Back to Journals » Journal of Inflammation Research » Volume 14
Changes in Plasma Growth Differentiation Factor-15 After Laparoscopic Sleeve Gastrectomy in Morbidly Obese Patients: A Prospective Study
Authors Salman A , Shaaban HED , Salman M , M Seif El Nasr S, Soliman A, Salem A, Tag El-Din M, Mikhail HMS, El Domiaty H, Abd Allah N, GabAllah GMK, Youssef A
Received 5 February 2021
Accepted for publication 22 March 2021
Published 12 April 2021 Volume 2021:14 Pages 1365—1373
DOI https://doi.org/10.2147/JIR.S304929
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Monika Sharma
Ahmed Salman,1 Hossam El-Din Shaaban,2 Mohamed Salman,3 Sayed M Seif El Nasr,1 Ahmed Soliman,1 Abdoh Salem,4 Mohamed Tag El-Din,4 Hani Maurice Sabri Mikhail,3 Heba El Domiaty,5 Nesrin Abd Allah,6 Ghada MK GabAllah,7 Ahmed Youssef1
1Internal Medicine Department, Faculty of Medicine, Cairo University, Cairo, Egypt; 2Gastroenterology Department, National Hepatology and Tropical Medicine Research Institute, Cairo, Egypt; 3General Surgery Department, Faculty of Medicine, Cairo University, Cairo, Egypt; 4General Surgery Department, Faculty of Medicine, Al-Azhar University, Cairo, Egypt; 5Clinical Physiology Department, Faculty of Medicine, Menoufia University, Shebin El-Kom, Menoufia, Egypt; 6Anatomy and Embryology Department, Faculty of Medicine, Menoufia University, Shebin El-Kom, Menoufia, Egypt; 7Medical Biochemistry Department, Faculty of Medicine, Menoufia University, Shebin El-Kom, Menoufia, Egypt
Correspondence: Ahmed Salman
Internal Medicine Department, Faculty of Medicine, Cairo University, Cairo, Egypt
Tel +20 1000468664
Email [email protected]
Purpose: This study aimed to assess the potential changes of Growth differentiation factor 15 (GDF15) after laparoscopic sleeve gastrectomy (LSG) in morbidly obese patients.
Methods: We conducted a prospective study on 68 patients who underwent LSG and 58 cases, who were enrolled as a control group, to whom conservative measures of weight loss were adopted. Both groups were followed for 12 months.
Results: At the baseline, the serum GDF15 was comparable between LSG and conservative groups (409.93± 119 versus 385.8± 120.2 pg/mL, p =0.246). However, at 12 months after the operation, the serum GDF15 was significantly higher in the LSG than conservative groups (699.941 ± 193.5 versus 559 ± 159.7; p < 0.001). The degree of serum GDF15 increase was higher in the LSG group (290.01 ± 189.9 versus 173.14 ± 116.7; p < 0.001). The degree of serum GDF15 increase correlated negatively with the final BMI (r = − 0.352, p =0.001) and weight loss (r = − 0.793, p =0.001) at 12 months after the operation. The correlation analysis demonstrated that the initial GFD15 did not correlate with any baseline parameters. Multiple regression analysis of change in serum GDF15 showed a statistical significance of the weight loss after 12 months.
Conclusion: The present work confirms the impact of successful weight loss on the circulating level of GDF15. Our study demonstrated that the circulating GDF15 increased significantly after LSG and it was correlated to the degree of weight loss.
Keywords: bariatric surgery, laparoscopic sleeve gastrectomy, growth differentiation factor 15, appetite
Introduction
Morbid obesity is associated with a high risk of metabolic disorder and cardiovascular disease mortality and morbidity.1–3 Due to poor outcomes of pharmacological and non-pharmacological approaches to morbid obesity, physicians have applied surgical procedures, which demonstrate incredibly high efficacy in severe, morbidly obese patients.4 Bariatric procedures induce significant weight loss with several changes in the mechanisms of homeostatic, including glucose and lipid homeostasis, and enhance the hormone regulation system.5 Anatomic alterations in the gastrointestinal tract can induce changes in gut hormones, bile acids, adipokines, inflammatory cytokines, hepatokines, and gut microbiota. Bariatric operations decrease pro-inflammatory molecules and enhance anti-inflammatory molecules.6
In the recent years, bariatric procedures played as gastrointestinal metabolic surgery has been viewed as a new strategy for obesity‐associated type 2 diabetes mellitus (T2DM) for cases with BMI >35 kg/m2. Published data have shown that bariatric/metabolic surgery is an efficient and durable management option for obese T2DM subjects.7
Laparoscopic sleeve gastrectomy (LSG) is one of the most common restrictive bariatric surgery procedures, with more than 20% reduction in the original size of the stomach.8 For its simplicity, positive results and low complication rates, this technique is widely preferred. Some clinical trials for morbid obesity treatment have demonstrated better long-term survival after LSG than conventional care.9,10 Besides, it results in rapid and independent weight loss, T2DM remission and cardiovascular death reduction.11,12 LSG can improve glucose homeostasis in obese cases with T2DM through various mechanisms including the adipose tissue metalloproteinases 2, −7, and −9 independent pathways.13 Other mechanisms for improvement include amelioration of insulin resistance (due to a substantial decrease in calorie intake, and decrease in fat mass) and postoperative elevated levels of gut hormones, such as glucagon-like peptide-1 that enhance cell response to nutrients.14
Growth differentiation factor (GDF-15), also called macrophage inhibitory cytokine-1 (MIC-1), is a novel inflammatory biomarker. It is considered one of the divergent TGF-beta family members. GDF-15 is induced in hepatocytes by surgical and chemical injury and heat shock.15,16
In healthy individuals, this marker circulates in the plasma with a detectable concentration, which elevates significantly in cancer patients.17,18 GDF15 has a significant role in the cases of cancer-related weight loss, as it induces fat and lean body mass loss.19 Moreover, it has been used as a prognostic biomarker to predict the mortality in patients with cardiovascular disease.20 Kempf et al and Vila et al reported that GDF15 is a promising predictor for impaired glucose control and future insulin resistance in morbidly obese patients.21,22 However, the exact mechanism of GDF15 in obesity or the potential changes after bariatric surgeries is still unknown. Therefore, this study aimed to assess the potential changes of GDF15 after LSG in morbidly obese patients.
Materials and Methods
Study Design
We conducted a prospective study on 68 patients who underwent LSG and 58 cases, who were enrolled as a control group, to whom conservative measures of weight loss were adopted. Both patients and controls were recruited from the period March 2016 to May 2018. All patients gave written informed consents. The responsible local Ethical Committee of Cairo University Hospitals approved this study. This study was conducted in accordance with the Declaration of Helsinki.
Inclusion and Exclusion Criteria
We enrolled only adult patients (18–65 years) who were underwent LSG and were willing to participate in this study. There were no gender restrictions. Patients with serious cardiac conditions, severe coagulopathy, untreated eating disorders, inability to fulfill the nutritional requirements, pregnancy, or unwillingness to participate in the study have been ruled out.
Laparoscopic Sleeve Gastrectomy Procedure
In all cases, we applied the standard LSG procedure. The procedure was conducted through four trocar-port (5 mm, 5 mm, 10 mm, 12 mm). After inserting trocars, we used a Ligasure to split the greater omentum from the stomach. The first linear stapler was then fired at the beginning of a 4 cm from the gastroduodenal junction (Ethicon Echelon Flex Powered Endopath 60 mm). Until the completion of the LSG, we inserted a gastric calibration tube (36-Fr bougie) into the stomach. To complete gastric resection, a total of 5 stapler firings were required. Finally, we used a Covidien 3–0 V-Loc suture with seromuscular suturing to reinforce the entire staple line.
Determination of Obesity-Linked Comorbidities
The following criteria were adopted to determine the obesity-linked comorbidities. Diabetes was determined as the usage of antidiabetic drugs, fasting blood glucose ≥ 126 mg/dL, or hemoglobin A1c (HbA1C) level ≥ 6.5%. Hypertension was determined as the usage of antihypertensive drugs, systolic blood pressure ≥ 140 mmHg, or diastolic blood pressure ≥ 90 mmHg. Dyslipidemia was determined as the usage of lipid-lowering medications, total cholesterol ≥ 200 mg/dL, triglycerides ≥ 200 mg/dL, or low-density lipoprotein cholesterol (LDL-cholesterol) ≥ 120 mg/dL. The occurrence of the disease at start of the study and at the end of follow-up was calculated according to these criteria with comparison between the two arms.23
GDF15 Assay
Fasting blood was obtained in chilled, ethylenediaminetetraacetic acid (EDTA) tubes, for GDF15 assay. The serum was extracted at 80 Celsius for 10 minutes, after an immediate centrifuge at 3000 rpm, waiting for hormone analysis. The GDF15 assay was carried out using an enzyme-linked immunosorbent assay kit (ELISA; Cloud-Clone Corp, 1304 Langham Creek Dr, Suite 226, Houston, TX 77084 USA). The insulin resistance homeostasis (HOMA-IR) index was used for the calculation of insulin resistance [(Fasting Glucose-Fasting Insulin)/22.5.
Statistical Analysis
Statistical analysis was performed using SPSS version 22. Categorical data were summarized using numbers and percentages. Continuous data were tested for normality using Kolmogorov–Smirnov test. Normally distributed variables were presented as mean and standard deviations, while variables that were not normally distributed were expressed as median and interquartile range. Chi-squared test was used to compare the categorical data and an independent t-test was used to compare continuous data. The correlation between the change in BMI with GDF15 was assessed using Pearson Correlation. P-value less than 0.05 was defined as significant and the null hypothesis was rejected.
Results
The current study included 68 patients who underwent LSG. In addition, 58 cases were enrolled as a control group, to whom conservative measures of weight loss were adopted. The mean age in the LSG group was 42 ±7.9 years old compared to 38.76 ±8.3 years old in the conservative group (p =0.028). The frequencies of smokers (p =0.4), patients with history of coronary artery disease (p =0.92), diabetes (p =0.79), hypertension (p =0.15), and hyperlipidemia (p =0.85) were comparable between both groups at the baseline. Patients in LSG group had significantly higher BMI than the conservative group (44.8 ±3.5 versus 43.3 ±2.9Kg/m2; p =0.011). Similarly, patients in LSG group had significantly higher homeostatic model assessment for insulin resistance (HOMA-IR) levels (p =0.002), serum ALT (p <0.001), and lower LDL-C (p =0.009) than the control group. Other laboratory findings were comparable between both groups (Table 1).
 |
Table 1 Characteristics of the Both Groups Prior to Intervention |
At the end of follow-up, patients in LSG group exhibited significantly lower BMI than the conservative group (32.37 ±3.6 versus 38.84 ±3.3Kg/m2; p =0.001). Likewise, the extent of weight loss was significantly higher in the LSG group (38.1 ±12.2 versus 14.1 ±15.4Kg, p =0.001). The values of HbA1c, fasting blood glucose, HOMA-IR, and LDL were significantly lower in the LSG group (p >0.05; Table 2).
 |
Table 2 Follow-Up of the Metabolic Status Findings of Both Groups Post Intervention |
At the baseline, the serum GDF15 was comparable between LSG and conservative groups (409.93 ±119 versus 385.86 ±120.2pg/mL, p =0.246). However, at 12 months after the operation, the serum GDF15 was significantly higher in the LSG than conservative groups (699.941 ±193.5 versus 559 ±159.7; p <0.001). The degree of serum GDF15 increase was higher in the LSG group (290.01 ±189.9 versus 173.14 ±116.7; p <0.001; Figure 1). The degree of serum GDF15 increase correlated negatively with the final BMI (r = −0.352, p =0.001) and weight loss (r = −0.793, p =0.001) at 12 months after the operation (Figures 2 and 3).
 |
Figure 1 Degree of serum GDF15 increase in both groups. |
 |
Figure 2 Correlation of degree of serum GDF15 with the BMI. |
 |
Figure 3 Correlation of degree of serum GDF15 with the weight. |
The correlation analysis demonstrated that the initial GFD15 did not correlate with any baseline parameters (Table 3), while correlation between the change of serum GDF15 and change in other parameters showed significant correlation with FPG, fasting plasma insulin (FPI), HOMA-IR and ALT (Table 4).
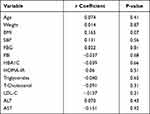 |
Table 3 Correlation Between the Pre-Intervention Level of GFD15 and Other Baseline Parameters |
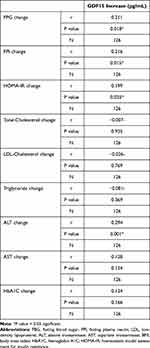 |
Table 4 Correlation of the Degree of Serum GDF15 Increase and Change in Other Parameters |
Analysis of the relationship between change in serum GDF15 and change in each parameter in multiple regression analysis showed statistical significance of the weight loss after 12 months (Table 5).
 |
Table 5 Analysis of Relationship Between Change in Serum GDF15 and Change in Each Parameter in Multiple Regression Analysis |
As regards the LSG group, there was a significant correlation between changes in GDF15 and changes in weight and BMI (Table 6). The correlation with weight loss was more significant in the LSG group than the conservative group (Table 7). Multi-regression analysis showed that the only independent predictor of GDF increase in LSG group was weight loss (Table 8).
 |
Table 6 Correlation Between Change in GDF15 and Change in Weight and BMI Only in LSG Group |
 |
Table 7 Correlation Coefficient of Change in GDF15 with Change in Weight Between LGS and Conservative Groups |
 |
Table 8 Multi-Regression Analysis for Factors Affecting Change in GDF15 After LSG |
Discussion
To date, the exact mechanism of GDF15 in obesity or the potential changes after bariatric surgeries is still unknown. In the current work, we showed that the GDF15 level increased significantly after LSG, the extent of GDF15 increase was significantly higher in patients who underwent LSG than patients who followed conservative treatment regimen. Notably, we found that the degree of change in GDF15 correlated significantly with the degree of weight loss and BMI 12 months after the operation.
GDF15 is a proinflammatory cytokine that presents at low level in various body organs; this cytokine is an important mediator whose level increases significantly during inflammatory process, tissue injury, ageing, and carcinogenesis.24 Moreover, a cumulative body of evidence demonstrated that circulating GDF15 is closely correlated with components of metabolic syndrome such as cardiovascular diseases, diabetes, and insulin resistance.23,25 Since the 2007 study by Johnen et al19 it has been shown that GDF15, through binding to (GDNF Family Receptor Alpha Like) GFRAL receptor in the brain stem, is a potent regulator of appetite as well; circulating GDF15 was found to induce anorexia, and weight loss in mice with overexpression of GDF15.26,27 Therefore, it seems logical to assume significant alterations in circulating GDF15 levels following any obesity-control measure; and that circulating GDF15 can be used as a proxy of the sensible weight loss and resolution of metabolic abnormalities. In the present study, we found that the serum GDF15 increased significantly following LSG to an extent that was higher than the increase following conservative management. In addition, degree of change in GDF15 correlated significantly with the degree of weight loss. Our findings are in line with a recent report by Dolo et al28 who demonstrated a significant increase in the circulating GDF15 levels after LSG. Another study showed that bariatric surgery significantly increase circulating GDF15 levels 12 months after surgery, this increase correlated significantly with weight loss.29 Another work showed similar findings.22
Nonetheless, it should be noted that the exact impact of circulating GDF15 on weight in human has not been fully understood yet with conflicting data. For example, contradictory to the results that demonstrated a regulatory role of GDF15 in response to obesity, previous study showed that overexpression of GDF15 following starvation, but not overfeeding.30 Therefore, further studies are still needed to highlight the role of GDF15 in feeding regulation.
In conclusion, the present work confirms the impact of successful weight loss on the circulating level of GDF15. Our study demonstrated that the circulating GDF15 increased significantly after LSG and it was correlated to the degree of weight loss. The current work could serve as a seminal study in the future, given the originality of the contribution and its clinically relevant nature. Most importantly, given the statistical results, the study underlines the fact that LSG has a measurable impact on plasma GDF15. Consequently, this shall reinforce the concept that the effects of LSG are primarily metabolic and physiological rather than merely anatomical/mechanistic.
Novelty of the Study
To our knowledge, scarce studies addressed the topic of the changes of GDF15 patients after LSG and so this article adds momentum to the literature regarding this novel topic. In addition, among the strengths of this study is the reasonable number of cases with a quite acceptable follow-up period. This point may make our work fairly unique as it is often difficult to get patients back for a second follow-up after such interval.
Furthermore, it is sensible to say that this work may point to potential mechanisms that may be underlying the weight loss after LSG and this shall provide novel horizons into the worth of GDF15; and possibly make it an attractive goal for new therapeutic approaches in disorders related to obesity.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest for this work.
References
1. Abushouk AI, El-Husseny MWA, Bahbah EI, et al. Peroxisome proliferator-activated receptors as therapeutic targets for heart failure. Biomed Pharmacother. 2017;95:692–700. doi:10.1016/j.biopha.2017.08.083
2. Pucci G, Alcidi R, Tap L, et al. Sex- and gender-related prevalence, cardiovascular risk and therapeutic approach in metabolic syndrome: a review of the literature. Pharmacol Res. 2017;120:34–42. doi:10.1016/j.phrs.2017.03.008
3. Milić S, Lulić D, Štimac D. Non-alcoholic fatty liver disease and obesity: biochemical, metabolic and clinical presentations. World J Gastroenterol. 2014;20(28):9330–9337. doi:10.3748/wjg.v20.i28.9330
4. Bennett JMH, Mehta S, Rhodes M. Surgery for morbid obesity. Postgrad Med J. 2007;83(975):8–15. doi:10.1136/pgmj.2006.048868
5. Dall’Asta C, Paganelli M, Morabito A, et al. Weight loss through gastric banding: effects on TSH and thyroid hormones in obese subjects with normal thyroid function. Obesity. 2010;18(4):854–857. doi:10.1038/oby.2009.320
6. Syu Y, Inui A, Chen C. A perspective on metabolic surgery from a gastroenterologist. J Pharmacol Sci. 2017;133(2):61–64. doi:10.1016/j.jphs.2017.01.001
7. Lee WJ, Owaid Almalki O. Recent advancements in bariatric/metabolic surgery. Ann Gastroenterol Surg. 2017;1(3):171–179. doi:10.1002/ags3.12030
8. Gentileschi P. Laparoscopic sleeve gastrectomy as a primary operation for morbid obesity: experience with 200 patients. Gastroenterol Res Pract. 2012;2012:1–4. doi:10.1155/2012/801325
9. Fuks D, Verhaeghe P, Brehant O, et al. Results of laparoscopic sleeve gastrectomy: a prospective study in 135 patients with morbid obesity. Surgery. 2009;145(1):106–113. doi:10.1016/j.surg.2008.07.013
10. Lombardo V, Baratta R, Giannone G. Laparoscopic sleeve gastrectomy for morbid obesity. Our initial experience. Ann Ital Chir. 2010;81(1):17–20.
11. Rosenthal RJ. International sleeve gastrectomy expert panel consensus statement: best practice guidelines based on experience of >12,000 cases. Surg Obes Relat Dis. 2012;8(1):8–19. doi:10.1016/j.soard.2011.10.019
12. Emadeldin D, Ramadan A, Fala SY, et al. Disease modifying efficacy of memantine in Alzheimer’s disease; a pooled analysis of 13 randomized controlled trials. J Neurol Sci. 2017;381:767. doi:10.1016/j.jns.2017.08.2166
13. Wu W, Lee W, Lee T, Chen S, Chen C. Do different bariatric surgical procedures influence plasma levels of matrix metalloproteinase-2, −7, and −9 among patients with type 2 diabetes mellitus? World J Diabetes. 2020;11(6):252–260. doi:10.4239/wjd.v11.i6.252
14. Wang Y, Song Y, Chen J, et al. Roux-en-Y gastric bypass versus sleeve gastrectomy for super super obese and super obese: systematic review and meta-analysis of weight results, comorbidity resolution. Obes Surg. 2019;29(6):1954–1964. doi:10.1007/s11695-019-03817-4
15. Lerner L, Hayes TG, Tao N, et al. Plasma growth differentiation factor 15 is associated with weight loss and mortality in cancer patients. J Cachexia Sarcopenia Muscle. 2015;6(4):317–324. doi:10.1002/jcsm.12033
16. Zimmers TA, Jin X, Hsiao EC, McGrath SA, Esquela AF, Koniaris LG. Growth differentiation factor-15/macrophage inhibitory cytokine-1 induction after kidney and lung injury. Shock. 2005;23(6):543–548.
17. Brown DA, Lindmark F, Stattin P, et al. Macrophage inhibitory cytokine 1: a new prognostic marker in prostate cancer. Clin Cancer Res. 2009;15(21):6658–6664. doi:10.1158/1078-0432.CCR-08-3126
18. Meyer-Siegler KL, Bellino MA, Tannenbaum M. Macrophage migration inhibitory factor evaluation compared with prostate specific antigen as a biomarker in patients with prostate carcinoma. Cancer. 2002;94(5):1449–1456. doi:10.1002/cncr.10354
19. Johnen H, Lin S, Kuffner T, et al. Tumor-induced anorexia and weight loss are mediated by the TGF-β superfamily cytokine MIC-1. Nat Med. 2007;13(11):1333–1340. doi:10.1038/nm1677
20. Schopfer DW, Ku IA, Regan M, et al. Growth differentiation factor 15 and cardiovascular events in patients with stable ischemic heart disease (The Heart and Soul Study). Am Heart J. 2014;167(2):186–192.e1. doi:10.1016/j.ahj.2013.09.013
21. Kempf T, Guba-Quint A, Torgerson J, et al. Growth differentiation factor 15 predicts future insulin resistance and impaired glucose control in obese nondiabetic individuals: results from the XENDOS trial. Eur J Endocrinol. 2012;167(5):671–678. doi:10.1530/EJE-12-0466
22. Vila G, Riedl M, Anderwald C, et al. The relationship between insulin resistance and the cardiovascular biomarker growth differentiation factor-15 in obese patients. Clin Chem. 2011;57(2):309–316. doi:10.1373/clinchem.2010.153726
23. Park JY, Yoonseok Heo Y, Kim YJ, et al. Long-term effect of bariatric surgery versus conventional therapy in obese Korean patients: a multicenter retrospective cohort study. Ann Surg Treat Res. 2019;96(6):283–289. doi:10.4174/astr.2019.96.6.283
24. Fairlie WD, Moore AG, Bauskin AR, et al. MIC-1 is a novel TGF-β superfamily cytokine associated with macrophage activation. J Leukoc Biol. 1999;65(1):2–5. doi:10.1002/jlb.65.1.2
25. Lind L, Wallentin L, Kempf T, et al. Growth-differentiation factor-15 is an independent marker of cardiovascular dysfunction and disease in the elderly: results from the Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) study. Eur Heart J. 2009;30(19):2346–2353. doi:10.1093/eurheartj/ehp261
26. Wakchoure S, Swain TM, Hentunen TA, et al. Expression of macrophage inhibitory cytokine-1 in prostate cancer bone metastases induces osteoclast activation and weight loss. Prostate. 2009;69(6):652–661. doi:10.1002/pros.20913
27. Hsu JY, Crawley S, Chen M, et al. Non-homeostatic body weight regulation through a brainstem-restricted receptor for GDF15. Nature. 2017;550(7675):255–259. doi:10.1038/nature24042
28. Dolo PR, Yao L, Liu PP, et al. Effect of sleeve gastrectomy on plasma growth differentiation factor-15 (GDF15) in human. Am J Surg. 2020;220(3):725–730. doi:10.1016/j.amjsurg.2020.01.041
29. Kleinert M, Bojsen-Møller KN, Jørgensen NB, et al. Effect of bariatric surgery on plasma GDF15 in humans. Am J Physiol Metab. 2019;316:E615–E621.
30. Patel S, Alvarez-Guaita A, Melvin A, et al. GDF15 provides an endocrine signal of nutritional stress in mice and humans. Cell Metab. 2019;29(3):707–718.e8. doi:10.1016/j.cmet.2018.12.016
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
