Back to Journals » Drug Design, Development and Therapy » Volume 17
Changes in Drug Clinical Trials of Thyroid Diseases in China, 2009–2022
Authors Li C, Hao J, Wang C, Yang J, Zheng Y , Zhang K, Hui W, Meng X, Gao J, Li W, Tang YD
Received 8 March 2023
Accepted for publication 7 July 2023
Published 3 August 2023 Volume 2023:17 Pages 2315—2324
DOI https://doi.org/10.2147/DDDT.S409617
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Qiongyu Guo
Chen Li,1,* Jun Hao,2,* Chuangshi Wang,2 Jie Yang,1 Yitian Zheng,1 Kuo Zhang,3 Wen Hui,4 Xiangbin Meng,1 Jun Gao,1 Wei Li,2 Yi-Da Tang1,5
1Department of Cardiology and Institute of Vascular Medicine, Peking University Third Hospital; Key Laboratory of Molecular Cardiovascular Science, Ministry of Education, Beijing, People’s Republic of China; 2Medical Research and Biometrics Center, National Clinical Research Center for Cardiovascular Diseases, Fuwai Hospital, National Center for Cardiovascular Diseases, Peking Union Medical College and Chinese Academy of Medical Sciences, Beijing, People’s Republic of China; 3Department of Cardiology, State Key Laboratory of Cardiovascular Disease, Fuwai Hospital, National Center for Cardiovascular Diseases, Peking Union Medical College and Chinese Academy of Medical Sciences, Beijing, People’s Republic of China; 4Department of Science and Technology, West China Hospital, Sichuan University, Chengdu, Sichuan, People’s Republic of China; 5Research Unit of Medical Science Research Management/Basic and Clinical Research of Metabolic Cardiovascular Diseases, Chinese Academy of Medical Sciences, Beijing People’s Republic of China
*These authors contributed equally to this work
Correspondence: Wei Li; Yi-Da Tang, Email [email protected]; [email protected]
Objective: The incidence rate of thyroid diseases increased worldwide. This study aims to overview the changing landscape of drug clinical trials on thyroid disease during 2009– 2022.
Methods: The detailed information of thyroid disease drug trials registered on the National Medical Products Administration (NMPA) Registration and Information Disclosure Platform for Drug Clinical Studies was searched and collected. The thyroid drug clinical trials were analyzed by the characteristics, time trends, indications, and geographical distribution.
Results: Sixty-five thyroid disease drug clinical trials were launched from 2009 to 2022 in China, which included 21 trials in nontumorous thyroid disease and 44 trials in thyroid carcinoma. The number of registered trials of thyroid diseases including thyroid carcinoma and nontumorous thyroid disease increased steadily from 2009 to 2020. Bioequivalence studies accounted for the largest proportion (32[49.2%]), while phase I and Phase II studies both only accounted for 18.5% (12/65). A significant difference was observed in the trials phase, and randomization between thyroid carcinoma and nontumorous thyroid disease. In terms of clinical indications and drug mechanisms, the number of trials in multi-target tyrosine kinase inhibitors for thyroid carcinoma (n=35) ranked first, followed by thyroid hormone for hypothyroidism (n=7), thyrotropin for thyroid carcinoma (n=6). Sixty-five trials were led by 36 principal investigator (PI) units, and more than 30% of PI-leading units were located in Shanghai (n=7) and Beijing (n=4).
Conclusion: During the past 13 years, the development of thyroid diseases drugs trials has achieved certain progress in thyroid carcinoma, especially the molecular targeted therapy, yet the development of drug trials on nontumorous thyroid disease was very slow.
Keywords: thyroid disease, thyroid carcinoma, nontumorous thyroid disease, China, drug clinical trial
Introduction
Thyroid diseases, including thyroid carcinoma and nontumorous thyroid disease, are prevalent diseases of the endocrine system worldwide. The incidence rates of thyroid carcinoma and nontumorous thyroid disease were significantly increased in China.1,2 As the routine screening of thyroid function is not indicated in the present guidelines, the incidence rate of nontumorous thyroid disease may be underestimated.3,4 For the rare terminal thyroid carcinoma which cannot be treated surgically, chemotherapy and targeted therapy is the main strategy. Thyroid hormones and calcium intake should also be taken for life to maintain endocrine homeostasis in patients after thyroidectomy or with radioiodine treatment for hyperthyroidism, autoimmune thyroid disease, congenital hypothyroidism or toxic goiter. In terms of nontumorous thyroid diseases, drug therapy is the only option. The development of drug clinical trials for thyroid disease is important for the optimization of clinical therapy strategies and improvement in patients’ prognosis and long-term quality of life.
The National Medical Products Administration (NMPA) established a Registration and Information Disclosure Platform for Drug Clinical Studies in 2013 and required all the clinical trials conducted in China and approved by the NMPA should be registered and disclosed mandatorily on this platform. If the drug had been approved before the date of this announcement, the applicant must complete the supplemental registration on the platform within 3 months. Therefore, the registration on the platform became the essential way to market for drugs in China. The move accelerated the review process of drug applications and approval. The Chinese government also issued a series of policies during 2013–2017 to support the development of innovative drugs and clinical trials, which has made great progress in drug research and marketing.5 Although some reviews have focused on and summarized the clinical trials of lung cancer, lymphoma, pediatric drugs, etc.6–9 There is still a lack of relevant analysis on the trends and characteristics of drug trials for thyroid diseases. This study aimed to analyze the changes in the drug clinical trials for thyroid diseases in China and to provide useful information for investigators, pharmaceutical enterprises, policymakers and other stakeholders.
Materials and Methods
Data Source and Collection
We searched the NMPA Registration and Information Disclosure Platform for Drug Clinical Studies website (http://www.chinadrugtrials.org.cn) using the word “thyroid” as a keyword. After that, we checked all candidate clinical trials by manual screening to make sure all eligible clinical trials were relevant to the thyroid disease.
Search Strategy and Selection Criteria
The included trials must meet several criteria: (1) the clinical indication must be thyroid diseases. (2) Drug trials should be registered between January 1, 2009, and December 31, 2022. (3) The location of drug trials should be in China. Detailed information on drug trials was collected, including trial title, leading unit, registered date, trial status, trial phase, type and mechanism of tested drug, indications, trial objective, the number of participating centers, geographical location of the principal clinical trial units, key inclusion criteria and exclusion criteria, date of first ethical review, and first enrolment. The data selection and extraction process were conducted by two investigators (C.L. and J.H.). In case of disagreement, all investigators will discuss and reach a consensus under the moderation of a senior investigator (Y-D.T.). A total of 65 drug trials on thyroid diseases were included in the following analysis (Figure 1).
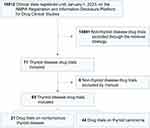 |
Figure 1 Flowchart of the selection process. Abbreviation: NMPA, National Medical Products Administration. |
Statistical Analysis
We classified the drug clinical trials of thyroid diseases into thyroid carcinoma and nontumorous thyroid disease according to pathological types. For descriptive analyses, quantitative variables were expressed as frequency and percentages (%). We analyzed the 13-year characteristic and trends of clinical trials, the distribution of indication and geography. A two-sided p < 0.05 was considered statistically significant. Differences were compared using Pearson’s chi-square test or Fisher’s exact test for dichotomous variables. All statistical analyses were conducted using SAS software, version 9.4 (SAS Institute).
Results
Time Trends of Initiated Trials
A total of 65 drug clinical trials were registered on this platform of NMPA, involving 21 trials on nontumorous thyroid disease and 44 trials in thyroid carcinoma. The annual number of drug trial registration in thyroid disease by year is demonstrated in Figure 2. During 2009–2017, the number of trial registration was quite small in both thyroid carcinoma and nontumorous thyroid disease, but it increased significantly between 2018 and 2021. The total number of trials on thyroid disease increased by 167%.
 |
Figure 2 Annual numbers of initiated thyroid diseases drug clinical trials by major indications in China, 2009–2022. |
Characteristics of Registered Trials
The detailed information on the drug trial’s characteristics is summarized in Table 1. It is of significant difference in the phase, and randomization (p<0.05) between nontumorous thyroid disease and thyroid carcinoma. In terms of status, the trials in nontumorous thyroid disease were all completed or still in proceeding, while there are two trials in thyroid carcinoma suspended and terminated (Figure 3). The bioequivalence study accounted for 66.7% of nontumorous thyroid disease trials. However, it only accounted for 40.9% of thyroid carcinoma, followed by 15.9% in phase I, 22.7% in phase II and 20.5% in phase III. In the study design, 71.4% of trials in nontumorous thyroid disease were cross-over studies.
 |
Table 1 Characteristics of Thyroid Diseases Drug Clinical Trials in China (n=65) |
 |
Figure 3 Distribution of phase and status for drug clinical trials. Numbers beside each column of the chart represent the actual number of trials. |
Distribution of Clinical Trials by Pathological Types and Drug Mechanisms
Among all the 65 trials, the proportion of drugs targeting differentiated thyroid cancer (44.6% [29/65]) accounted for the largest, followed by medullary thyroid carcinoma (13.8% [9/65]), hypothyroidism (10.8% [7/65]), and hyperthyroidism (9.2% [6/65]) (Figure 4A). In contrast, the number of drugs for Graves’ disease (n=1), anaplastic thyroid cancer (n=2), and thyroiditis was quite small (n=2).
In terms of the drug mechanisms and clinical indications, the number of multi-target tyrosine kinase inhibitors ranked first (n=35) (Figure 4B). The definite drugs of multi-target tyrosine kinase inhibitors are summarized in Table 2. As for differentiated thyroid cancer, the number of multi-target tyrosine kinase inhibitors in clinical trials accounted for the largest proportion (35.4% [23/65]) (Figure 4B), including sorafenib, anlotinib, dabrafenib, donafenib, lenvatinib, surufatinib, vandetanib. For undifferentiated thyroid carcinoma, an anti-PD-1 antibody (Hx008) and a BRAF V600E small molecule inhibitor (Hlx208) were applied for clinical trials. For medullary thyroid carcinoma, all drug trials were multi-target tyrosine kinase inhibitors including cabozantinib, LOXO-292, Blu-667 and HA121-28 and TY-1091. For advanced thyroid cancer, surufatinib, SYHA1813, YP01001 and selective RET inhibitor (Hec169096) are all Class I new drugs for the treatment of advanced solid tumors. In nontumorous thyroid disease trials, drugs for hyperthyroidism and hypothyroidism were levothyroxine sodium, propylthiouracil, methimazole and metoprolol which have been commonly used for many years. The only new drug trial was the neonatal Fc receptor antibody for Graves’ ophthalmopathy (GO).
 |
Table 2 The Registered Drugs Trials for Thyroid Diseases by Disease Topic and Drug Class |
Geographical Distribution of Trial Leading Units in China
Overall, 65 trials were conducted in 17 provinces, autonomous regions or municipalities in China (Figure 5; Supplementary Table 1). A total of 15 of 36 (41.7%) principal investigator (PI) leading units were from Shanghai (n=6), Beijing (n=4), and Anhui (n=4). In contrast, there was no PI leading unit in the northwest of China, which reflected an uneven geographical distribution in clinical trials for thyroid disease in China (Figure 5). In addition, trial leading units in thyroid carcinoma were more widely distributed in China than those in nontumorous thyroid disease.
 |
Figure 5 Geographical distribution of thyroid diseases drug clinical trials and leading units in China, 2009–2022. |
Discussion
The drug trials of thyroid diseases in China are related to millions of patients. However, the current situation of drug clinical trials for thyroid diseases in China is still unclear. This study is aimed at analyzing the current situation and the progress for thyroid drug trials in China. Thyroid diseases include thyroid carcinoma and nontumorous thyroid diseases. Thyroid carcinoma is the most common cancer in the endocrine system.10 In the last century, surgery and radioiodine therapy were the main strategies for thyroid carcinoma, but there is no effective treatment for refractory differentiated thyroid cancer, undifferentiated thyroid cancer and medullary thyroid carcinoma. However, molecular targeted therapy shed light on these refractory thyroid cancers and provided a more efficient and accurate treatment strategy.11 In the past ten years, due to changes in China’s drug research and development and approval policies, as well as a large amount of capital investment, trials of molecular targeted drugs on thyroid carcinoma have made much progress in China, which provided the Chinese patients with more options. In terms of nontumorous thyroid disease, including hyperthyroidism, hypothyroidism and Graves’ disease, there is also high prevalence in the Chinese population.3 Apart from the commonly used anti-thyroid drugs and levothyroxine in the clinic, targeted therapy and immunomodulation therapy are potential research and development directions in the future. However, there was no previous research providing analysis and prospect of drug clinical trials on thyroid diseases in China to date. To the best of our knowledge, this is the first study to comprehensively analyze changes in drug clinical trials on thyroid diseases in China.
We analyzed 65 trials including 21 trials in nontumorous thyroid disease and 44 trials in thyroid carcinoma from 2009 to 2022 in China. Compared with the rapid development in drug trials on thyroid cancer, drug trials on nontumorous thyroid diseases have developed slowly.
A significant difference was observed in the trial phase and randomization between thyroid carcinoma and nontumorous thyroid disease. In terms of clinical indications and drug mechanisms, the number of trials in multi-target tyrosine kinase inhibitors for thyroid carcinoma (n=35) ranked first. Sixty-five trials were led by principal investigator (PI) units in 17 provinces, which exhibited a severely uneven geographical distribution.
From 2015 to 2017, the State Council of China successively issued a series of reform measures, including “Opinions on the Reform and Approval System of Drugs and Medical Devices” and “Opinions on Deepening the Reform of the Evaluation and Approval System and Encouraging the Innovation of Pharmaceutical and Medical Devices”.12,13 In this study, we also found that the numbers of both thyroid carcinoma and nontumorous thyroid disease increased gradually from 2009 to 2021. In 2018, the NMPA also announced that clinical trials can be carried out if no negative and questionable comments or opinions are received within sixty working days after the application.14 What is more, the government provided clear opinions on the consistency evaluation and encouraged enterprises to carry out bioequivalence studies.15 With the support of the above policies, drug clinical trials on thyroid diseases have also achieved much development. We found a significant increase in the number of registered trials for both thyroid carcinoma and nontumorous thyroid diseases since 2018. However, the bioequivalence studies accounted for the largest proportion (32[49.2%]), while phase I studies only accounted for 18.5% (12/65), suggesting the deficiency in original drug research and development.
Drug therapy is of great significance for both thyroid carcinoma and nontumorous thyroid disease. In thyroid carcinoma, molecular targeted therapy is the most promising therapeutic strategy for advanced thyroid cancer, medullary thyroid carcinoma and undifferentiated thyroid cancer.16,17 Basic research confirmed that gene mutation of the tyrosine kinase family was closely related to thyroid carcinoma. Mutations of RET and VEGFR genes existed in medullary thyroid carcinoma and follicular thyroid carcinoma widely, while mutations of the BRAF gene were found in undifferentiated thyroid and follicular thyroid carcinoma.18,19 The targeted therapy also focuses on these genes. Sorafenib was the first approved drug in 2013 by US Food and Drug Administration (FDA) for differentiated thyroid cancer therapy.20 Several drug trials have proved that targeted therapy including multi-target tyrosine kinase inhibitors, rearranged during transmission (RET) inhibitors, BRAF kinase inhibitors and tropomyosin receiver kinase (TRK) inhibitors significantly improved the patient’s medium progression-free survival (PFS).21,22 In this study, we found that half of the trials (n=35) were multi-target tyrosine kinase inhibitor, which indicated the extensive therapeutic potential and competitive development of this target. It should also be noted that selective RET inhibitor (Hec169096) and multi-target tyrosine kinase inhibitor (SYHA1813, YP01001) applied by the Chinese pharmaceutical enterprises are all first-in-class new drugs. In the research and development of thyroid carcinoma drugs, China has gradually caught up and approached the international level.
Compared with the thyroid carcinoma trials, the number of drug trials on nontumorous thyroid diseases was less (n=21). The vast majority of drug trials were generic drugs. In the thyroid hormone drugs, there was only levothyroxine sodium and was lack of triiodothyronine, a highly efficient active form for rapid therapy in hypothyroidism and myxedema coma. A combination therapy including LT4 and T3 was also used in the trials for Hashimoto’s thyroiditis, but the advantage was still controversial, mainly due to the risk of arrhythmia.23,24 In terms of the dosage form, there is only the tablet form, without any oral solution. The levothyroxine needs to be converted into the effective active form T3 by deiodinase in the body and intestinal absorption is often affected by many factors. It is a difficult problem to avoid over-treatment and under-treatment in the clinic. Recently, several studies have shown that liquid or hydrogel dosage forms are more conducive to promoting the absorption of levothyroxine and improving patient compliance.25,26 The improvement in the dosage form should also be strengthened in this field.
In anti-thyroid drugs, propylthiouracil and methimazole have been found and applied for the clinic since the 1940s.27,28 Although the efficacy of these anti-thyroid drugs in hyperthyroidism, Graves’ disease and other thyroid diseases have been widely recognized. The side effects may not be ignored. Propylthiouracil and methimazole could induce liver injury and agranulocytosis.29 Apart from propylthiouracil and methimazole, there were only commonly used drugs including β-blocker, immunosuppressant azathioprine and glucocorticoid in the trial applications and lack of specific TSHR targeted drugs. What is more, propylthiouracil was recommended for the pregnancy due to its low placenta permeability, and there is still a clear risk in fetal birth defects.30 Due to the lack of new drugs and related clinical trials, how to avoid harm to the fetus and mother as much as possible during the treatment of hyperthyroid pregnant women is a long-term pending problem.31–33 Trials in pregnancy require strict ethical approval and a safety review process. Compared with other countries, there is still a lack of drug clinical trials on thyroid diseases during pregnancy in China, and the recommendations also lack clinical trial evidence support. New therapies such as thyroid stimulating hormone receptor (TSHR) targeted therapy and immune cell therapy were still lacking trial application in China. Compared with trials on thyroid carcinoma (n=44), trials on nontumorous thyroid diseases (n=21) were much less, and most of the trials were bioequivalent studies. The new drug trials were the Monoclonal Antibody (HBM9161) and Insulin-like growth factor 1 receptor (IGF-1R) antibody (IBI311, ZB001 and PHP1003) targeting Graves’ Ophthalmopathy (GO). It is worth mentioning that PHP1003 was also the first independently innovative targeted drug for GO in China. At present, there are no approved targeted drugs for GO by NMPA. These drug trials will bring great improvement to the clinical treatment of GO patients in China. Consistent with the previous studies, our results also showed that the original drugs are mainly concentrated in the cancer field.5,34 On the one hand, more new targets have been found in cancer research, and on the other hand, it is easier for cancer drug trials application and endpoints observation.
Despite the progress in the drug clinical trials on thyroid diseases, there remain shortcomings that should not be ignored. First, the number of international multi-center trials was few. International multi-center trials could improve the utilization of new drugs in Chinese patients, and provide evidence for the efficacy and safety evaluation in Chinese patients. Second, doctors are the actual executants in drug trials. There have been strong policy incentives for drug development enterprises and hospitals, but not enough for doctors. Third, in the development of anti-thyroid cancer drugs, there were a large number of “me too” drugs, which followed the first-in-class drugs. There is a lack of first-in-class drugs. The underlying reasons may be the insufficient original basic research and lack of enterprises with research and development capability and sufficient capital. Fourthly, the geographical distribution of the leading units of drug trials was quite uneven, which reflects the imbalance distribution of high-quality medical resources. Hospitals with drug clinical trial units were gathered in Beijing, Shanghai and other economically developed provinces, which is uncoordinated with the geographical distribution of patients with thyroid diseases in China.
Conclusions
This study provided a comprehensive analysis of drug trials for thyroid diseases in China in the past 12 years. The rapid growth in the number of drug trials reflected the progress in the clinical trial capacity of new drug research and development for thyroid diseases. The most prominent progress lies in drug targeted therapy for thyroid carcinoma, but there are a lot of homogeneous trials targeting the same hot spot target and lack of innovative “first in class” drugs. More research and investment are needed in nontumorous thyroid diseases. In general, the development of drug trials has provided more optimized drug options for patients with thyroid diseases in China. Considering the increased prevalence of thyroid diseases in China, a sustained and concerted effort development is required to improve the innovative drug development and drug optimization for thyroid diseases from relevant policymakers, investigators, pharmaceutical enterprises and hospital clinical trial organizations.
Data Sharing Statement
The data will be made available by the authors, without undue reservation.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the National Natural Science Foundation of China (81825003, 91957123, 81800327, 81900272, 82270376), the National Key Research and Development Program of China (2020YFC2004700), the Beijing Nova Program (Z201100006820002) from Beijing Municipal Science & Technology Commission, CAMS Innovation Fund for Medical Sciences (2021-I2M-5-003). The funding source of this study does not influence or involve in study design; collection, analysis, and interpretation of data; the writing of the report; and the decision to submit the paper for publication. All authors had full access to the data and had final responsibility for the decision to submit for publication.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Deng Y, Li H, Wang M, et al. Global burden of thyroid cancer from 1990 to 2017. JAMA Netw Open. 2020;3(6):e208759. doi:10.1001/jamanetworkopen.2020.8759
2. Wang J, Yu F, Shang Y, et al. Thyroid cancer: incidence and mortality trends in China, 2005-2015. Endocrine. 2020;68(1):163–173. doi:10.1007/s12020-020-02207-6
3. Li Y, Teng D, Ba J, et al. Efficacy and Safety of Long-Term Universal Salt Iodization on Thyroid Disorders: epidemiological Evidence from 31 Provinces of Mainland China. Thyroid. 2020;30(4):568–579. doi:10.1089/thy.2019.0067
4. Shan Z, Chen L, Lian X, et al. Iodine Status and Prevalence of Thyroid Disorders After Introduction of Mandatory Universal Salt Iodization for 16 Years in China: a Cross-Sectional Study in 10 Cities. Thyroid. 2016;26(8):1125–1130. doi:10.1089/thy.2015.0613
5. Su X, Wang H, Zhao N, et al. Trends in innovative drug development in China. Nat Rev Drug Discov. 2022;21(10):709–710. doi:10.1038/d41573-022-00077-3
6. Chen H, Zhou Y, Han X, et al. The changing landscape of anti-lymphoma drug clinical trials in mainland China in the past 15 years (2005-2020): a systematic review. Lancet Reg Health West Pac. 2021;8:100097. doi:10.1016/j.lanwpc.2021.100097
7. Zhong Q, Tao Y, Chen H, et al. The changing landscape of anti-lung cancer drug clinical trials in mainland China from 2005 to 2020. Lancet Reg Health West Pac. 2021;11:100151. doi:10.1016/j.lanwpc.2021.100151
8. Song L, Jia Y, Ran S, et al. Current situation of pediatric clinical trials in China: focus on trials for drug marketing application and administrative approval. BMC Pediatr. 2022;22(1):144. doi:10.1186/s12887-022-03208-2
9. Li N, Huang HY, Wu DW, et al. Changes in clinical trials of cancer drugs in mainland China over the decade 2009-18: a systematic review. Lancet Oncol. 2019;20(11):e619–e26. doi:10.1016/S1470-2045(19)30491-7
10. La Vecchia C, Negri E. Thyroid cancer: the thyroid cancer epidemic - overdiagnosis or a real increase? Nat Rev Endocrinol. 2017;13(6):318–319. doi:10.1038/nrendo.2017.53
11. Priya SR, Dravid CS, Digumarti R, et al. Targeted therapy for medullary thyroid cancer: a review. Front Oncol. 2017;7:238. doi:10.3389/fonc.2017.00238
12. Opinions on Deepening the Reform of the Evaluation and Approval System and Encouraging the Innovation of Pharmaceutical and Medical Devices; 2017. Available from: http://www.gov.cn/xinwen/2017-10/08/content_5230105.htm?from=groupmessage&isappinstalled=0.
13. Opinions on Reforming the Evaluation and Approval System of Pharmaceutical and Medical Devices; 2015. Available from: http://www.gov.cn/zhengce/content/2015-08/18/content_10101.htm.
14. Announcement of the National Medical Products Administration on adjusting the approval process for drug clinical trial evaluation; 2018. Available from: https://www.nmpa.gov.cn/directory/web/nmpa/xxgk/ggtg/ywlchshyjgrdgg/20180727172901286.html.
15. Opinions on the evaluation of consistency of quality and efficacy of generic drugs; 2016. Available from: http://www.gov.cn/zhengce/content/2016-03/05/content_5049364.htm.
16. Filetti S, Durante C, Hartl DM, et al. ESMO Clinical Practice Guideline update on the use of systemic therapy in advanced thyroid cancer. Ann Oncol. 2022;33(7):674–684. doi:10.1016/j.annonc.2022.04.009
17. Attwood MM, Fabbro D, Sokolov AV, et al. Trends in kinase drug discovery: targets, indications and inhibitor design. Nat Rev Drug Discov. 2021;20(11):839–861. doi:10.1038/s41573-021-00252-y
18. Nagura S, Katoh R, Miyagi E, et al. Expression of vascular endothelial growth factor (VEGF) and VEGF receptor-1 (Flt-1) in Graves disease possibly correlated with increased vascular density. Hum Pathol. 2001;32(1):10–17. doi:10.1053/hupa.2001.21139
19. Prabhu M, Shakya S, Ballal S, et al. RET gene mutation analysis and long-term clinical outcomes of medullary thyroid cancer patients. Nucl Med Commun. 2020;41(11):1136–1142. doi:10.1097/MNM.0000000000001264
20. Brose MS, Nutting CM, Jarzab B, et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, Phase 3 trial. Lancet. 2014;384(9940):319–328. doi:10.1016/S0140-6736(14)60421-9
21. Elisei R, Schlumberger MJ, Muller SP, et al. Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol. 2013;31(29):3639–3646. doi:10.1200/JCO.2012.48.4659
22. Schlumberger M, Tahara M, Wirth LJ, et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med. 2015;372(7):621–630. doi:10.1056/NEJMoa1406470
23. Nygaard B, Jensen EW, Kvetny J, et al. Effect of combination therapy with thyroxine (T4) and 3,5,3’-triiodothyronine versus T4 monotherapy in patients with hypothyroidism, a double-blind, randomised cross-over study. Eur J Endocrinol. 2009;161(6):895–902. doi:10.1530/EJE-09-0542
24. Jonklaas J, Bianco AC, Cappola AR, et al. Evidence-Based Use of Levothyroxine/Liothyronine Combinations in Treating Hypothyroidism: a Consensus Document. Eur Thyroid J. 2021;10(1):10–38. doi:10.1159/000512970
25. Castellana M, Castellana C, Giovanella L, et al. Prevalence of gastrointestinal disorders having an impact on tablet levothyroxine absorption: should this formulation still be considered as the first-line therapy? Endocrine. 2020;67(2):281–290. doi:10.1007/s12020-019-02185-4
26. Ducharme M, Scarsi C, Bettazzi E, et al. A Novel Levothyroxine Solution Results in Similar Bioavailability Whether Taken 30 or Just 15 Minutes Before a High-Fat High-Calorie Meal. Thyroid. 2022;32(8):897–904. doi:10.1089/thy.2021.0604
27. Astwood EB, Vanderlaan WP. Treatment of hyperthyroidism with propylthiouracil. Ann Intern Med. 1946;25(5):813–821. doi:10.7326/0003-4819-25-5-813
28. Reveno WS, Rosenbaum H. Treatment of hyperthyroidism with 1-methyl-2-mercaptoimidazole. J Am Med Assoc. 1950;143(16):1407–1408. doi:10.1001/jama.1950.02910510025006
29. Corvilain B, Hamy A, Brunaud L, et al. Treatment of adult Graves’ disease. Ann Endocrinol (Paris). 2018;79(6):618–635. doi:10.1016/j.ando.2018.08.003
30. Kahaly GJ, Bartalena L, Hegedus L, et al. 2018 European Thyroid Association Guideline for the Management of Graves’ Hyperthyroidism. Eur Thyroid J. 2018;7(4):167–186. doi:10.1159/000490384
31. Tsakiridis I, Giouleka S, Kourtis A, et al. Thyroid Disease in Pregnancy: a Descriptive Review of Guidelines. Obstet Gynecol Surv. 2022;77(1):45–62. doi:10.1097/OGX.0000000000000960
32. Illouz F, Luton D, Polak M, et al. Graves’ disease and pregnancy. Ann Endocrinol (Paris). 2018;79(6):636–646. doi:10.1016/j.ando.2018.08.004
33. De Groot L, Abalovich M, Alexander EK, et al. Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(8):2543–2565. doi:10.1210/jc.2011-2803
34. Li G, Liu Y, Hu H, et al. Evolution of innovative drug R&D in China. Nat Rev Drug Discov. 2022;21(8):553–554. doi:10.1038/d41573-022-00058-6
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

