Back to Journals » Infection and Drug Resistance » Volume 16
Changes of Serum Pyruvate Kinase M2 Level in Patients with Sepsis and Its Clinical Value
Authors Wang L , Tang D , Zhang P
Received 6 July 2023
Accepted for publication 21 September 2023
Published 28 September 2023 Volume 2023:16 Pages 6437—6449
DOI https://doi.org/10.2147/IDR.S429314
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Li Wang, Dongling Tang, Pingan Zhang
Department of Clinical Laboratory, Institute of Translational Medicine, Renmin Hospital of Wuhan University, Wuhan, Hubei, 430060, People’s Republic of China
Correspondence: Pingan Zhang, Department of Clinical Laboratory, Institute of Translational Medicine, Renmin Hospital of Wuhan University, Wuhan, Hubei, 430060, People’s Republic of China, Tel +86 13971196429, Email [email protected]
Purpose: The glucose metabolic reprogramming is an important pathological mechanism in sepsis, which involves a series of enzymes including Pyruvate kinase M2 (PKM2). The purpose of this study is to explore the diagnostic and prognostic value of serum PKM2 in sepsis patients.
Patients and Methods: This study recruited 143 sepsis patients, 91 non-sepsis patients, and 65 physical examiners, divided into sepsis group, non-sepsis group, and control group. Measure the serum PKM2 concentration of subjects, collect and analyze clinical and laboratory indicators of all subjects. Independent risk factors were selected by Logistic regression analysis. The area under curve (AUC) was calculated by plotting the receiver operating characteristic (ROC) curve to determine the diagnostic and prognostic value of biomarkers.
Results: Compared with non-sepsis and control groups, the serum PKM2 levels in the sepsis group were significantly increased (both P< 0.001). PKM2 was an independent risk factor for sepsis and had the best diagnostic efficacy when combined with procalcitonin, with the AUC value of 0.9352. Patients with high levels of PKM2 were more likely to experience organ damage and had a higher incidence of septic shock. On the 1st and 3rd days of admission, the serum PKM2 levels in the septic shock group were higher than those in the sepsis group (both P< 0.05), with AUC values of 0.7296 and 0.6247, respectively. On the 3rd and 7th days of admission, the serum PKM2 levels in the non-survival group were significantly higher than those in the survival group (both P< 0.001), with AUC values of 0.7033 and 0.8732, respectively.
Conclusion: The serum PKM2 levels in sepsis patients are significantly increased and correlated with disease severity and clinical outcomes. PKM2 may be a new diagnostic and prognostic biomarker for sepsis.
Keywords: sepsis, PKM2, glucose metabolic reprogramming, diagnosis, prognosis
Introduction
Sepsis is a complex disease caused by a dysregulated host immune response to infection, ultimately leading to life-threatening acute organ dysfunction.1 Sepsis is characterized by high morbidity and mortality, resulting in huge medical and economic burdens. According to epidemiological surveys, there are nearly 49 million sepsis patients worldwide each year, of which 11 million die, accounting for approximately 20% of all deaths worldwide.2 At present, the management of clinical sepsis patients still relies mainly on traditional treatment measures such as fluid resuscitation, antibiotics, and vasopressors.3 Early identification and timely management play a decisive role in improving the clinical outcomes of sepsis patients.4 Biomarkers can be used for early infection diagnosis, prognosis, and treatment guidance in sepsis patients, providing clinical information superior to other examination methods.5
The pathophysiological mechanism of sepsis is complex, and glucose metabolic reprogramming in immune cells is one of the important pathogenic mechanisms. The glucose metabolic reprogramming, also known as the “Warburg effect”, was originally discovered by biochemist Otto Warburg, which means that cells use glucose in the way of glycolysis under aerobic conditions.6 Activated immune cells mainly rely on aerobic glycolysis to rapidly produce a large amount of ATP and biosynthetic intermediates, which are used to support cell proliferation and inflammatory factor production.7 The glycolysis process involves a series of enzymes, of which Pyruvate kinase M2 (PKM2) is a rate-limiting enzyme. PKM2, the M2 isoform of pyruvate kinase, participates in the final stage of glucose metabolic reprogramming by dephosphorylating phosphoenolpyruvate to pyruvate, and can also enter the nucleus to regulate the expression of hypoxia-inducible factor-1α (HIF-1α), programmed cell death ligand 1 (PD-L1) and other genes.8,9 PKM2, as the key mediator and potential therapeutic target for inflammation and metabolic disorders, has received increasing attention.10,11 However, there are no research reports on the clinical value of serum PKM2 as a biomarker for sepsis. Therefore, in this study, we measured serum PKM2 levels in sepsis patients, analyzed the correlation between PKM2 and disease severity, inflammation indicators, and organ damage scores, and evaluated the clinical value of PKM2 as a biomarker for sepsis.
Materials and Methods
Study Population and Grouping
A total of 299 subjects were recruited in the Renmin Hospital of Wuhan University from April 2022 to April 2023. All subjects were divided into three groups, including 143 in the sepsis group, 91 in the non-sepsis group, and 65 in the control group. Patients in the sepsis group came from the intensive care unit (ICU), and the inclusion criteria refer to sepsis 3.0 definition: sepsis is a life-threatening organ dysfunction caused by the host’s dysfunctional response to infection.1 Patients in the sepsis group were further divided into sepsis and septic shock groups with the following criteria: after adequate fluid resuscitation, vasoactive drugs were still required to maintain a mean arterial pressure (MAP) ≥65 mmHg (1 mmHg = 0.133kPa) and serum lactate >2mmol/L (>18mg/dL). At the same time, we divided sepsis patients into survival and non-survival groups according to their survival outcomes at 28 days after admission. The non-sepsis group referred to patients who received treatment in the ICU during the same period but were not diagnosed with sepsis (supplementary materials for admission diagnosis, Table S1). The exclusion criteria for the sepsis and non-sepsis groups are as follows: patients under 18 years old, pregnant, with malignant tumors, readmitted with secondary infections, or treated at another hospital. The control group consisted of medical examination personnel who came to the hospital during the same period. The exclusion criteria were those under 18 years old, pregnant, with organ dysfunction, and those who had infection or had taken antibiotics in the past month. This study was approved by the Ethics Committee of the Renmin Hospital of Wuhan University (No. WDRY2022-K157).
Blood Sample Preparation
Collect serum samples from all subjects and store them in a refrigerator at −80 °C until analysis. These serum samples are all clinical samples preserved in department of clinical laboratory in the daily work.
Laboratory Indicator Testing
The serum PKM2 concentration was measured using a commercial Elisa reagent kit (JL48224, Jianglai Biological, China). Roche Diagnostics Cobas 8000e801 automatic chemiluminescence immunoanalyzer detects serum procalcitonin (PCT). Shenzhen Xilaiheng Company’s H780-3 fully automatic protein analyzer detects C-reactive protein (CRP) and serum amyloid A (SAA). The Japanese Sysmex XE-2100 automatic blood cell analyzer detects red blood cell (RBC), white blood cell (WBC), platelet (PLT) counts, and hemoglobin (Hb). Siemens ADVIA 2400 biochemical analyzer detects aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin (TBIL), blood urea nitrogen (BUN), creatinine (Crea) and lactate dehydrogenase (LDH). Sysmex CA7000 automatic coagulation analyzer detects prothrombin time (PT) and thrombin time (TT). All reagents used for testing were completed within the time specified in the instruction manual and strictly followed the relevant operating procedures of the experiment. The personnel conducting the measurement are unaware of the grouping and allocation of serum samples.
Clinical Data Collection
During hospitalization, baseline demographic and clinical data were collected for all subjects, including age, gender, underlying disease, site of infection, hospital stay time, ICU stay time, mechanical ventilation (MV), blood pressure, blood glucose (Glu), blood lactate (Lac), Glasgow Coma Scale (GCS) scores, and microbiological culture results. Calculate the Sequential Organ Failure Assessment (SOFA) score and acute physiology and chronic health evaluation II (APACHE II) score based on clinical and laboratory data obtained within 24 hours of admission.
Statistical Analysis
SPSS 25.0, GraphPad Prism 9.0, and MedCalc 20.1 statistical software were used to statistically analyze the experimental data. Qualitative variables were expressed as values and percentages, and the chi-square test was used for comparison between groups. The quantitative variable uses the single sample Kolmogorov–Smirnov method to test whether the data of each group conform to the Normal distribution. Normal distribution data is expressed by mean ± standard deviation ( ). Independent sample T-test is used for comparison between two groups, and one-way ANOVA is used for comparison between multiple groups. Non-normal distribution data are represented by M (Q1, Q3), two groups are compared by Mann Whitney U-test, three groups and above are tested by Kruskal Wallis H-test, and the Spearman correlation coefficient is used to represent the correlation between two variables. Logistic regression models were used to analyze the relationship between each indicator and the occurrence of sepsis; the diagnostic efficacy of each indicator was analyzed using the receiver operating characteristic (ROC) curve, and the area under curve (AUC) was compared by using Delong’s method. AUC between 0.5 and 0.7 indicates poor diagnostic value, while 0.7 to 0.9 indicates moderate diagnostic value, and over 0.9 indicates high diagnostic value. When the Youden index (sensitivity+specificity−1) is maximized, the optimal cutoff value is determined. The difference is statistically significant as P<0.05.
). Independent sample T-test is used for comparison between two groups, and one-way ANOVA is used for comparison between multiple groups. Non-normal distribution data are represented by M (Q1, Q3), two groups are compared by Mann Whitney U-test, three groups and above are tested by Kruskal Wallis H-test, and the Spearman correlation coefficient is used to represent the correlation between two variables. Logistic regression models were used to analyze the relationship between each indicator and the occurrence of sepsis; the diagnostic efficacy of each indicator was analyzed using the receiver operating characteristic (ROC) curve, and the area under curve (AUC) was compared by using Delong’s method. AUC between 0.5 and 0.7 indicates poor diagnostic value, while 0.7 to 0.9 indicates moderate diagnostic value, and over 0.9 indicates high diagnostic value. When the Youden index (sensitivity+specificity−1) is maximized, the optimal cutoff value is determined. The difference is statistically significant as P<0.05.
Results
Study Population Characteristics
As shown in Table 1, a total of 299 subjects were included in this study, including 143 in the sepsis group, 91 in the non-sepsis group, and 65 in the control group. There were no statistically significant differences in gender, drinking, smoking, hospital stay time, and underlying diseases among the three groups (all P>0.05). Compared with the non-sepsis group, the sepsis group had the higher probability of using mechanical ventilation, lower oxygenation index, and longer ICU stay time (all P<0.05). The age, WBC, and Glu levels in the sepsis group and non-sepsis group were significantly higher than those in the control group (all P<0.05). The SOFA score, Crea, CRP, SAA, PCT, PKM2, and LDH levels in the sepsis group were significantly higher than those in the non-sepsis group, and the non-sepsis group was significantly higher than the control group (all P<0.05). RBC and Hb were significantly lower in the sepsis group and non-sepsis group than in the control group (all P<0.05). The GCS score and PLT of the sepsis group were significantly lower than that of the non-sepsis group, and the non-sepsis group was significantly lower than the control group (P<0.05). The lactate and MAP levels in the sepsis group were significantly higher than those in the non-sepsis group and control group (all P<0.05). The TBIL in the sepsis group was significantly higher than that in the control group (P<0.05). Among patients with sepsis, the most common site of infection is the lung (53.85%), followed by the abdominal cavity (29.37%) and urinary tract (11.89%).
 |
Table 1 Baseline Demographics and Clinical Characteristics of Study Population |
PKM2 and Infection Site and Pathogen
Based on the location of the primary infection, we divided sepsis patients into four groups: lung, abdominal cavity, urinary tract, and other. As shown in Figure 1A, there was no difference in serum PKM2 levels among patients with sepsis at different infection sites (all P>0.05), but they were significantly higher than those in the non-sepsis group and control group (all P<0.05). In addition, according to the microbial test results, we further divided sepsis patients into Gram-positive bacteria (G+), Gram-negative bacteria (G−), fungi, viruses, mixed infection, and undefined groups. As shown in Figure 1B, there was no statistically significant difference among the sepsis groups (all P>0.05), but both were higher than those in the non-sepsis and control groups (all P<0.05).
PKM2 and Diagnosis
To determine the impact of PKM2 on sepsis in ICU patients, we conducted a univariate logistic regression analysis. The results showed that WBC, PLT, CRP, SAA, PCT, PKM2, SOFA score, Glu, Lac, LDH, mechanical ventilation, oxygenation index, MAP, and GCS were all influencing factors for sepsis (all P<0.05). However, multivariate logistic regression analysis showed that only PCT and PKM2 were independent risk factors for sepsis in ICU patients (both P<0.01) (Table 2). We further analyzed the diagnostic value of PCT, PKM2, and combination in sepsis by drawing ROC curves. As shown in Figure 2 and Table 3, AUC comparison: combination>PKM2>PCT, sensitivity comparison: combination>PKM2>PCT, specificity comparison: combination>PKM2>PCT, and the optimal cut-off values for PKM2 and PCT diagnosis were 6.865ng/mL and 3.960ng/mL, respectively. The AUC detected by PKM2 alone was significantly higher than that detected by PCT (Z=3.013, P<0.01), and the AUC detected by combination was significantly higher than that detected by PKM2 and PCT alone (Z=2.541, 4.775, both P<0.05).
 |
Table 2 Univariate and Multivariate Regression Analysis for Predicting Sepsis |
 |
Table 3 ROC Curve Analysis of the Diagnostic Value of PKM2, PCT, and Combination for Sepsis |
 |
Figure 2 Receiver operating characteristic curves of PKM2, PCT and combinations for the diagnosis of sepsis. |
PKM2 and Clinical Indicators
To further analyze the relationship between PKM2 and clinical features, patients were divided into four groups based on the quartile of PKM2 (Q1, <5.61; Q2,5.61–17.31; Q3,17.31–40.54; Q4, ≥40.54). As shown in Table 4, compared with the other three groups, the Q4 group had the highest APACHE II score, SOFA score, septic shock incidence, WBC, PCT, AST, ALT, BUN, Lac, and LDH concentrations, longer PT and TT times, lowest oxygenation index, and highest use rate of vasopressors. However, there was no significant difference in the 28-day mortality rate among the groups. In addition, we found the positive correlation between PKM2 levels and traditional inflammatory indicators (PCT, WBC, CRP), Glu, Lac, LDH, and SOFA scores through Spearman correlation analysis. The driving factors for differences in total SOFA scores include respiratory, cardiovascular, coagulation, hepatic, and renal components (Figures S1-S3).
 |
Table 4 Clinical Characteristics and Laboratory Results Stratified by Quartiles of PKM2 |
PKM2 Distinguishes Sepsis from Septic Shock
We divided the sepsis group patients into two groups: the sepsis group and the septic shock group. We collected the serum of the two groups of patients on the 1st, 3rd, and 7th days after admission, and dynamically analyzed the PKM2 level. As shown in Figure 3, the serum PKM2 concentration of patients in the sepsis group and septic shock group gradually decreased with the increase of admission time and was lower on the 7th day than on the 1st and 3rd days (P<0.01 in the sepsis group, P<0.05 in the septic shock group). On the 1st and 3rd days of admission, the serum PKM2 levels in the septic shock group were significantly higher than those in the sepsis group (both P<0.05). ROC curve analysis results show that PKM2 on the 1st and 3rd days had the value of distinguishing sepsis from septic shock (both P<0.05), and the AUCs were 0.7296 and 0.6247, respectively (Figure 4, Table 5).
 |
Table 5 ROC Curve Analysis of Dynamic Monitoring PKM2 in Sepsis and Septic Shock |
 |
Figure 3 Dynamic changes of PKM2 levels in patients with sepsis and septic shock. ***P < 0.001, *P<0.05, compared with sepsis group; ns, not statistically different compared with sepsis group. |
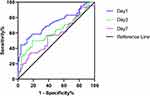 |
Figure 4 Receiver operating characteristic curves for PKM2 dynamic levels differentiating sepsis from septic shock. |
PKM2 Distinguishes Sepsis Survivors from Non-Survivors
Based on the 28-day survival outcome after admission, we further divided the sepsis group patients into survival group and non-survival groups, and collected serum samples from patients on the 1st, 3rd, and 7th days after admission to detect PKM2 levels. As shown in Figure 5, with the increase of hospitalization days, the PKM2 levels of patients in the survival group gradually decreased, significantly lower on the 7th day than on the 3rd day and significantly lower on the 3rd day than on the 1st day (both P<0.05). However, there was no significant difference in serum PKM2 levels among non-survival group patients at three time points (P=0.235). On the 3rd and 7th days, the concentration of PKM2 in the non-survival group was significantly higher than that in the survival group (both P<0.001). At the same time, ROC curve analysis showed that PKM2 had prognostic value on the 3rd and 7th days (both P<0.001), and AUC, sensitivity and specificity on the 7th day were greater than those on the 3rd day (Figure 6, Table 6).
 |
Table 6 ROC Curve Analysis of PKM2 Dynamic Level in Predicting Survivors and Non-Survivors |
 |
Figure 6 Receiver operating characteristic curves of PKM2 dynamic levels in distinguishing sepsis survival and non-survival groups. |
Discussion
Sepsis, as a critical illness in the ICU, has the characteristics of rapid progression and high mortality rate. Although the clinical understanding of the pathological mechanisms of sepsis continues to deepen and the medical level continues to improve, this critical illness remains the main cause of death in the ICU.12,13 Currently, the diagnosis of sepsis still lacks a “gold standard”, and exploring reliable diagnostic biomarkers remains an important aspect of research in recent years. Studying the pathophysiological mechanisms and key molecules of sepsis is an important foundation for developing new diagnostic biomarkers and therapeutic targets. The main purpose of this study is to analyze whether PKM2, which has been identified as a key molecule for glucose metabolism reprogramming in sepsis in preclinical experiments, can become a biomarker for sepsis patients. In this study, we found that (1) The serum PKM2 level was significantly elevated in patients with sepsis, but not in the non-sepsis group and control group; (2) PKM2 had a high diagnostic value for sepsis; (3) PKM2 was associated with commonly used clinical indicators in sepsis patients; (4) Dynamic monitoring of PKM2 levels had a prognostic value for sepsis patients. Therefore, we speculate that serum PKM2 may be a novel biomarker for the diagnosis and prognosis of sepsis patients.
In the early stage of sepsis, infection causes the activation of immune cells and the production and release of inflammatory factors. The released inflammatory factors will induce the production and release of new cytokines, forming a “Cytokine storm”, which will eventually lead to multiple organ function damage.14 Glucose metabolic reprogramming is an important mechanism for immune cell activation, proliferation, and differentiation. In the resting state, innate immune cells and adaptive immune cells mainly produce ATP through Oxidative phosphorylation. When activated to a pro-inflammatory state, aerobic glycolysis is the main metabolic mode.15 Aerobic glycolysis can provide sufficient energy and biological raw material basis for the activation of immune cells and the synthesis and release of inflammatory factors.16 From this perspective, limiting aerobic glycolysis can reduce the inflammatory damage of sepsis. As one of the key enzymes of aerobic glycolysis, PKM2 has been identified as a key target for sepsis treatment in preclinical studies. Inhibiting PKM2 can reduce the production of inflammatory factors and tissue and organ damage in septic mice, and reduce mortality.17–20 Our data further supports these findings. We found that serum PKM2 levels were significantly increased in sepsis patients and correlated with indicators of infection and the severity of the disease. In addition, the positive correlation between PKM2 and blood glucose, lactate, and LDH suggested that human sepsis patients may share the PKM2-centered glucose metabolism reprogramming pathway identified in preclinical models.
Although there have been reports that the primary infection site and pathogen type are associated with host-specific response disorders,21,22 we did not find any differences in PKM2 among sepsis patients with different primary infection sites or pathogen types. According to reports, PKM2 is elevated in various inflammatory diseases, as well as bacteria, viruses, and parasites can induce upregulation of PKM2 expression in cells.23,24 Combined with the findings of this study, we speculate that PKM2 is a non-specific serum infection indicator in the early stages of sepsis. Of course, further experimental verification is needed to determine whether there is a difference in the upregulation of the PKM2 gene during the process of immune cell glucose metabolic reprogramming caused by different pathogens. PCT is one of the most studied and used biomarkers of sepsis to date, which significantly increases during bacterial infection but does not increase during viral infection. Our study found that PKM2 increases in sepsis caused by various pathogens, making up for the shortcomings of PCT in this regard.
PKM2 is not only a rate-limiting enzyme of glycolysis, but also a transcription activator of inflammatory mediators. It can participate in the pathogenesis of many diseases by regulating the Warburg effect and promoting inflammatory response.25,26 At present, research on novel drugs targeting PKM2 has been reported in various diseases and has made some progress. However, there are few reports on using serum PKM2 as a biomarker for disease diagnosis.27–30 In this study, multivariate regression analysis showed that PKM2 and PCT were independent risk factors for sepsis in ICU patients; the ROC curve analysis showed that the AUC of PKM2 in diagnosing sepsis alone was 0.9154, with a high diagnostic value. Given the limitations of a single indicator, we combined PKM2 with PCT for the diagnosis of sepsis, achieving the highest clinical diagnostic value.
During the treatment of sepsis patients admitted to the ICU, closely monitoring the dynamic changes of various indicators helps to timely understand the patient’s response to treatment and conduct risk stratification.31,32 Some studies have found that PKM2-dependent aerobic glycolysis in mononuclear macrophages can increase the release of high mobility group protein B1 (HMGB1, the late mediator of fatal systemic inflammation), and promote septic shock and death in mice.33 This study also found that sepsis patients in the high PKM2 level group had a higher incidence of septic shock and more severe condition when stratifying sepsis patients according to quartiles of admission PKM2. However, there was no statistical difference in the 28-day mortality rate among the groups, presumably due to the inclusion of patients in the non-survival group who were less severe on the day of admission and subsequently progressed to severe to death. In order to better explain the relationship between PKM2 and adverse outcomes in sepsis patients, we dynamically monitored serum PKM2 levels according to the severity of the disease. In the sepsis group and septic shock group, as the hospitalization time prolonged, the patient’s condition improved and PKM2 showed a downward trend. On the 1st and 3rd day of admission, PKM2 levels in septic shock patients were significantly higher than those in sepsis patients, indicating diagnostic value. On the contrary, in the sepsis non-survival group, as the hospitalization time increased, the patient’s condition worsened and PKM2 levels gradually increased. On the 3rd and 7th days, the PKM2 levels in the non-survival group were significantly higher than those in the survival group. PKM2 on both the 3rd and 7th days had prognostic value, and AUC on the 7th day was higher than on the 3rd day. This result suggests that sustained high levels of PKM2 are associated with poor prognosis, which may mean that cells producing PKM2 are unable to turn off aerobic glycolysis, while cells in patients with decreased PKM2 levels can switch back to oxidative phosphorylation, which may be the basis for affecting the treatment of this axis. If the serum PKM2 level in sepsis patients does not decrease or continues to increase, it suggests that clinical doctors should take corresponding measures in a timely manner to reduce mortality.
Finally, because this study is only a single-center study in a short period of time, the cut-off values obtained by ROC curves in this paper can only provide a reference and cannot be used clinically. We call for a larger study by a qualified research group to determine whether PKM2 can become the next common clinical inflammatory indicator for sepsis.
Conclusion
In this study, we found that serum PKM2 levels were increased in sepsis patients and had a high diagnostic value for sepsis. The serum level of PKM2 was correlated with the severity of the disease and could predict the clinical outcomes of patients. In summary, serum PKM2 has the potential to become a new biomarker for sepsis.
Ethics Approval and Consent to Participate
This study is in accordance with the Declaration of Helsinki. This study was approved by the Ethics Committee of Renmin Hospital of Wuhan University (NO. WDRY2022-K157). The blood samples collected in this study were sourced from clinical samples stored in the department of clinical laboratory in the daily work, and the clinical data collected in this study strictly followed the requirements of the ethics committee. No personal identification information was collected, so it was approved to exempt patients from informed consent.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research was funded by the National Natural Science Foundation of China (No. 81773444) and the Hubei Provincial Health Commission Natural Project (No. WJ2023M073).
Disclosure
All authors have disclosed no conflicts of interest in this work.
References
1. Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801–810. doi:10.1001/jama.2016.0287
2. Rudd KE, Johnson SC, Agesa KM, et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the Global Burden of Disease Study. Lancet. 2020;395(10219):200–211. doi:10.1016/S0140-6736(19)32989-7
3. Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021;47(11):1181–1247. doi:10.1007/s00134-021-06506-y
4. Chaudhuri D, Herritt B, Lewis K, et al. Dosing Fluids in Early Septic Shock. Chest. 2021;159(4):1493–1502. doi:10.1016/j.chest.2020.09.269
5. Komorowski M, Green A, Tatham KC, et al. Sepsis biomarkers and diagnostic tools with a focus on machine learning. EBioMedicine. 2022;86:104394. doi:10.1016/j.ebiom.2022.104394
6. Warburg O, Wind F, Negelein E. THE METABOLISM OF TUMORS IN THE BODY. J Gen Physiol. 1927;8(6):519–530. doi:10.1085/jgp.8.6.519
7. Wasyluk W, Zwolak A. Metabolic Alterations in Sepsis. J Clin Med. 2021;10(11):2412. doi:10.3390/jcm10112412
8. Liu Z, Le Y, Chen H, Zhu J, Lu D. Role of PKM2-Mediated Immunometabolic Reprogramming on Development of Cytokine Storm. Front Immunol. 2021;12:748573. doi:10.3389/fimmu.2021.748573
9. Rao J, Wang H, Ni M, et al. FSTL1 promotes liver fibrosis by reprogramming macrophage function through modulating the intracellular function of PKM2. Gut. 2022;71(12):2539–2550. doi:10.1136/gutjnl-2021-325150
10. Yuan L, Wang Y, Chen Y, et al. Shikonin inhibits immune checkpoint PD-L1 expression on macrophage in sepsis by modulating PKM2. Int Immunopharmacol. 2023;121:110401. doi:10.1016/j.intimp.2023.110401
11. Wang J, Yang P, Yu T, et al. Lactylation of PKM2 Suppresses Inflammatory Metabolic Adaptation in Pro-inflammatory Macrophages. Int J Biol Sci. 2022;18(16):6210–6225. doi:10.7150/ijbs.75434
12. Font MD, Thyagarajan B, Khanna AK. Sepsis and Septic Shock - Basics of diagnosis, pathophysiology and clinical decision making. Med Clin North Am. 2020;104(4):573–585. doi:10.1016/j.mcna.2020.02.011
13. Cecconi M, Evans L, Levy M, et al. Sepsis and septic shock. Lancet. 2018;392(10141):75–87. doi:10.1016/S0140-6736(18)30696-2
14. Chousterman BG, Swirski FK, Weber GF. Cytokine storm and sepsis disease pathogenesis. Semin Immunopathol. 2017;39(5):517–528. doi:10.1007/s00281-017-0639-8
15. Liu J, Zhou G, Wang X, Liu D. Metabolic reprogramming consequences of sepsis: adaptations and contradictions. Cell Mol Life Sci. 2022;79(8):456. doi:10.1007/s00018-022-04490-0
16. Petrasca A, Phelan JJ, Ansboro S, et al. Targeting bioenergetics prevents CD4 T cell-mediated activation of synovial fibroblasts in rheumatoid arthritis. Rheumatology. 2020;59(10):2816–2828. doi:10.1093/rheumatology/kez682
17. Zhang Q, Luo P, Xia F, et al. Capsaicin ameliorates inflammation in a TRPV1-independent mechanism by inhibiting PKM2-LDHA-mediated Warburg effect in sepsis. Cell Chem Biol. 2022;29(8):1248–1259.e6. doi:10.1016/j.chembiol.2022.06.011
18. Pei L, Le Y, Chen H, et al. Cynaroside prevents macrophage polarization into pro-inflammatory phenotype and alleviates cecal ligation and puncture-induced liver injury by targeting PKM2/HIF-1α axis. Fitoterapia. 2021;152:104922. doi:10.1016/j.fitote.2021.104922
19. Pan L, Hu L, Zhang L, et al. Deoxyelephantopin decreases the release of inflammatory cytokines in macrophage associated with attenuation of aerobic glycolysis via modulation of PKM2. Int Immunopharmacol. 2020;79:106048. doi:10.1016/j.intimp.2019.106048
20. Xie M, Yu Y, Kang R, et al. PKM2-dependent glycolysis promotes NLRP3 and AIM2 inflammasome activation. Nat Commun. 2016;7(1):13280. doi:10.1038/ncomms13280
21. Wang Q, Li X, Tang W, et al. Differential Gene Sets Profiling in Gram-Negative and Gram-Positive Sepsis. Front Cell Infect Microbiol. 2022;12:801232. doi:10.3389/fcimb.2022.801232
22. Peters-Sengers H, Butler JM, Uhel F, et al. Source-specific host response and outcomes in critically ill patients with sepsis: a prospective cohort study. Intensive Care Med. 2022;48(1):92–102. doi:10.1007/s00134-021-06574-0
23. Li M, Lu H, Wang X, et al. Pyruvate kinase M2 (PKM2) interacts with activating transcription factor 2 (ATF2) to bridge glycolysis and pyroptosis in microglia. Mol Immunol. 2021;140:250–266. doi:10.1016/j.molimm.2021.10.017
24. Ren L, Zhang W, Zhang J, et al. Influenza A Virus (H1N1) Infection Induces Glycolysis to Facilitate Viral Replication. Virol Sin. 2021;36(6):1532–1542. doi:10.1007/s12250-021-00433-4
25. Grant MM. Pyruvate Kinase, Inflammation and Periodontal Disease. Pathogens. 2021;10(7):784. doi:10.3390/pathogens10070784
26. Alquraishi M, Puckett DL, Alani DS, et al. Pyruvate kinase M2: a simple molecule with complex functions. Free Radic Biol Med. 2019;143:176–192. doi:10.1016/j.freeradbiomed.2019.08.007
27. Zahra K, Dey T, Ashish S, et al. Pyruvate Kinase M2 and Cancer: the Role of PKM2 in Promoting Tumorigenesis. Front Oncol. 2020;10:159. doi:10.3389/fonc.2020.00159
28. Tan C, Li L, Han J, et al. A new strategy for osteoarthritis therapy: inhibition of glycolysis. Front Pharmacol. 2022;13:1057229. doi:10.3389/fphar.2022.1057229
29. Zuo J, Tang J, Lu M, et al. Glycolysis Rate-Limiting Enzymes: novel Potential Regulators of Rheumatoid Arthritis Pathogenesis. Front Immunol. 2021;12:779787. doi:10.3389/fimmu.2021.779787
30. Almousa AA, Morris M, Fowler S, et al. Elevation of serum pyruvate kinase M2 (PKM2) in IBD and its relationship to IBD indices. Clin Biochem. 2018;53:19–24. doi:10.1016/j.clinbiochem.2017.12.007
31. De Backer D, Dorman T. Surviving Sepsis Guidelines: a Continuous Move Toward Better Care of Patients With Sepsis. JAMA. 2017;317(8):807–808. doi:10.1001/jama.2017.0059
32. Lin CY, Wang YH, Chen YM, et al. Dynamic monitoring of kidney injury status over 3 days in the intensive care unit as a sepsis phenotype associated with hospital mortality and hyperinflammation. Biomed J. 2022;45(4):665–674. doi:10.1016/j.bj.2021.08.006
33. Huang J, Liu K, Zhu S, et al. AMPK regulates immunometabolism in sepsis. Brain Behav Immun. 2018;72:89–100. doi:10.1016/j.bbi.2017.11.003
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


