Back to Journals » Journal of Pain Research » Volume 16
Central Sensitization in Patients with Chronic Pain Secondary to Carpal Tunnel Syndrome and Determinants
Authors Feng B , Gong C , You L, Lin Y, Wang Y, Ip WY, Wang Y
Received 25 September 2023
Accepted for publication 12 December 2023
Published 19 December 2023 Volume 2023:16 Pages 4353—4366
DOI https://doi.org/10.2147/JPR.S441786
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Natalie Strand
Beibei Feng,1– 4,* Chen Gong,1,3,4,* Longfei You,1,3,4,* Yangyang Lin,1,3,4 Yafei Wang,1,3,4 Wing Yuk Ip,2 Yuling Wang1,3,4
1Department of Rehabilitation Medicine, the Sixth Affiliated Hospital, Sun Yat-sen University, Guangzhou, People’s Republic of China; 2Department of Orthopaedics & Traumatology, the University of Hong Kong, Hong Kong, Special Administrative Regions, People’s Republic of China; 3Guangdong Provincial Clinical Research Center for Rehabilitation Medicine, Guangzhou, People’s Republic of China; 4Biomedical Innovation Center, the Sixth Affiliated Hospital, Sun Yat-sen University, Guangzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yuling Wang, Department of Rehabilitation Medicine, the Sixth Affiliated Hospital, Sun Yat-sen University, No. 26, Yuancun 2nd Cross Road, Guangzhou, 510655, People’s Republic of China, Tel + 86-20-38476737, Fax +86-20-38254221, Email [email protected] Wing Yuk Ip, Department of Orthopaedics & Traumatology, the University of Hong Kong, Hong Kong, Special Administrative Regions, People’s Republic of China, Tel +852-22554581, Fax +852-28174392, Email [email protected]
Purpose: Central sensitization (CS) is commonly seen in chronic pain disorders, including neuropathic pain. However, there exist inconsistencies concerning the presence of CS in chronic pain secondary to carpal tunnel syndrome (CTS). CS and neuropathic pain manifestations in CTS remain not well established. Therefore, this study aims to investigate the CS and pain profiles in patients with CTS and to explore the potential determinants associated with CS.
Patients and Methods: Patients with suspected CTS symptoms lasting 3 months or above and healthy controls were enrolled. History, physical examinations, and nerve conduction studies were employed to confirm the diagnosis and severity of median nerve dysfunction. The central sensitization inventory (CSI) was used to screen CS. Other outcomes included neuropathic pain, CTS-specific symptom severity and functions, emotion, and health-related quality of life. Between-group comparisons were conducted in terms of the CS presence. Logistic regression analysis was performed to identify determinants associated with CS.
Results: Over 60% of participants with CTS were found with clinical CS, significantly higher than that in the control group. More than 70% of the CTS participants were identified to have possible or very likely neuropathic pain components. In addition, one-fourth of CTS cases had depression or anxiety. Anxiety was associated with an increased risk of developing CS in CTS (adjusted OR=1.31, 95% CI 1.08– 1.59), whereas higher self-perceived general health rating was negatively associated with the presence of CS (adjusted OR=0.92, 95% CI 0.88– 0.97) in the multivariate adjusted regression model.
Conclusion: CS is prevalent in patients with CTS. Predominant neuropathic pain characteristics were uncovered in CTS patients as well as comorbid psychological distress. Significant association was found between anxiety and CS presence. Self-perceived general health was inversely related to CS. Further research is warranted to explore the mechanisms of anxiety and central pain processing in painful entrapment neuropathy.
Keywords: central sensitization, chronic pain, carpal tunnel syndrome, determinants
Introduction
Central sensitization (CS), an abnormal condition of the central nervous system, is characterized by pain hypersensitivity manifestations such as hyperalgesia and allodynia, which is usually associated with the persistence of chronic pain.1–3 The presence of CS has been reported to be related to augmented neural signal processing in the cortex and spinal cord.1,2 CS is frequently reported in population with various chronic pain disorders, including osteoarthritis, low back pain, and peripheral neuropathies, and it may explain the widespread pain beyond the affected lesions, and worse still, place an adverse effect on the efficacy of pain management strategies.4–8
Carpal tunnel syndrome (CTS) is regarded as the most common type of peripheral nerve entrapment disorder that inflicts a number of people in the world.9–11 Patients with CTS often complain of numbness, tingling, and pain symptoms in areas innervated by the median nerve, especially during the nighttime that may affect their normal sleep, and some may even experience weakness at hand with thenar muscle atrophy in severe cases.9–11 CS has been identified in patients with CTS by previous relevant studies, which could be associated with a poorer functional recovery after carpal-release surgeries or other conservative interventions.7,12–16 Aberrant pain sensitivity was found in CTS patients, with signatures of CS such as extraterritorial pain, lowered pain threshold, hyperalgesia to temperature and mechanical stimuli.14,17–20
Although few studies were conducted previously to examine the presence of CS in CTS, there exist several inconsistencies in the findings on sensory phenotypes of CS among CTS patients.18–25 Several trials revealed differences in parameters including cold, mechanical, and vibration detection thresholds, heat pain threshold, and pressure pain threshold in CTS patients, compared with normal controls.20,24,25 However, no significant between-group differences were discovered in other reports with regard to cold, heat, mechanical, or pressure pain thresholds between participants with and without CTS.23,26 Furthermore, different psychophysiological factors may play a role in the development of CS among those with CTS, namely clinical pain patterns and magnitudes, body mass index (BMI), depression and/or anxiety, and so forth, however, few studies have considered these factors and controlled the possible confounding effects.14,15
The overall pain and CS profiles of patients with CTS have not been fully understood thus far, given the heterogeneous findings in previous studies. Also, the determinants of CS in CTS have yet to be well established. Therefore, the present study aimed to first detect the presence of CS in CTS by the validated Chinese Central Sensitization Inventory (CSI-C)27 as well as to investigate the overall neuropathic pain profiles and clinical characteristics of participants with CTS. Moreover, this study would also like to explore the determinants associated with the CS presence among CTS patients.
Material and Methods
This study was part of a prospective cohort trial, with consecutive sampling from public cluster hospitals in Hong Kong. Ethics approval was obtained from Institutional Review Board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster, and it was preregistered at the clinical trial registry of The University of Hong Kong (HKUCTR-2828) prior to the start of the study. Participants were fully informed about the purpose of the study, and their written consent was obtained before joining the study. All procedures in the study were conducted in accord with the Declaration of Helsinki.
Participants
Patients with suspected CTS symptoms, which lasted at least 3 months were enrolled in two public hospitals in Hong Kong from December 2020 to February 2022. The inclusion criteria for the CTS group were as follows: a) clinically diagnosed as CTS confirmed by nerve conduction study (distal motor latency of the median nerve >3.8 m/s and/or sensory or motor conduction velocity of the median nerve <50 m/s) with positive clinical provocative tests such as Phalen’s test and Tinel Sign;28–30 b) duration of the clinical symptoms lasting no less than 3 months; c) symptom severity score on the visual analogue scale (VAS) greater than 4/10 points; d) willing to join and able to comply with the study protocol.
Those patients who met any of the following conditions were excluded from the study: a) with a history of a wrist or hand surgery or trauma within the past 6 months or intra-articular injection of corticosteroids within the past 3 months; b) having severe or unstable comorbidities including stroke, spinal cord injury, Parkinson’s disease, gout, diabetes mellitus, ischemic heart disease, hypertension, chronic kidney disease, cancer, and so on; c) having other concurrent chronic central or peripheral neuropathic pain conditions; d) having other concurrent chronic musculoskeletal pain disorders; e) abnormal cognitive or mental status, unable to cooperate with the procedures; f) other reasons leading to a failure to comply with the experimental procedures.
A pain-free healthy control group with age- and gender-matched was also recruited in this study. The inclusion criteria for the healthy control group: a) having no pain complaints in the past 3 months; b) having no history of arthritis, fibromyalgia, CTS, or other nerve injuries or dysfunction or other chronic musculoskeletal pain disorders; c) reporting no anxiety or depression in the past 3 months. The exclusion criteria for the healthy control group were similar to that mentioned for the CTS group. Besides, the healthy controls were excluded if they presented acute pain at the screening visit.
Electro-Diagnostic Examination
Nerve conduction studies were employed for a confirmation of the diagnosis of CTS among the participants. The nerve conduction study was conducted in the following standardized steps previously validated by our team at the Clinical Electro-diagnostic Unit, Tung Wah Hospital.31 First, the median sensory nerve conduction was tested. Participants were asked to wash their hands with warm water and dry up. The stimulating ring electrode is placed over the proximal interphalangeal joint of the index finger, and the reference ring electrode over the distal interphalangeal joint of the index finger. The recording electrodes were placed between the flexor carpi radialis and the palmaris longus tendons at the wrist, which is about 12 cm proximal to the ring electrode, as well as at a point just proximal to the distal wrist crease. The ground electrode was positioned between the stimulation and the recording sites. Then, supramaximal electrical stimulation to the median nerve was applied through the stimulating electrode over the index finger for ten times.31 For a better comparison, the ulnar sensory nerve was also measured.
Median motor nerve conduction was evaluated as follows: The recording electrode was placed at the motor point of the abductor pollicis brevis at the thenar area, and the referencing electrode was placed over the proximal phalanx of the thumb. Electrical stimuli were given at several different sites, including the middle of palm, which is about 3–4 cm distal to the distal wrist crease, 6.5 cm proximal to the recording point at the wrist between flexor carpi radialis and palmaris longus tendons, as well as the medial aspect of the antecubital space of the elbow, respectively. The distal motor latency, CMAP amplitude, and conduction velocities of the median nerve were collected. Similarly, the ulnar motor nerve conduction was also measured for a contrast. The recording electrode was placed over the belly of the abductor digiti minimi, and the reference electrode to the distal phalanx of the little finger. Electrical stimulation was performed at the wrist, 7cm proximal to the recording electrode, as well as below and above the elbow, 5cm distal and proximal to the ulnar groove.
The diagnosis of CTS was determined based on the nerve conduction studies of the median nerve, as well as the clinical history and the specific provocative physical examinations such as the Tinel sign and Phalen’s test, in accord with the internationally established guidelines.9,29,30 The severity grading of CTS ranging from very mild to severe was made depending on the neurophysiological results, including the distal motor latency of the median motor nerve and/or the conduction velocity and amplitude of median sensory nerve.29,30
Self-Reported Clinical Outcomes
Several self-reported outcome measures were adopted in the present study to investigate the pain sensitization, neuropathic pain features, pain magnitude and related functions and quality of life, as well as mental status of the participants, which will be elaborated as below.
Central Sensitization Inventory
Central sensitization inventory (CSI) was employed in this study to detect signs of central pain sensitization in patients with CTS.27,32,33 CSI is comprised of two parts. The first part includes 25 questions related to the sensitization symptom severity. For each item, five levels of options including “never, rarely, sometimes, often, and always” are provided. A cutoff score of 30 is commonly used to differentiate between subclinical and clinical central sensitization.34 The second part provides a checklist of several central sensitivity syndromes as well as depression and anxiety. Cultural adaptation and validation of Chinese CSI were conducted prior to implementation in the present study, which demonstrated good reliability and validity.27
PainDETECT Instrument
The neuropathic pain component among the participants was identified by the PainDETECT instrument (PD), which is an efficient screening tool in clinical practice with a recognized sensitivity (85%) and specificity (80%).35–37 PD embodies seven specific questions concerning typical neuropathic pain symptoms, including numbness, burning sensation, tingling or pricking, electric shock pain, pain sensitivity to light touch, temperature, and pressure. Each question was rated in a 6-point Likert scale, namely “never”, “hardly noticed”, “slightly”, “moderately”, “strongly”, and “very strongly”. In addition, pain radiation and the pattern of pain symptoms on a body chart were also recorded. The presence of a neuropathic-like pain component by PD is determined by the final scoring, that is, 0–12 points indicate an unlikely neuropathic pain component; 13–18 points mean possible neuropathic pain, and 19 points or above imply a very likely neuropathic pain component.
Visual Analogue Scale
Visual analogue scale was used to assess the pain intensity, which employs a 100-mm line to allow participants to rate their pain intensity across a continuum from “no pain at all” to “extremely intolerable pain”.38 Participants enrolled were asked to give three pain ratings based on their actual experiences, namely current pain intensity and the worst, and average pain intensities experienced over the past 1 month.
Boston Carpal Tunnel Syndrome Questionnaire
Boston carpal tunnel questionnaire (BCTQ) was adopted in this study to evaluate the specific disease-related symptom severity and function for CTS.9,39,40 It consists of two subscales, namely symptom severity scale (SSS) and functional scale (FS).40 The former has 11 questions concerning the severity of clinical presentations of hand/wrist symptoms such as numbness, tingling, pain, and weakness, while the latter includes eight items related to hand functions. The BCTQ used in this study was the validated Chinese version by Fok, Leung, and Lee.41
Hospital Anxiety and Depression Scale
Mental states of anxiety and depression were measured by the Hospital Anxiety and Depression Scale (HADS).42 HADS contains 14 items assessing anxiety and depression, respectively. Each item on the questionnaire can be scored from 0 to 3 on a 4-point Likert scale. The subscales of anxiety and depression were calculated independently. The rating criteria are as follows: 0–7 points means no anxiety or depression, while 8 points or above represents a positive case of anxiety or depression.42
EQ-5D-5L
EQ-5D-5L, a popular generic and standardized measure of health status, was utilized to evaluate health-related quality of life.43,44 The EQ-5D-5L includes five dimensions of mobility, self-care, usual activities, pain/discomfort, and anxiety/depression with five rating levels (ranging from “no difficulty” to “extremely difficult”). An overall grading of self-perceived health status through a 0–100 scale is also included.44 Official permission of the Chinese version of EQ-5D-5L was obtained from EuroQol.org before using it in the study.
Statistical Analyses
Shapiro–Wilk test was used to assess the normality of data distribution. For data that were not normally distributed, median and interquartile range (IQR) are presented for continuous variables and categorical variables are reported with a frequency distribution. Non-parametric statistical tests were employed for the relevant statistical analyses. The presence rate of central sensitization by CSI was calculated for both the CTS and control group. Kruskal–Wallis test was used to compare the demographic and clinical outcomes between the two groups. Cross tabulation and chi-square statistics were used to detect associations between nominal and categorical variables. Binary logistic regression was performed to estimate the odds ratio (OR) and 95% confidence interval (95% CI) for the presence of a central sensitization component in CTS patients. Univariate logistic regression analysis was carried out for the initial selection of potential predictors associated with the presence of central sensitization. Those predictors screened in univariate regression with significance and met the theoretical hypotheses underlying central sensitization in CTS and are then incorporated in the multivariate regression model. Prior to running the multivariate logistic regression, correlations between the candidate variables were examined to avoid multicollinearity. In addition, the multivariate model was adjusted for the covariate of age and gender. All data analyses were conducted using IBM SPSS Statistics for Macintosh, Version 28.0 (Chicago: SPSS Inc.) and the significance level was set at 0.05.
Results
Demographic and Clinical Characteristics
Totally 168 patients with wrist/hand complaints were screened in the outpatient clinic. Of them, 155 were confirmed with diagnosis of CTS after physical examinations and nerve conduction studies, and eligible for enrollment. In addition, 57 healthy controls were recruited. The average age of the CTS group was 63 years, and the majority of them were female. Similarly, the control group had a mean age of 59 years and over 90% were women. Participants with CTS complained of wrist/hand symptoms for more than 30 months on average. According to the grading system of CTS severity, over 70% of the CTS participants were classified as moderate or severe CTS.29,30
The demographic and clinical characteristics of CTS participants and healthy controls are displayed in Table 1.
 |
Table 1 Demographic and Clinical Characteristics of Participants |
Presence of Central Sensitization
The presence of central sensitization is determined by the CSI-C.27 A threshold score of 30 was used to discriminate between subclinical and clinical central sensitizations.45 As seen in Table 2, over 60% of participants with CTS in the study were present with clinical central sensitization, compared to that of 8.7% in the control group (p<0.001). Moreover, severity levels of central sensitization among the CTS population ranged from mild to extreme (Table 2), with regard to previously established classification system.34
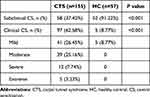 |
Table 2 The Presence of Clinical Central Sensitization of Participants |
Neuropathic Pain Features
The neuropathic pain components classified by PainDETECT between patients with CTS and healthy controls were compared. Table 3 demonstrates that approximately three-quarters of CTS patients experienced possible or very likely neuropathic pain components in this study, while all participants in the control group were classified as having unlikely neuropathic pain.
 |
Table 3 Neuropathic Pain Components Among Participants |
Prevalence of Depression and Anxiety
Abnormal mental status including depression and anxiety by HADS was detected among the participants enrolled in the study. As shown in Table 4, over 25% of CTS patients were presented with depression and about 28% with anxiety. No depression or anxiety was reported in the healthy control group.
 |
Table 4 Depression and Anxiety Status of Participants |
Determinants of Central Sensitization
The predictors associated with the presence of central sensitization in participants with CTS were explored using logistic regression. Univariate regression analyses showed several factors associated with the presence of central sensitization (see Table 5). Potential variables with statistical significance in univariate regression model included sex, employment status, maximal pain intensity in the past month, neuropathic pain features by PD, depression and anxiety, BCTQ scoring, as well as self-perceived general health rating. The severity level of CTS was with a borderline significance value.
 |
Table 5 Univariate Analyses of Central Sensitization in Participants with Carpal Tunnel Syndrome |
Taking into account the hypotheses underlying the presence of central sensitization as well as considering the issue of multicollinearity in multiple regression models, 10 predictors were incorporated into the multivariate logistic regression model, adjusted by age (Table 6). The multivariate analysis demonstrates that anxiety is a risk factor associated with the presence of central sensitization in CTS patients with an elevated OR value of 1.31 (p=0.007; 95% CI, 1.08 to 1.59). In addition, the self-perceived general health rating is a protective factor in the development of central sensitization among CTS participants (p=0.001, OR=0.92, 95% CI, 0.88 to 0.97).
 |
Table 6 Multivariate Analyses of Central Sensitization in Participants with Carpal Tunnel Syndrome |
Discussion
To the best of our knowledge, the present study firstly looked at the profiles of CS and neuropathic pain features using CSI among the Chinese population with the most prevalent entrapment neuropathy of CTS. More than 63% of the participants with CTS in this study were found to be present with clinical CS, significantly higher than that in the normal control group. A large majority of CTS participants were identified to have possible or very likely neuropathic pain components. In addition, quite a few CTS cases exhibited depression or anxiety. The multivariate regression model demonstrated that anxiety was associated with an increased risk of developing CS in CTS, while higher self-perceived general health rating was negatively associated with the occurrence of CS.
Characterized by amplified central processing of pain, CS has been popularly discovered in a variety of chronic disorders with ongoing pain.4–8 Patients with CTS were also found to experience CS, who complained of extraterritorial pain apart from the typical symptoms of paraesthesia, tingling, as well as pain in areas innervated by the median nerve.14,17–20 Although several previous studies have employed quantitative sensory testing to detect CS among CTS patients, controversial results are noted across different trials.18–26 Our findings showed over 60% of CTS participants were recognized as having clinical CS through CSI classification.27,34 In previous relevant reports, over a quarter of CTS patients were identified to be with CS using clinical rules of prediction based on quantitative sensory testing.7 The high prevalence of CS found in the present study could be partly due to the subjective nature of the CSI questionnaire, which echoed similar reports on presence rate of neuropathic pain based on the individual perception of pain symptoms in CTS.25,46–48 Nonetheless, it was claimed that CSI scoring was not related to CS signs of different sensory thresholds.49 The insignificant association between subjective and objective measurements for CS could be probably related to the variances in demographic and clinical features of participants involved, including the age range, the CTS symptom severity, as well as functional limitations. For instance, in that study, CTS patients were relatively younger and of less severe CTS symptomatology.49 CSI is a convenient scale to screen for CS in clinical practice,27 which would be a supplementary alternative to quantitative sensory testing. However, the lack of quantitative sensory testing in this study has limited a further comparison of the exact association between CSI scores and somatosensory profiles of CTS patients, which deserves additional efforts in the future.
Given the definition of neuropathic pain,50 pain symptoms secondary to CTS, commonly with altered sensation, should be naturally identified as neuropathic pain. However, previous studies noted that some CTS patients’ dominant presentations fell into the type of nociceptive pain rather than neuropathic pain.51 The prevalence of neuropathic pain among patients with CTS varies across different trials, ranging from 30% to 80%.25,46,48,52 In our study, as high as 75% of participants with CTS were classified as having possible or very likely neuropathic pain via the PainDETECT tool, which is consistent with previous study,25 despite different measuring instruments used for classifying neuropathic pain. Moreover, those CTS of positive neuropathic pain character seemed more vulnerable to develop CS symptomatology, and negative correlations were found between the severity of neuropathic pain and sensory function as well as mental health status in CTS.25 Our univariate analysis found that neuropathic pain by the PainDETECT score was significantly associated with CS presence; however, it was no longer a predictor of significance in the multivariate regression model with age and gender adjusted. Since neuropathic pain severity is related to an impaired emotional health such as anxiety or depression,53,54 its predicting effect in the multiple regression model could probably be attenuated as anxiety turned out to be a significant risk factor for the presence of CS in CTS in the present study.
Negative emotions such as depression and anxiety have been found to be correlated with the persistence of chronic pain and abnormal pain sensitization.14,15,54,55 In the present study, about 26% and 28% of CTS patients were classified as depression cases and anxiety cases, respectively. The average depression and anxiety scores of CTS participants were 5.9 and 5.7, which are significantly higher than the normal control group. Similarly, previous studies have demonstrated significantly higher rates of depression and anxiety in those with CTS, compared to the general population.30,56 The population with CTS is reported to be associated with a heightened risk to develop anxiety and depressive symptoms.30 The comorbidity of anxiety and/or depression with chronic pain is not uncommon,54,57 and the underlying pathophysiological mechanisms may be due to overlapping regions and neural networks associated with chronic pain and psychological distress in the brain.58–61 Those with anxiety or depression may be more likely to experience abnormal pain sensitization such as CS and less responsive to pain relieving treatments, which merits further attention in practice.7,15,16
Since centrally mediating pain mechanisms in CTS patients can have an adverse impact on the therapeutic efficacy of clinical treatments for CTS,15,62 it is of crucial importance to identify relevant predictors of CS. Due to its complex pathophysiological mechanisms, several different factors could be influencing CS in CTS, including symptom intensity, severity of the median nerve dysfunction, emotional well-being, as well as BMI and smoking.14,15 In this study, a multivariate regression analysis showed that anxiety scores were positively associated with CS presence in CTS, with an elevated OR of 1.31 (95% CI 1.08–1.59) adjusted for age and sex. As mentioned above, patients with CTS had a higher risk of developing negative emotions like depression and anxiety.30,56 In return, those CTS patients with coexisting anxiety may be associated with an increased risk of experiencing abnormal pain sensitization. The interplay between affective distress such as anxiety and depressive symptoms and central sensitization is complicated and not well understood, which could play an important role in the chronification of refractory pain syndromes.60,61 In addition, good self-perceived general health seems to be a protective factor to develop CS among those with CTS in the study, with an OR of 0.92 (95% CI 0.88–0.97). It can be explained that those patients without CS tend to have a more satisfactory health-related quality of life. Knowledge of the clinical predictors associated with CS in CTS may serve to supply first-line clinicians with evidence-based reference when making clinical decisions on management strategies for different patients with CTS, as the existence of CS should affect the responsiveness of clinical treatments.62
There are both strengths and limitations in this study. First, the study included a sample of more than 150 older people with CTS (mean age of 63 years), having a prolonged duration of pain symptoms. Of these, the majority are female, with an increased BMI on average, which is in fact a risk factor for CTS. Furthermore, the CTS participants in the study presented with moderate-to-severe pain, and more than half of the patients were diagnosed as having moderate-to-severe CTS. Hence, the present study represented a clinical sample of Chinese CTS patients with medium to high severity and more afflicting pain magnitude lasting for a relatively long period of time. This study also employed the validated Chinese CSI to investigate the CS among the population of CTS, providing additional evidence for CS screening through the self-administered scale that is easily used in clinical practice. In addition, neuropathic pain features and mood disorders such as depression and anxiety of participants were also evaluated. Several limitations also exist. Since quantitative sensory testing was not incorporated in the study, no correlational analysis was done to compare the CS presence between two approaches via objective assessments and subjective measurements. However, as existing evidence has proved the presence of CS by quantitative sensory testing, the principal objective of this study was to investigate the utility of our validated CSI-C to screen CS in chronic pain related to the entrapment neuropathy of CTS. In addition, only two factors were significantly associated with CS presence in the multivariate regression model, though quite a few variables were of statistical significance in the univariate analyses. A larger sample size may be needed in the future for further validation.
Conclusion
We conclude that CS is prevalent in chronic pain secondary to CTS. Predominant neuropathic pain characteristics were uncovered in CTS patients as well as comorbid psychological distress. Significant association was found between anxiety score and the presence of CS in CTS. The self-perceived general health status was inversely related to CS. Future studies should be warranted to explore the underlying mechanisms of anxiety and central pain processing.
Abbreviation
BCTQ, Boston carpal tunnel questionnaire; BMI, body mass index; CI, confidence interval; CS, central sensitization; CSI, central sensitization inventory; CSI-C, the validated Chinese Central Sensitization Inventory; CTS, carpal tunnel syndrome; FS, functional scale; HC, healthy control; IQR, interquartile range; NP, neuropathic pain; OR, odds ratio; PD, the PainDETECT instrument; SD, standard deviation; SSS, symptom severity scale; VAS, the visual analogue scale.
Data Sharing Statement
The datasets used and analyzed during the current study are available from the first author (Beibei Feng, [email protected] or [email protected]) on reasonable request for academic purpose.
Acknowledgments
The authors would like to thank all the participants in the study.
Author Contributions
All authors made a significant contribution to the current manuscript, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by the Guangzhou Municipal Basic and Applied Basic Research Program (2024A04J3986), the program of Guangdong Provincial Clinical Research Center for Rehabilitation Medicine (2023B110003), and the Guangdong Hopson-Pearl River Education Development Foundation (No. H20190116202012724). The funders above were not involved in any research aspects such as study design, data collection and analysis, report writing and paper submission.
Disclosure
All authors declare no conflicts of interest.
References
1. Nijs J, George SZ, Clauw DJ, et al. Central sensitisation in chronic pain conditions: latest discoveries and their potential for precision medicine. Lancet Rheumatol. 2021;3(5):e383–e392. doi:10.1016/S2665-9913(21)00032-1
2. Gatchel RJ, Neblett R. Central Sensitization: a Brief Overview. J Appl Biobehav Res. 2018;23(2):e12138. doi:10.1111/jabr.12138
3. Woolf CJ. Evidence for a central component of post-injury pain hypersensitivity. Nature. 1983;306(5944):686–688. doi:10.1038/306686a0
4. Lluch E, Nijs J, Courtney CA, et al. Clinical descriptors for the recognition of central sensitization pain in patients with knee osteoarthritis. Disabil Rehabil. 2018;40(23):2836–2845. doi:10.1080/09638288.2017.1358770
5. Nijs J, Apeldoorn A, Hallegraeff H, et al. Low back pain: guidelines for the clinical classification of predominant neuropathic, nociceptive, or central sensitization pain. Pain Physician. 2015;18(3):E333–E345.
6. Zanette G, Cacciatori C, Tamburin S. Central sensitization in carpal tunnel syndrome with extraterritorial spread of sensory symptoms. Pain. 2010;148(2):227–236. doi:10.1016/j.pain.2009.10.025
7. Fernández-de-Las-Peñas C, Fernández-Muñoz J, Navarro-Pardo E, Da-Silva-Pocinho R, Ambite-Quesada S, Pareja J. Identification of Subgroups of Women with Carpal Tunnel Syndrome with Central Sensitization. Pain Med. 2016;17(9):1749–1756. doi:10.1093/pm/pnw054
8. Arendt-Nielsen L, Morlion B, Perrot S, et al. Assessment and manifestation of central sensitisation across different chronic pain conditions. Eur J Pain. 2018;22(2):216–241. doi:10.1002/ejp.1140
9. Padua L, Coraci D, Erra C, et al. Carpal tunnel syndrome: clinical features, diagnosis, and management. Lancet Neurol. 2016;15(12):1273–1284. doi:10.1016/S1474-4422(16)30231-9
10. Atroshi I, Gummesson C, Johnsson R, Ornstein E, Ranstam J, Rosén I. Prevalence of Carpal Tunnel Syndrome in a General Population. JAMA. 1999;282(2):153–158. doi:10.1001/jama.282.2.153
11. Feng B, Chen K, Zhu X, et al. Prevalence and risk factors of self-reported wrist and hand symptoms and clinically confirmed carpal tunnel syndrome among office workers in China: a cross-sectional study. BMC Public Health. 2021;21(1):57. doi:10.1186/s12889-020-10137-1
12. Fernández-de-Las-Peñas C, Plaza-Manzano G. Carpal tunnel syndrome: just a peripheral neuropathy? Pain Management. 2018;8(3):209. doi:10.2217/pmt-2017-0063
13. Soon B, Vicenzino B, Schmid A, Coppieters M. Facilitatory and inhibitory pain mechanisms are altered in patients with carpal tunnel syndrome. PLoS One. 2017;12(8):e0183252. doi:10.1371/journal.pone.0183252
14. Sobeeh MG, Ghozy S, Elshazli RM, Landry M. Pain mechanisms in carpal tunnel syndrome: a systematic review and meta-analysis of quantitative sensory testing outcomes. Pain. 2021. doi:10.1097/j.pain.0000000000002566
15. Liew BXW, de-la-Llave-Rincón AI, Arias-Buría JL, Ortega-Santiago R, Fernández-de-Las-Peñas C. Understanding the Psychophysiological Mechanisms Related to Widespread Pressure Pain Hyperalgesia Underpinning Carpal Tunnel Syndrome: a Network Analysis Approach. Pain Med. 2021;22(11):2708–2717. doi:10.1093/pm/pnab241
16. Roh YH. Influence of centrally mediated symptoms on functional outcomes after carpal tunnel release. Sci Rep. 2018;8(1):11134.
17. Bialosky JE, Bishop MD, Robinson ME, Price DD, George SZ. Heightened pain sensitivity in individuals with signs and symptoms of carpal tunnel syndrome and the relationship to clinical outcomes following a manual therapy intervention. Manual ther. 2011;16(6):602–608. doi:10.1016/j.math.2011.06.003
18. Fernández-de-las-Peñas C, de la Llave-Rincón AI, Fernández-Carnero J, Cuadrado ML, Arendt-Nielsen L, Pareja JA. Bilateral widespread mechanical pain sensitivity in carpal tunnel syndrome: evidence of central processing in unilateral neuropathy. Brain. 2009;132(Pt 6):1472–1479. doi:10.1093/brain/awp050
19. Schmid AB, Soon BT, Wasner G, Coppieters MW. Can widespread hypersensitivity in carpal tunnel syndrome be substantiated if neck and arm pain are absent? Eur J Pain. 2012;16(2):217–228. doi:10.1016/j.ejpain.2011.06.003
20. Tampin B, Vollert J, Schmid AB. Sensory profiles are comparable in patients with distal and proximal entrapment neuropathies, while the pain experience differs. Curr Med Res Opin. 2018;34(11):1899–1906. doi:10.1080/03007995.2018.1451313
21. Schmid AB, Bland JD, Bhat MA, Bennett DL. The relationship of nerve fibre pathology to sensory function in entrapment neuropathy. Brain. 2014;137(Pt 12):3186–3199. doi:10.1093/brain/awu288
22. de la Llave-Rincón AI, Fernández-de-las-Peñas C, Fernández-Carnero J, Padua L, Arendt-Nielsen L, Pareja JA. Bilateral hand/wrist heat and cold hyperalgesia, but not hypoesthesia, in unilateral carpal tunnel syndrome. Exp Brain Res. 2009;198(4):455–463. doi:10.1007/s00221-009-1941-z
23. Baselgia LT, Bennett DL, Silbiger RM, Schmid AB. Negative Neurodynamic Tests Do Not Exclude Neural Dysfunction in Patients With Entrapment Neuropathies. Arch Phys Med Rehabil. 2017;98(3):480–486. doi:10.1016/j.apmr.2016.06.019
24. Kennedy DL, Vollert J, Ridout D, Alexander CM, Rice ASC. Responsiveness of quantitative sensory testing-derived sensory phenotype to disease-modifying intervention in patients with entrapment neuropathy: a longitudinal study. Pain. 2021;162(12):2881–2893. doi:10.1097/j.pain.0000000000002277
25. Matesanz L, Hausheer AC, Baskozos G, Bennett DLH, Schmid AB. Somatosensory and psychological phenotypes associated with neuropathic pain in entrapment neuropathy. Pain. 2021;162(4):1211–1220. doi:10.1097/j.pain.0000000000002102
26. Baskozos G, Sandy-Hindmarch O, Clark AJ, et al. Molecular and cellular correlates of human nerve regeneration: ADCYAP1/PACAP enhance nerve outgrowth. Brain. 2020;143(7):2009–2026. doi:10.1093/brain/awaa163
27. Feng B, Hu X, Lu WW, Wang Y, Ip WY. Cultural Validation of the Chinese Central Sensitization Inventory in Patients with Chronic Pain and its Predictive Ability of Comorbid Central Sensitivity Syndromes. J Pain Res. 2022;15:467–477. doi:10.2147/JPR.S348842
28. Deng X, Chau PL-H, Chiu S-Y, Leung K-P, Hu Y, Ip W-Y. Neural plasticity secondary to carpal tunnel syndrome: a pseudo-continuous arterial spin labeling study. Neural Regeneration Res. 2021;16(1):56.
29. Bland JD. A neurophysiological grading scale for carpal tunnel syndrome. Muscle Nerve. 2000;23(8):1280–1283. doi:10.1002/1097-4598(200008)23:8<1280::aid-mus20>3.0.co;2-y
30. McCallum LM, Damms NA, Sarrigiannis PG, Zis P. Anxiety and depression in patients with suspected carpal tunnel syndrome - A case controlled study. Brain Behav. 2019;9(7):e01342. doi:10.1002/brb3.1342
31. Deng X, Chau LP, Chiu SY, Leung KP, Hu Y, Ip WY. Screening of Axonal Degeneration in Carpal Tunnel Syndrome Using Ultrasonography and Nerve Conduction Studies. J Vis Exp. 2019;(143). doi:10.3791/58681
32. Cuesta-Vargas AI, Neblett R, Chiarotto A, et al. Dimensionality and Reliability of the Central Sensitization Inventory in a Pooled Multicountry Sample. J Pain. 2018;19(3):317–329. doi:10.1016/j.jpain.2017.11.006
33. Ohashi Y, Fukushima K, Inoue G, et al. Central sensitization inventory scores correlate with pain at rest in patients with Hip osteoarthritis: a retrospective study. BMC Musculoskelet Disord. 2020;21(1):595. doi:10.1186/s12891-020-03630-6
34. Neblett R, Hartzell MM, Mayer TG, Cohen H, Gatchel RJ. Establishing Clinically Relevant Severity Levels for the Central Sensitization Inventory. Pain Practice. 2017;17(2):166–175. doi:10.1111/papr.12440
35. Attal N, Bouhassira D, Baron R. Diagnosis and assessment of neuropathic pain through questionnaires. Lancet Neurol. 2018;17(5):456–466. doi:10.1016/s1474-4422(18)30071-1
36. Bouhassira D. Neuropathic pain: definition, assessment and epidemiology. Rev Neurol. 2019;175(1):16–25. doi:10.1016/j.neurol.2018.09.016
37. Freynhagen R, Baron R, Gockel U, Tölle TR. painDETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr. Med. Res. Opin. 2006;22(10):1911–1920. doi:10.1185/030079906X132488
38. Kliger M, Stahl S, Haddad M, Suzan E, Adler R, Eisenberg E. Measuring the Intensity of Chronic Pain: are the Visual Analogue Scale and the Verbal Rating Scale Interchangeable? Pain Pract. 2015;15(6):538–547. doi:10.1111/papr.12216
39. Ortiz-Corredor F, Calambas N, Mendoza-Pulido C, Galeano J, Díaz-Ruíz J, Delgado O. Factor analysis of Carpal Tunnel Syndrome Questionnaire in relation to nerve conduction studies. Clin Neurophysiol. 2011;122(10):2067–2070. doi:10.1016/j.clinph.2011.02.030
40. Levine DW, Simmons BP, Koris MJ, et al. A self-administered questionnaire for the assessment of severity of symptoms and functional status in carpal tunnel syndrome. J Bone Joint Surg Am. 1993;75(11):1585–1592. doi:10.2106/00004623-199311000-00002
41. Fok M, Leung HB, Lee WM. Evaluation of a Hong Kong Chinese version of a self-administered questionnaire for assessing symptom severity and functional status of carpal tunnel syndrome: cross-cultural adaptation and reliability. Hong Kong Med J. 2007;13(5):342–347.
42. Leung CM, Wing YK, Kwong PK, Lo A, Shum K. Validation of the Chinese-Cantonese version of the Hospital Anxiety and Depression Scale and comparison with the Hamilton Rating Scale of Depression. Acta Psychiatr Scand. 1999;100(6):456–461. doi:10.1111/j.1600-0447.1999.tb10897.x
43. Feng YS, Kohlmann T, Janssen MF, Buchholz I. Psychometric properties of the EQ-5D-5L: a systematic review of the literature. Qual Life Res. 2021;30(3):647–673. doi:10.1007/s11136-020-02688-y
44. Zhu J, Yan XX, Liu CC, et al. Comparing EQ-5D-3L and EQ-5D-5L performance in common cancers: suggestions for instrument choosing. Qual Life Res. 2021;30(3):841–854. doi:10.1007/s11136-020-02636-w
45. Neblett R. The central sensitization inventory: a user’s manual. J Appl Biobehav Res. 2018;23(2). doi:10.1111/jabr.12123
46. Oteo-álvaro Á, Marín MT. Predictive factors of the neuropathic pain in patients with carpal tunnel syndrome and its impact on patient activity. Pain Manag. 2018;8(6):455–463. doi:10.2217/pmt-2018-0045
47. Sonohata M, Tsuruta T, Mine H, et al. The Effect of Carpal Tunnel Release on Neuropathic Pain in Carpal Tunnel Syndrome. Pain Res Manag. 2017;2017:8098473. doi:10.1155/2017/8098473
48. Gürsoy AE, Kolukısa M, Yıldız GB, Kocaman G, Celebi A, Koçer A. Relationship between electrodiagnostic severity and neuropathic pain assessed by the LANSS pain scale in carpal tunnel syndrome. Neuropsychiatr Dis Treat. 2013;9:65–71. doi:10.2147/ndt.S38513
49. Matesanz-García L, Cuenca-Martínez F, Simón AI, et al. Signs Indicative of Central Sensitization Are Present but Not Associated with the Central Sensitization Inventory in Patients with Focal Nerve Injury. J Clin Med. 2022;11(4). doi:10.3390/jcm11041075
50. Finnerup NB, Haroutounian S, Kamerman P, et al. Neuropathic pain: an updated grading system for research and clinical practice. Pain. 2016;157(8):1599–1606. doi:10.1097/j.pain.0000000000000492
51. Lazaro RP. Neuropathic symptoms and musculoskeletal pain in carpal tunnel syndrome: prognostic and therapeutic implications. Surg Neurol. 1997;47(2):115–117. doi:10.1016/s0090-3019(95)00457-2
52. Sonohata M, Tsuruta T, Mine H, et al. Clinical characteristics of neuropathic pain in patients with carpal tunnel syndrome. Hand Surg. 2014;19(1):43–48. doi:10.1142/s0218810414500087
53. Cohen SP, Mao J. Neuropathic pain: mechanisms and their clinical implications. Br Med J. 2014;348. doi:10.1136/bmj.f7656
54. Roughan WH, Campos AI, García-Marín LM, et al. Comorbid Chronic Pain and Depression: shared Risk Factors and Differential Antidepressant Effectiveness. Front Psychiatry. 2021;12:643609. doi:10.3389/fpsyt.2021.643609
55. Suzuki K, Haruyama Y, Kobashi G, et al. Central Sensitization in Neurological, Psychiatric, and Pain Disorders: a Multicenter Case-Controlled Study. Pain Res Manag. 2021;2021:6656917. doi:10.1155/2021/6656917
56. Beleckas CM, Prather H, Guattery J, Wright M, Kelly M, Calfee RP. Anxiety in the orthopedic patient: using PROMIS to assess mental health. Quality Life Res. 2018;27(9):2275–2282. doi:10.1007/s11136-018-1867-7
57. Cherif F, Zouari HG, Cherif W, Hadded M, Cheour M, Damak R. Depression Prevalence in Neuropathic Pain and Its Impact on the Quality of Life. Pain Res Manag. 2020;2020:7408508. doi:10.1155/2020/7408508
58. Zhuo M. Long-term cortical synaptic changes contribute to chronic pain and emotional disorders. Neurosci Lett. 2019;702:66–70. doi:10.1016/j.neulet.2018.11.048
59. McIlwrath SL, Montera MA, Gott KM, et al. Manganese-enhanced MRI reveals changes within brain anxiety and aversion circuitry in rats with chronic neuropathic pain- and anxiety-like behaviors. Neuroimage. 2020;223:117343. doi:10.1016/j.neuroimage.2020.117343
60. McCarberg B, Peppin J. Pain Pathways and Nervous System Plasticity: learning and Memory in Pain. Pain Med. 2019;20(12):2421–2437. doi:10.1093/pm/pnz017
61. Gómez Penedo JM, Rubel JA, Blättler L, et al. The Complex Interplay of Pain, Depression, and Anxiety Symptoms in Patients With Chronic Pain: a Network Approach. Clin J Pain. 2020;36(4):249–259. doi:10.1097/ajp.0000000000000797
62. Shi Q, Bobos P, Lalone EA, Warren L, MacDermid JC. Comparison of the Short-Term and Long-Term Effects of Surgery and Nonsurgical Intervention in Treating Carpal Tunnel Syndrome: a Systematic Review and Meta-Analysis. Hand. 2020;15(1):13–22. doi:10.1177/1558944718787892
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
