Back to Journals » Journal of Pain Research » Volume 16
Central Mechanism of Acupuncture Treatment in Patients with Migraine: Study Protocol for Randomized Controlled Neuroimaging Trial
Authors Jia J , Yan C, Zheng X, Shi A, Li Z , Xu L, Hui Z, Chen Y, Cao Z, Wang J
Received 3 June 2022
Accepted for publication 8 December 2022
Published 18 January 2023 Volume 2023:16 Pages 129—140
DOI https://doi.org/10.2147/JPR.S377289
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Houman Danesh
Jingnan Jia, Chaoqun Yan, Xiancheng Zheng, Anqi Shi, Zhijun Li, Lufan Xu, Zhiyuan Hui, Yichao Chen, Zimin Cao, Jun Wang
Dongzhimen Hospital, Beijing University of Chinese Medicine, Beijing, People’s Republic of China
Correspondence: Chaoqun Yan; Jun Wang, Department of Acupuncture and Moxibustion, Dongzhimen Hospital, Beijing University of Chinese Medicine, Hai Yun Cang on the 5th Zip, Dongcheng District, Beijing, 100700, People’s Republic of China, Tel +86-10-84013161, Email [email protected]; [email protected]
Purpose: Acupuncture has been recognized as an effective and safe alternative therapy for migraine, but its central mechanism has not yet been adequately explained. Meanwhile, research into the clinical efficacy and central mechanism of true acupuncture (TA) and sham acupuncture (SA) is lacking. It is necessary to investigate whether TA has better efficacy than SA, and how they achieve different effects. This study aims to evaluate the efficacy of TA and SA, observe the brain response caused by TA and SA, and further investigate the central nervous mechanism of TA and SA treatment for patients with migraine.
Patients and Methods: This is a randomized controlled neuroimaging trial combining acupuncture treatment with functional magnetic resonance imaging, with patients and outcome assessors blinded. A total of 60 patients with migraine will be randomly allocated to receive 12 sessions of either TA or SA treatments (three sessions per week for 4 weeks), and 30 healthy participants will be recruited as the healthy control (HC) group. Outcome assessment and neuroimaging will be conducted before and after the entire intervention. A headache diary and questionnaires of life quality and psychological properties will be used to evaluate clinical efficacy. Multimodal magnetic resonance imagining data analysis will be used to investigate the central mechanism of TA or SA in treating migraine. Pearson’s correlation analysis will be used to reveal the relationship between the brain response and clinical improvements.
Conclusion: The results of this study will reveal the brain response to TA and SA in patients with migraine and contribute to further expanding the knowledge of their central mechanism.
Study Registration: This trial has been approved by the ethics committee of Dongzhimen Hospital affiliated to Beijing University of Chinese Medicine (DZMEC-KY-2020-38) and registered in the Chinese Clinical Trial Registry (registration number ChiCTR2000033995).
Keywords: migraine, acupuncture, functional magnetic resonance imaging, protocol
Introduction
Migraine is a common and disabling disorder that typically manifests as episodic attacks of headache lasting 4 to 72 hours, and is often accompanied by nausea, phonophobia, or photophobia.1 As a worldwide public health issue, the prevalence of migraine has been estimated to be 14.4% overall, with 18.9% for women and 9.8% for men.2 Migraine without aura (MwoA) is the most common form of migraine. The high prevalence and the unavoidable disability of migraine place an enormous burden on individuals and society; migraine has been ranked as the second largest contributor of neurological disability, after stroke.3
Currently, the management of migraine is mainly based on pharmacological treatments, comprising drugs for acute treatment and prevention.4 However, these medications are often associated with side effects and adverse events (AEs), and their overuse increases the risk of clinical progression from episodic to chronic daily headache. Thus, effective nonpharmacological therapeutic strategies for migraine, such as acupuncture, have gradually but widely gained support in many countries. As recognized and recommended by the World Health Organization (WHO),5 acupuncture can be a safe and effective alternative therapy for migraine, and its positive effect has been confirmed by evidence-based medicine studies.6,7 However, whether the effect of true acupuncture (TA) is superior to that of sham acupuncture (SA) remains a highly controversial point,8–10 and the underlying mechanisms of acupuncture in treating migraine are still unclear. These factors partly restrict the widespread application of acupuncture in treating and preventing migraine.
With the development of neuroimaging technology, a brand-new perspective is possible.11–14 Functional magnetic resonance imaging (fMRI), which is characterized by high-quality temporal and spatial resolution without the use of ionizing radiation, has rapidly become an optimal neuroimaging method to investigate the central mechanism of acupuncture.15–17 Increasing numbers of fMRI-based studies of migraine have been conducted, and pathological changes in brain function and structure have been described; it is reported that acupuncture can be used to improve clinical symptoms, as well as to promote functional reorganization of the brain.14,18,19 However, structural remodeling in the brain, induced by acupuncture in patients with migraine, has only been observed in few studies. Meanwhile, reports of these neuroimaging studies often describe the cerebral response induced by TA,20,21 while scarcely focusing on the different central mechanisms of TA and SA. Moreover, diverse brain regions or networks modulated by TA are often reported, with different conclusions.20,22,23 Thus, soundly designed studies of the clinical efficacy and brain response, incorporating multimodal neuroimaging data analysis and correlation analysis, are worth conducting.
In our previous research on TA treatment for migraine,24 we found that, based on the classic Gen-Jie (root–knot) theory of traditional Chinese medicine (TCM), acupuncture of the Jing-Well points in the three Yang meridians of the foot (including GB44, BL67, and ST45) could not only have good clinical efficacy in treating patients with migraines in different headache regions, but could also adjust the functional connection of the default mode network. Therefore, with the purpose of better exploring the clinical efficacy and brain response to both TA and SA, as well as investigating further the central mechanism of acupuncture in treating migraine, we designed this randomized controlled, clinical neuroimaging trial with multimodal magnetic resonance imaging (MRI) analysis (Figure 1).
 |
Figure 1 Trial profile. (A) Participants and intervention. (B) MRI scan. (C) Brain response. |
Methods
Study Design
This is a clinical randomized controlled neuroimaging trial, with three groups: a TA group, an SA group, and a healthy control (HC) group. Participants, including 60 patients with MwoA and 30 healthy subjects (forming the HC group), will be recruited from our clinical center. The total observation period for patients with MwoA will be 20 weeks: 4 weeks of baseline, 4 weeks of treatment, and 12 weeks of follow-up. During the 4-week treatment, patients in the two acupuncture groups will receive 12 sessions of TA or SA treatment. Both the clinical outcome assessments and the MRI scans for patients with MwoA will be conducted at the baseline and at the end of the acupuncture treatments. During the follow-up period, clinical outcome assessments will be conducted every 4 weeks. All of the subjects in the HC group (who will be matched to the TA and SA groups by age, sex, and education) will undergo only the baseline fMRI scan (Figure 2).
 |
Figure 2 Study flowchart. |
The protocol has been registered with the Chinese Clinical Trial Registry (registration number ChiCTR2000033995) and will be conducted in accordance with the Declaration of Helsinki. The study will be reported in accordance with the Consolidated Standards of Reporting Trials (CONSORT) statement and its relevant extensions to randomized trials.25,26 This protocol is reported in accordance with the Standard Protocol Items: Recommendations for Intervention Trials (SPIRIT) guidelines,27 (Supplementary Table 1).
Participants and Recruitment
A total of 60 patients with MwoA, meeting the diagnostic criteria according to the third edition of the International Classification of Headache Disorders of the International Headache Society (IHS),1 will be recruited at our clinical centers, at Dongzhimen Hospital affiliated to Beijing University of Chinese Medicine. In addition, 30 healthy participants will be recruited to the HC group.
The trial will be advertised by the official account on WeChat (China’s most popular social media platform) of Dongzhimen Hospital affiliated to Beijing University of Chinese Medicine. Interested participants will be screened in the clinic against the inclusion or exclusion criteria. Information flyers introducing the details of the trial will be posted at outpatient clinics, for greater exposure.
All participants will receive a study information sheet, detailing the design, procedure, benefits, and risks of the study. Participants will also be instructed to complete a headache diary and will be asked to provide written informed consent before enrollment. Acupuncture treatment and MRI scans will be provided to participants without charge.
Randomization and Blinding
Patients with MwoA will be randomly assigned (on a 1:1 ratio) to either the TA or the SA group after signing written informed consent forms. The randomization sequence will be computer-generated by independent research staff using SAS 9.3 software (SAS Institute, Cary, NC, USA), using block randomization with a block size of 6 through a central randomization system. The randomization list will be stored by an uninvolved investigator and concealed from other study personnel. The allocation schedule will use a telephone randomization procedure. Participants will be informed that they will receive one of two effective interventions randomized after enrollment (acupuncture following the principles of TCM or another type of acupuncture).
Since SA will produce the same stimulation as TA, participants will be blinded to their group allocation. Acupuncturists will not be blinded to the treatment assignments, given the nature of the interventions. Outcome assessors and personnel involved in data analysis will be blinded to the group allocation throughout the entire trial.
To test the blinding to TA or SA treatment and to assess the credibility of the respective treatment methods, assessment of blinding will be used and the Bang index28,29 will be calculated in the middle of the treatment phase and at its end.
Inclusion Criteria
Inclusion Criteria for TA and SA Groups
- Diagnosed as having MwoA, according to the criteria specified by the IHS1;
- Male and female, aged 18–65, right-handed;
- Initial onset of migraines prior to the age of 50 years;
- Experience of acute migraine attacks at a frequency of 2 to 8, but less than 15, days of attacks per month during the previous 3 months and during baseline measurement;
- Experience of migraine attacks for at least 1 year;
- Provision of written, informed consent.
Inclusion Criteria for HC Group
- Male and female, aged 18–65, with a normal physical examination and with no family history of severe disease;
- No history of neurological disease, and no history of major physical disease or trauma;
- Right-handed, roughly matched with the sex, age, and education of the participants with MwoA selected;
- No contraindications for MRI scans, such as pacemakers, cardiac defibrillators, cochlear implants, or claustrophobia.
Exclusion Criteria
Prospective participants will be excluded for the following reasons:
- Headache caused by organic disorders (eg, subarachnoid hemorrhage, cerebral hemorrhage, cerebral embolism, cerebral thrombosis, vascular malformation, arteritis, hypertension, or arteriosclerosis);
- Neurological disease, immunodeficiency, bleeding disorders, or allergies;
- Prophylactic headache treatment with drugs during the previous 3 months;
- Women with a long history of dysmenorrhea, or who are pregnant or lactating, or have plans to become pregnant within 6 months;
- Involvement in other clinical trials;
- History of alcohol or drug abuse or addiction;
- Any contraindications for acupuncture (such as a bleeding tendency) or MRI scans.
During the trial period, patients who meet the following criteria will be dropped out of the study:
- Taking medication or receiving additional treatment other than acupuncture and ibuprofen;
- Occurrence of a serious AE, because of which the doctors consider the treatment should be terminated;
- Withdrawal of consent for study participation by patient for subjective or objective reasons.
If the patients drop out, the researchers should contact them, using as much effort as practicable, to ask the reason, and to make an appointment for follow-up. These patients’ final acupuncture time and relevant assessments should be recorded, for both retention and statistical analysis.
Interventions
The treatment strategies were developed based on TCM theory, by consensus with experienced acupuncture practitioners. All acupuncturists have been trained for at least 5 years and are licensed with at least 3 years of clinical experience. Patients in the TA and SA groups will receive 12 sessions of acupuncture treatment over 4 weeks (three times a week) with each session lasting 30 min. Sterile, single-use filiform acupuncture needles (size 0.25 mm × 25 mm, Ande, Guizhou, China) will be used in the treatment.
For the TA group, only one acupoint will be selected, according to the syndrome differentiation of meridians of the headache region. For temporal headache (defined as Shaoyang headache in TCM), the Zuqiaoyin (GB44) acupoint will be selected. For posterior headache (defined as Taiyang headache in TCM), the Zhiyin (BL67) acupoint will be selected. For headache in the region of the forehead and the brow bone (defined as Yangming headache in TCM), the Lidui (ST45) acupoint will be selected (Figure 3).
 |
Figure 3 Syndrome differentiation of meridians in headache region and acupoint selection for TA group. (A) Headache region. (B) Corresponding acupoint of TA. |
The needle will be inserted in the acupoint at a depth of about 2 to 3 mm, and will be twisted for about 5 s. Each point will be acupunctured to achieve the deqi sensation (a sensation of soreness, numbness, distention, or radiation that indicates effective needling).
For the SA group, the choice of acupuncture needle and the duration of treatment will be the same as in the TA group, but there will be no attempt to induce the deqi sensation. The corresponding non-acupoints will be chosen for the SA group, 4 cm away from the acupoints used for patients in the TA group, on the dorsalis pedis. Moreover, the non-acupoints for patients in the SA group are located between the two meridians instead of on the same meridian as the acupoints for patients in the TA group (Figure 4 and Table 1). All acupoints and non-acupoints will be punctured bilaterally using disposable needles.
 |
Table 1 Details of Acupoints and Non-Acupoints of TA and SA Groups |
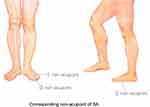 |
Figure 4 Syndrome differentiation of meridians in headache region and corresponding non-acupoint selection for SA group. |
Patients will be asked to fill in headache diaries during the whole study period, including the baseline phase, the intervention phase, and the follow-up period. According to the guidelines of the IHS on clinical trials in migraine,30,31 headache diaries are designed to record the details of migraine attacks, including the time of headache attack and cease, the intensity, frequency, location, and cause of the headache, associated symptoms in each migraine attack, and the use of medication.
Patients will not be allowed to take any prophylactic medications during the study period. In cases of intolerable headache, the patients will be allowed to take ibuprofen (300-mg capsules with sustained release) as a rescue medication, and the use of ibuprofen will be documented in the headache diary, including the time taken, the time of pain relief, and details of any side effects.
MRI Protocol
The MRI scans will be performed at the Beijing Hospital of Traditional Chinese Medicine Affiliated to Capital Medical University using a Siemens 3.0 T scanner (Skyra, Siemens, Erlangen, Germany) equipped with a standard head coil. The scanning procedure, including resting-state blood oxygen level dependent fMRI (BOLD fMRI), diffusion tensor imaging (DTI), and three-dimensional T1-weighted imaging (3D-T1WI), is designed to elucidate cerebral functional and structural changes. An echo planar imaging (EPI) sequence will be used for BOLD fMRI scanning: repetition time (TR) = 2000 ms; echo time (TE) = 30 ms; field of view = 224 mm × 224 mm; flip angle (FA) = 90°; layer thickness = 3.5 mm; axial slices = 32; size of voxel = 3.5 mm × 3.5 mm × 3.5 mm; in-plane resolution = 64 × 64; volumes = 240. Sagittal structural images will be acquired using a magnetization prepared rapid gradient-echo 3D-T1WI sequence with the following parameters: TR/TE = 2530/2.98 ms; FA = 7°; inversion time = 1100 ms; matrix = 256 × 256; 1 mm slice thickness without slice gap. For DTI, a double spin-echo EPI sequence will be used: TR/TE = 12,000/77 ms; FA = 90°; volume interval = 12; 2 mm thick axial slices.
MwoA patients will undergo MRI scans at baseline and at the end of intervention. Before scanning, participants will be required to put on earplugs and helmet and remove all magnetic and metal items. The participants will be asked to adopt a comfortable supine position and to keep awake. A foam pad will be used to reduce head movement. All scans will be reviewed qualitatively by two radiologists to screen for possible brain lesions or structural abnormalities.
Outcome Measures
Clinical Outcome Assessments
Clinical efficacy will be assessed from the changes in clinical characteristics, based on the patients’ headache diaries during the trial and questionnaires of life quality and psychological factors, as follows: (1) frequency of migraine attacks; (2) number of days with migraine; (3) visual analog scale score (where 0 indicates no pain and 10 indicates the worst pain ever); (4) acute pain medication intake; (5) summary scores on the Migraine-Specific Quality-of-Life Questionnaire (MSQ), Migraine Disability Assessment (MIDAS), State-Trait Anxiety Inventory (STA-I), and Beck Depression Inventory (BDI).31,32
Any AEs will be recorded for safety assessment. The outcome measures shown here will be measured at baseline and 4, 8, 12, and 16 weeks after randomization. Detailed time points of outcome assessments are given in Table 2.
 |
Table 2 Timetable of Treatments and Outcomes Collection |
Neuroimaging Data Evaluation
In this study, the functional and structural brain response induced by treatment in the TA and SA groups will be observed using MRI. These neuroimaging outcomes will be acquired after the MRI scanning and analysis. After preprocessing, data from BOLD fMRI will be analyzed to describe the changes of brain activity and function. Group differences in cerebral structure will be assessed by means of DTI and T1WI.
Before the acupuncture treatment, patients will complete the first fMRI scan within 3 days. They will undergo the second fMRI scan within 3 days after the completion of the intervention (Table 2).
Safety and Monitoring
Treatment-related AEs will be compared for patients in the TA and SA groups. Any AE, and how it is dealt with, will be recorded during the 4 treatment weeks and 12 follow-up weeks. Adverse events include bleeding, hematoma, fainting, severe pain, and local infection. If patients report any serious AEs, all details will be documented. The reason for any drop-out or withdrawal, as well as the participant’s compliance, will also be documented.
All researchers, including the acupuncturists, outcome assessors, data collector, data manager, data entry personnel, and statistician, will receive special training regarding the standard procedure and data management. To ensure consistency, all of the MRI scans will be conducted using the same machine by the same operator at baseline and the end of treatment; this operator has been technically trained by a professional engineer from the Chinese Association of Brain Imaging. Another member of the professional staff will check the imaging data for quality and protocol conformity after each scanning session. Additionally, to maintain quality control, a regular report of trial progress will be made, and the research leader will check all trial processes.
Sample Size and Data Management
The sample size of the neuroimaging study was calculated according to studies of the sample size estimation method of fMRI, using the simulation-based method suggested by Desmond et al33 and Hayasaka et al;34 for a stricter α of 0.000002, approximately 25 subjects are required in each group. In this study, allowing for a 20% drop-out rate and the possible occurrence of excessive head motions in the fMRI scanning, we plan to recruit 30 subjects in each group.
The researchers will be required to follow the requirements of a case report form (CRF) and fill in relevant information in a timely and accurate manner. Clinical data will be managed using printed and electronic CRFs. The data in the CRFs will be verified for accuracy, missing data, and data consistency by a clinical research associate. Only outcome assessors have access to CRFs. Hence, personal information of potential and enrolled participants will be protected.
Statistical Analysis
Clinical Data Analysis
The clinical data will be statistically analyzed using SPSS software (SPSS V25.0 for Windows). Data will be described utilizing the mean (standard deviation) when following a normality assumption and the median (quartile spacing) when the normality assumption is violated. Categorical variables will be described using percentages (%) and analyzed using a χ²-test or Fisher’s exact test. Continuous variables will be analyzed using the t-test or the Wilcoxon rank-sum. Analysis of covariance will be used for outcomes with inconsistent baselines to avoid the influence of these factors on the results. All analyses will be conducted on the intention-to-treat (ITT) population (any randomized participant who receives at least one assessment after baseline) and per-protocol (PP) population (any randomized participants who complete all of the assessments). Missing data will be replaced according to the principle of the last observation carried forward. Statistical significance is defined as P < 0.05 in all comparisons, and all statistical testing is two-sided.
MRI Data Analysis
Imaging data will be analyzed using the Data Processing Assistant and Resting-State fMRI (DPARSF) toolbox, in MATLAB 8.6 (Mathworks, Inc., Natick, MA, USA).35 Data preprocessing will include DICOM format conversion, removal of the first 10 volumes, slice timing, head-motion correction, and spatial normalization. After data preprocessing, whole-brain functional connectivity (FC), the amplitude of low-frequency fluctuation (ALFF), degree centrality (DC) and regional homogeneity (ReHo) will be computed. The diffusion tensor will be calculated from the images using a linear model, and fractional anisotropy (FA), axial diffusivity (AD), mean diffusivity (MD), and radial diffusivity (RD) whole-brain maps will be derived. DTI will be analyzed using PANDA package.36 Other structural MRI data like cortical thickness, gray matter volume (GMV) and white matter volume (WMV) will be analyzed using the voxel-based morphometry (VBM) toolbox within Statistical Parametric Mapping 12 (SPM12, Institute of Neurology, University College London). Multiple comparisons will be corrected using Gaussian random field. For visualization, DPARSF-viewer and Brain Net Viewer will be used.
Categorical variables, such as sex-distribution differences between patients with migraine and participants in the HC group will be tested using Pearson’s χ²-test. The independent t-test will be used to compare the distribution of continuous variables, such as age and years of education. The MRI data comparison between patients with migraine and participants in the HC group or between participants in the TA and SA groups will be analyzed using a two-sample t-test. Statistical significance will be considered for P < 0.05, and all hypothesis tests are two-tailed. In addition, the association between the difference in brain function and structure for the TA and SA groups and the clinical variables will be computed using Pearson’s correlation analysis (two-sided test).
Ethics and Dissemination
This trial will be conducted in accordance with the principles of the Declaration of Helsinki37 and has been approved by the ethics committee of Dongzhimen Hospital affiliated to Beijing University of Chinese Medicine (approval number, DZMEC-KY-2020-38). This study was registered on the Chinese Clinical Trial Registry website (registration number ChiCTR2000033995). If any modifications or decisions are made, amendments will be reviewed and approved by the ethics committee, and new protocols will be uploaded to the Chinese Clinical Trial Registry website (http://www.chictr.org.cn/). Written informed consent will be obtained from every participant. In addition, all researchers have been trained and have signed a commitment to protect the confidentiality of study participants.
Discussion
We have presented the protocol for this study for the following two reasons. On the one hand, although acupuncture has proven an effective and safe alternative therapy for migraine, there is no satisfactory explanation of its central mechanism, and there is a disconnect between practice and theory; this partly restricts the widespread application of acupuncture in the treatment and prevention of migraine.38–40 On the other hand, the results of some randomized controlled trials have led to the suggestion that there is no obvious difference in clinical efficacy between TA and SA.41,42 Whether SA intervention will facilitate the same cerebral functional reorganization and structural remodeling as TA remains worthy of further exploration. Therefore, we have designed this trial to investigate both the efficacy and the central mechanism of TA and SA in treating migraine.
In this study, strengths are shown in the following aspects. First, the combined assessments and correlation analyses of clinical outcomes and MRI data will promote to expand our knowledge of the mechanism of acupuncture for treating migraine in the neuroimaging field. Second, the adoption of scales for mental states, disability, and life quality will elaborate on the clinical efficacy of TA and SA, and contribute to reveal the functional and emotion-related brain response. Multimodal fMRI analyses will more comprehensively and objectively describe the acupuncture-induced brain response, regarding functional reorganization and structural remodeling. Third, based on literature research and the consensus of experts, we will include a HC group. A comparison of the fMRI data of patients with MwoA and HC subjects will help to distinguish central nervous system differences between them, and explore whether TA or SA can specifically correct abnormalities in the migraine-affected brain.
Moreover, we will adopt a single acupoint as the intervention for both TA and SA groups; this method has been shown in our previous research to be safe and effective.24 Compared with treatment using several acupoints, the selection of a single acupoint will be simpler and more convenient, will benefit clinical promotion, and will make the research more easily repeatable In addition, statistical analysis of questionnaires of blinding tests and the results of a credibility assessment will also help to avoid severe bias caused by unblinding of the TA or SA groups.31,43
One potential limitation of this study might be that the SA intervention is designed as penetrative SA (PSA), which involves superficial needling on non-acupoints without deqi (the deqi sensation is considered to be crucial to efficacy in traditional Chinese acupuncture). There have long been dissenting views, challenging SA in the past, arguing that its physiological effect of piercing the skin could result in false-negative outcomes.44 However, recent findings are inconsistent with these views. The latest research shows that penetrative SA (PSA) and nonpenetrative SA (NPSA) have a similar weak effect and are appropriate for use as an inert placebo control in randomized controlled trials for pain research. Meanwhile, PSA has been increasingly used because it produces the additional physiological effect of piercing the skin.45 Moreover, compared with NPSA methods, such as the use of nonpenetrating placebo needles with a blunt tip placed on an adhesive pads, PSA will be more effective when blinding Chinese patients, who often have personal experience of acupuncture treatment and expect to experience certain sensations during acupuncture treatment. Therefore, based on expert consensus and literature research, we choose PSA with sham acupoints as the placebo control intervention in this study, consistent with some presented clinical trials of migraine.46–48
Conclusion
In this protocol, a randomized controlled neuroimaging trial is outlined, of which the main purposes are to evaluate the efficacy of TA and SA (acupuncture and placebo acupuncture) treatment for migraine and to investigate the central mechanisms of TA and SA. The results and findings of this study will help provide a new perspective to illustrate the mechanism of acupuncture treatment, thereby promoting the widespread application of acupuncture and offering more benefits for patients affected by migraine in the future.
Abbreviations
AE, adverse event; BOLD, blood oxygen level dependent; CONSORT, Consolidated Standards of Reporting Trials; CRF, case report form; DPARSF, Data Processing Assistant and Resting-State fMRI; DTI, diffusion tensor imaging; EPI, echo planar imaging; FA, flip angle; fMRI, functional magnetic resonance imaging; HC, healthy control; HIS, International Headache Society; MRI, magnetic resonance imaging; MwoA, migraine without aura; NPSA, nonpenetrative sham acupuncture; PSA, penetrative sham acupuncture; SA, sham acupuncture; SPIRIT, Standard Protocol Items: Recommendations for Intervention Trials; TA, true acupuncture; TCM, traditional Chinese medicine; TE, echo time; TR, repetition time; WHO, World Health Organization; 3D-T1WI, three-dimensional T1-weighted imaging.
Data Sharing Statement
The datasets analyzed during this study will be available from the corresponding author on reasonable request after they have been published in a peer-reviewed international journal.
Ethics and Dissemination
This trial has been approved by the ethics committee of Dongzhimen Hospital affiliated to Beijing University of Chinese Medicine (DZMEC-KY-2020-38). The results of this trial will be disseminated through peer-reviewed publications and scientific conferences.
Acknowledgments
The authors sincerely thank all medical workers on the front line for fighting the COVID-19 epidemic.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work. Among them, JNJ contributed most to this work.
Funding
This study was supported by grants from the National Natural Science Foundation of China Youth Fund (82004197) and Project for New Teachers of Beijing University of Chinese Medicine (2020-JYB-XJSJJ-045).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders, 3rd edition. Cephalalgia. 2018;38(1):1–211. doi:10.1177/0333102417738202
2. Stovner LJ, Nichols E, Steiner TJ, et al. Global, regional, and national burden of migraine and tension-type headache, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol. 2018;17(11):954–976. doi:10.1016/s1474-4422(18)30322-3
3. Feigin VL, Nichols E, Alam T, et al. Global, regional, and national burden of neurological disorders, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol. 2019;18(5):459–480. doi:10.1016/s1474-4422(18)30499-x
4. Snow V, Weiss K, Wall E, Mottur-Pilson C. Pharmacologic management of acute attacks of migraine and prevention of migraine headache. Ann Intern Med. 2002;137(10):840–849. doi:10.7326/0003-4819-137-10-200211190-00014
5. NIH Consensus Conference. Acupuncture. JAMA. 1998;280(17):1518–1524. doi:10.1001/jama.280.17.1518
6. Giovanardi C, Cinquini M, Aguggia M, et al. Acupuncture vs. pharmacological prophylaxis of migraine: a systematic review of randomized controlled trials. Front Neurol. 2020;11:576272. doi:10.3389/fneur.2020.576272
7. Xu S, Yu L, Luo X, et al. Manual acupuncture versus sham acupuncture and usual care for prophylaxis of episodic migraine without aura: multicentre, randomised clinical trial. BMJ. 2020;368:m697. doi:10.1136/bmj.m697
8. Fan S, Jin S, Tang T, Chen M, Zheng H. Efficacy of acupuncture for migraine prophylaxis: a trial sequential meta-analysis. J Neurol. 2021;268(11):4128–4137. doi:10.1007/s00415-020-10178-x
9. Diener H, Kronfeld K, Boewing G, et al. Efficacy of acupuncture for the prophylaxis of migraine: a multicentre randomised controlled clinical trial. Lancet Neurol. 2006;5(4):310–316. doi:10.1016/s1474-4422(06)70382-9
10. Ni X, Dong L, Tian T, et al. Acupuncture versus various control treatments in the treatment of migraine: a review of randomized controlled trials from the past 10 years. J Pain Res. 2020;13:2033–2064. doi:10.2147/jpr.S259390
11. Schwedt TJ, Dodick DW. Advanced neuroimaging of migraine. Lancet Neurol. 2009;8(6):560–568. doi:10.1016/s1474-4422(09)70107-3
12. Sprenger T, Borsook D. Migraine changes the brain: neuroimaging makes its mark. Curr Opin Neurol. 2012;25(3):252–262. doi:10.1097/WCO.0b013e3283532ca3
13. Li Z, Liu M, Lan L, et al. Altered periaqueductal gray resting state functional connectivity in migraine and the modulation effect of treatment. Sci Rep. 2016;6(1):20298. doi:10.1038/srep20298
14. Kuangshi L, Yong Z, Yanzhe N, et al. The effects of acupuncture treatment on the right frontoparietal network in migraine without aura patients. J Headache Pain. 2015;16:518. doi:10.1186/s10194-015-0518-4
15. Bashir A, Lipton RB, Ashina S, Ashina M. Migraine and structural changes in the brain: a systematic review and meta-analysis. Neurology. 2013;81(14):1260–1268. doi:10.1212/WNL.0b013e3182a6cb32
16. Schwedt TJ, Chong CD. Functional imaging and migraine: new connections? Curr Opin Neurol. 2015;28(3):265–270. doi:10.1097/wco.0000000000000194
17. Catherine DC, Todd JS, David WD. Migraine: what imaging reveals. Curr Neurol Neurosci Rep. 2016;16(7):64. doi:10.1007/s11910-016-0662-5
18. Zhengjie L, Lei L, Fang Z, et al. The altered right frontoparietal network functional connectivity in migraine and the modulation effect of treatment. Cephalalgia. 2017;37(2):161–176. doi:10.1177/0333102416641665
19. Zhengjie L, Fang Z, Tao Y, et al. Acupuncture modulates the abnormal brainstem activity in migraine without aura patients. Neuroimage Clin. 2017;15:367–375. doi:10.1016/j.nicl.2017.05.013
20. Zou Y, Tang W, Li X, Xu M, Li J. Acupuncture reversible effects on altered default mode network of chronic migraine accompanied with clinical symptom relief. Neural Plast. 2019;2019:5047463. doi:10.1155/2019/5047463
21. Zhang Y, Li KS, Liu HW, et al. Acupuncture treatment modulates the resting-state functional connectivity of brain regions in migraine patients without aura. Chin J Integr Med. 2016;22(4):293–301. doi:10.1007/s11655-015-2042-4
22. Tu Y, Zeng F, Lan L, et al. An fMRI-based neural marker for migraine without aura. Neurology. 2020;94(7):e741–e751. doi:10.1212/wnl.0000000000008962
23. Chang CM, Yang CP, Yang CC, Shih PH, Wang SJ. Evidence of potential mechanisms of acupuncture from functional MRI data for migraine prophylaxis. Curr Pain Headache Rep. 2021;25(7):49. doi:10.1007/s11916-021-00961-4
24. Jia JN, Yan CQ, Qi XH, Zheng XC, Shi AQ, Wang J. 基于功能磁共振初探针刺对偏头痛患者默认模式网络的影响. [Effect of acupuncture on default mode network in patients with migraine based on functional magnetic resonance imaging: a preliminary study]. Zhongguo Zhen Jiu. 2021;41(10):1074–1078. Chinese. doi:10.13703/j.0255-2930.20201002-k0001
25. Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. Int J Surg. 2012;10(1):28–55. doi:10.1016/j.ijsu.2011.10.001
26. Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. PLoS Med. 2010;7(3):e1000251. doi:10.1371/journal.pmed.1000251
27. Chan A, Tetzlaff J, Altman D, Dickersin K, Moher D. SPIRIT 2013: new guidance for content of clinical trial protocols. Lancet. 2013;381(9861):91–92. doi:10.1016/s0140-6736(12)62160-6
28. Bang H, Ni L, Davis C. Assessment of blinding in clinical trials. Control Clin Trials. 2004;25(2):143–156. doi:10.1016/j.cct.2003.10.016
29. Diener H, Tassorelli C, Dodick D, et al. Guidelines of the international headache society for controlled trials of acute treatment of migraine attacks in adults: fourth edition. Cephalalgia. 2019;39(6):687–710. doi:10.1177/0333102419828967
30. Chan AW, Tetzlaff JM, Gøtzsche PC, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ. 2013;346(jan08 15):e7586. doi:10.1136/bmj.e7586
31. Diener HC, Tassorelli C, Dodick DW, et al. Guidelines of the international headache society for controlled trials of preventive treatment of migraine attacks in episodic migraine in adults. Cephalalgia. 2020;40(10):1026–1044. doi:10.1177/0333102420941839
32. Chang H, Jensen M, Yang C, Lai Y. Migraine-specific quality of life questionnaire Chinese version 2.1 (MSQv2.1-C): psychometric evaluation in patients with migraine. Health Qual Life Outcomes. 2019;17(1):108. doi:10.1186/s12955-019-1169-y
33. Desmond JE, Glover GH. Estimating sample size in functional MRI (fMRI) neuroimaging studies: statistical power analyses. J Neurosci Methods. 2002;118(2):115–128. doi:10.1016/S0165-0270(02)00121-8
34. Hayasaka S, Peiffer AM, Hugenschmidt CE, Laurienti PJ. Power and sample size calculation for neuroimaging studies by non-central random field theory. Neuroimage. 2007;37(3):721–730. doi:10.1016/j.neuroimage.2007.06.009
35. Chao-Gan Y, Yu-Feng Z. DPARSF: a MATLAB toolbox for “pipeline” data analysis of resting-state fMRI. Front Syst Neurosci. 2010;4:13. doi:10.3389/fnsys.2010.00013
36. Cui Z, Zhong S, Xu P, He Y, Gong G. PANDA: a pipeline toolbox for analyzing brain diffusion images. Front Hum Neurosci. 2013;7:42. doi:10.3389/fnhum.2013.00042
37. World Medical Association. World medical association declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. doi:10.1001/jama.2013.281053
38. Ma P, Dong X, Qu Y, et al. A narrative review of neuroimaging studies in acupuncture for migraine. Pain Res Manag. 2021;2021:9460695. doi:10.1155/2021/9460695
39. Peihong M, Yuzhu Q, Tao Y, et al. Neuroimaging in the understanding of acupuncture analgesia: a review of acupuncture neuroimaging study based on experimental pain models. Front Neurosci. 2021;15:648305. doi:10.3389/fnins.2021.648305
40. Liu S, Luo S, Yan T, et al. Differential modulating effect of acupuncture in patients with migraine without aura: a resting functional magnetic resonance study. Front Neurol. 2021;12:680896. doi:10.3389/fneur.2021.680896
41. Yang M, Du T, Long H, Sun M, Liang F, Lao L. Acupuncture for menstrual migraine: a systematic review. BMJ Support Palliat Care. 2020. doi:10.1136/bmjspcare-2019-002024
42. Linde K, Allais G, Brinkhaus B, et al. Acupuncture for the prevention of episodic migraine. Cochrane Database Syst Rev. 2016;6:CD001218. doi:10.1002/14651858.CD001218.pub3
43. Yin S, Chen Y, Lei D, et al. Cerebral mechanism of puncturing at He-Mu point combination for functional dyspepsia: study protocol for a randomized controlled parallel trial. Neural Regen Res. 2017;12(5):831–840. doi:10.4103/1673-5374.206655
44. Lund I, Näslund J, Lundeberg T. Minimal acupuncture is not a valid placebo control in randomised controlled trials of acupuncture: a physiologist’s perspective. Chin Med. 2009;4:1. doi:10.1186/1749-8546-4-1
45. Zhou R, Zhu YJ, Chen X, et al. Effect of sham acupuncture on chronic pain: a bayesian network meta-analysis. Pain Med. 2022. doi:10.1093/pm/pnac126
46. Liu L, Lyu TL, Fu MY, et al. Changes in brain connectivity linked to multisensory processing of pain modulation in migraine with acupuncture treatment. Neuroimage Clin. 2022;36:103168. doi:10.1016/j.nicl.2022.103168
47. Zhao L, Chen J, Li Y, et al. The long-term effect of acupuncture for migraine prophylaxis: a randomized clinical trial. JAMA Intern Med. 2017;177(4):508–515. doi:10.1001/jamainternmed.2016.9378
48. Zhang Y, Wang Z, Du J, et al. Regulatory effects of acupuncture on emotional disorders in patients with menstrual migraine without aura: a resting-state fMRI study. Front Neurosci. 2021;15:726505. doi:10.3389/fnins.2021.726505
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
