4. Shega JW, Hougham GW, Stocking CB, Cox-Hayley D, Sachs GA. Pain in community-dwelling persons with dementia: frequency, intensity, and congruence between patient and caregiver report. J Pain Symptom Manage. 2004;28(6):585–592. PMID: 15589083. doi:10.1016/j.jpainsymman.2004.04.012
5. Jensen-Dahm C, Vogel A, Waldorff FB, Waldemar G. Discrepancy between self- and proxy-rated pain in Alzheimer’s disease: results from the Danish Alzheimer intervention study. J Am Geriatr Soc. 2012;60(7):1274–1278. PMID: 22702408. doi:10.1111/j.1532-5415.2012.04036.x
6. Barry HE, Parsons C, Passmore AP, Hughes CM. Exploring the prevalence of and factors associated with pain: a cross-sectional study of community-dwelling people with dementia. Health Soc Care Community. 2016;24(3):270–282. PMID: 25708056. doi:10.1111/hsc.12204
7. Closs SJ, Barr B, Briggs M, Cash K, Seers K. A comparison of five pain assessment scales for nursing home residents with varying degrees of cognitive impairment. J Pain Symptom Manage. 2004;27(3):196–205. PMID: 15010098. doi:10.1016/j.jpainsymman.2003.12.010
8. International Association for the Study of Pain. Pain Assessment in Dementia. Available from: https://www.iasp-pain.org/resources/fact-sheets/pain-assessment-in-dementia/.
9. Abbey J, Piller N, De Bellis A, et al. The Abbey pain scale: a 1-minute numerical indicator for people with end-stage dementia. Int J Palliat Nurs. 2004;10(1):6–13. PMID: 14966439. doi:10.12968/ijpn.2004.10.1.12013
10. Lefebvre-Chapiro S. The DOLOPLUS 2 scale – evaluating pain in the older. Eur J Palliat Care. 2001;8(5):191–194.
11. Fuchs-Lacelle S, Hadjistavropoulos T. Development and preliminary validation of the pain assessment checklist for seniors with limited ability to communicate (PACSLAC). Pain Manag Nurs. 2004;5(1):37–49. PMID: 14999652. doi:10.1016/j.pmn.2003.10.001
12. Cappelleri JC, Jason Lundy J, Hays RD. Overview of classical test theory and item response theory for the quantitative assessment of items in developing patient-reported outcomes measures. Clin Ther. 2014;36(5):648–662. PMID: 24811753. doi:10.1016/j.clinthera.2014.04.006
13. Takai Y, Yamamoto-Mitani N, Chiba Y, Nishikawa Y, Hayashi K, Sugai Y. Abbey Pain Scale: development and validation of the Japanese version. Geriatr Gerontol Int. 2010;10(2):145–153. PMID: 20446928. doi:10.1111/j.1447-0594.2009.00568.x
14. Alimoradi Z, Lin CY, Ullah I, Griffiths MD, Pakpour AH. Item response theory analysis of the fear of COVID-19 scale (FCV-19S): a systematic review. Psychol Res Behav Manag. 2022;15:581–596. PMID: 35300204. doi:10.2147/PRBM.S350660
15. Walsh A, Cao R, Wong D, et al. Using item response theory (IRT) to improve the efficiency of the simple clinical colitis activity index (SCCAI) for patients with ulcerative colitis. BMC Gastroenterol. 2021;21(1):132. PMID: 33752610. doi:10.1186/s12876-021-01621-y
16. Woby SR, Roach NK, Urmston M, Watson PJ. Psychometric properties of the TSK-11: a shortened version of the Tampa Scale for Kinesiophobia. Pain. 2005;117(1–2):137–144. PMID: 16055269. doi:10.1016/j.pain.2005.05.029
17. Elston B, Goldstein M, Makambi KH. Item response theory analysis of the outpatient physical therapy improvement in movement assessment log (OPTIMAL). Phys Ther. 2013;93(5):661–671. PMID: 23431211. doi:10.2522/ptj.20120120
18. Prkachin KM. Facial pain expression. Pain Manag. 2011;1(4):367–376. PMID: 24645662. doi:10.2217/pmt.11.22
19. Strand LI, Gundrosen KF, Lein RK, et al. Body movements as pain indicators in older people with cognitive impairment: a systematic review. Eur J Pain. 2019;23(4):669–685. PMID: 30450680. doi:10.1002/ejp.1344
20. Helmer LML, Weijenberg RAF, de Vries R, et al. Crying out in pain-A systematic review into the validity of vocalization as an indicator for pain. Eur J Pain. 2020;24(9):1703–1715. PMID: 32573041. doi:10.1002/ejp.1623
21. Hadjistavropoulos T, Herr K, Prkachin KM, et al. Pain assessment in older adults with dementia. Lancet Neurol. 2014;13(12):1216–1227. PMID: 25453461. doi:10.1016/S1474-4422(14)70103-6
22. Zwakhalen SM, Hamers JP, Abu-Saad HH, Berger MP. Pain in older people with severe dementia: a systematic review of behavioural pain assessment tools. BMC Geriatr. 2006;6:3. PMID: 16441889. doi:10.1186/1471-2318-6-3
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

