Back to Journals » Cancer Management and Research » Volume 13
CD44+ Circulating Tumor Endothelial Cells Indicate Poor Prognosis in Pancreatic Ductal Adenocarcinoma After Radical Surgery: A Pilot Study
Authors Xing C, Li Y, Ding C, Wang S , Zhang H, Chen L, Li P, Dai M
Received 4 March 2021
Accepted for publication 15 May 2021
Published 1 June 2021 Volume 2021:13 Pages 4417—4431
DOI https://doi.org/10.2147/CMAR.S309115
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Harikrishna Nakshatri
Cheng Xing, Yatong Li, Cheng Ding, Shunda Wang, Hanyu Zhang, Lixin Chen, Pengyu Li, Menghua Dai
Department of General Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, 100730, People’s Republic of China
Correspondence: Menghua Dai
Department of General Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, No. 1, Shuai Fu Yuan, Dongcheng District, Beijing, 100730, People’s Republic of China
Tel +86-10-69152600
Email [email protected]
Background: Circulating tumor endothelial cells (CTECs) are cells that originate from tumor endothelial cells (TECs) of blood vessels and are shed into peripheral blood. Some studies have shown that CTECs are associated with tumor angiogenesis, growth and indicate prognosis in patients with malignant solid tumor. However, the role of CTECs especially the phenotype of CTECs in pancreatic adenocarcinoma (PDAC) is still not clear. We investigated the relationship between CTECs and patients’ prognosis.
Methods: A total of 73 patients with resectable PDAC were enrolled in our research and underwent radical surgery. Peripheral venous blood samples were collected before surgery, on postoperative day (POD) 7 and on postoperative month (POM) 1, respectively. We used integrated subtraction enrichment and immunostaining-fluorescence in situ hybridization (SE-iFISH) platform to identify and enumerate CTECs. Immunofluorescence was used to identify CTECs expressing CD44 and vimentin.
Results: In patients with early tumor recurrence (DFS< 6 months), the preoperative CD44+ CTEC levels showed significantly higher (P = 0.023). Univariate and multivariate analysis showed that history of diabetes [HR 2.656 (1.194– 5.908), P = 0.017], numbers of positive lymph nodes [HR 1.871 (1.388– 2.522), P < 0.001], preoperative numbers of CD44+ CTECs [HR 1.216 (1.064– 1.390), P = 0.004], and POM1 CA19-9 level [HR 1.002 (1.001– 1.002), P < 0.001] were independent prognostic factors for DFS.
Conclusion: The detection of CD44+CTECs in patients with resectable PDAC preoperatively could be an independent predictor of shorter DFS after radical surgery.
Keywords: circulating tumor endothelial cells, pancreatic adenocarcinoma, prognostic factor, CD44
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is one of the primary causes of cancer-related mortality worldwide.1,2 The only potentially curative treatment for PDAC is surgical resection. While patients with localized disease have been treated with integrated therapy based on radical surgery, the 5-year survival rate still remains 7%-8%.3 Many of these patients developed early postoperative metastatic recurrences due to micrometastatic foci occurred at the time of surgery. Many patients are understaged at diagnosis for the reason that these micrometastatic foci are not identified preoperatively.
Circulating tumor endothelial cells (CTECs)4,5 are cells that originate from tumor endothelial cells (TECs)6,7 of blood vessels and are shed into peripheral blood. It has been extensively investigated that TECs shows clinical significance in tumor growth and metastasis.8–10 Previous studies demonstrated that CTECs may play a part in tumor angiogenesis.11,12 CTECs have been recently reported to express multiple biomarkers such as tumor or stemness markers in patients with breast cancer13 and non-small-cell lung cancer (NSCLC).14,15
Several recent studies indicated that some circulating tumor cells (CTCs) have characteristics similar to circulating tumor stem cells (CTSCs).16 Since cancer growth depends on cancer stem cells (CSCs), which are commonly chemoresistant, CTSCs might be a more sensitive prognostic factor comparing to CTCs.17 Cluster of differentiation 44 (CD44) was a useful stemness marker as reported previously.18 A study demonstrated that gastric cancer patients with CTCs expressing CD44 were more inclined to develop disease recurrence and metastasis.19 Furthermore, another study showed that CTCs labeled with CD44 were independent prognostic factor of decreased disease-free survival (DFS) and overall survival (OS).20 A hypothesis has been proposed that CD44+ CTCs represent a more aggressive subset of CTCs with a more stem cell-like phenotype. Vimentin was considered as an important epithelial–mesenchymal transition (EMT) markers, and was correlated with cancer recurrence as well as decreased overall survival.21,22 Some studies found that CTCs expressing vimentin were more invasive and could promote metastases.23 However, the role of the stemness phenotype or mesenchymal phenotype of CTECs in patients with PDAC is still unclear.
The aim of the present study was to detect different phenotypes of CTECs in the peripheral blood of patients with PDAC, and to determine the prognostic value of CTECs.
Methods
Patients and Samples
From November 2017 to October 2020, patients with resectable PDAC who underwent radical surgery including pancreaticoduodenectomy, distal pancreatectomy with splenectomy, or total pancreatectomy were considered eligible for this study. 6 mL of peripheral venous blood samples were collected 1 day before surgery, postoperative day (POD) 7 and in postoperative month (POM) 1, respectively. All enrolled patients signed consent forms before blood sample collection. The study was approved by the Ethics Review Committees of Peking Union Medical College Hospital (PUMCH), and performed in compliance to the Declaration of Helsinki Principles. Inclusion criteria: (1) patients who underwent radical pancreatectomy (pancreaticoduodenectomy, distal pancreatectomy with splenectomy or total pancreatectomy); (2) patients’ postoperative pathological diagnosis was PDAC; (3) patients gave informed consented, complied with sample collection and follow-up. Exclusion criteria: (1) tumor was found unresectable or distal metastasis preoperatively or intraoperatively; (2) patients’ postoperative pathological diagnosis was not PDAC; (3) patients withdrawn informed consent, or patients were unable to comply with sample collection or follow-up. We collected the data regarding patients’ demographics, perioperative factors, pathologic details, surgical outcomes, survival, neoadjuvant or adjuvant therapy. The diagnosis of PDAC was confirmed by 2 independent pathologists. Pathological information including tumor size, differentiate degree, nodal status, margin status, perineural and perivascular invasion was recorded. Patients were followed up every 3–6 months postoperatively by the outpatient department. Contrast computed tomography of chest, abdomen and pelvis were routinely performed every 3–6 months to monitor the recurrence of tumor. The physicians were blinded to CTCs or CTECs information to ensure that the treatment plan was independent.
Subtraction Enrichment (SE)
We use Subtraction Enrichment Kit from Cytelligen (San Diego, CA, USA) to collect CTCs and CTECs. The procedure was performed according to the manufacturer’s protocol. The details of procedure was described in our previous study.24
Tumor Marker Immunostaining-Chromosome Fluorescence in situ Hybridization (i-FISH)
6-channel tri-marker (CD44/vimentin/CD31)-iFISH was used to identify CTCs or CTECs according to the manufacture’s protocol (Cytelligen).5 The dried monolayer cells on coated slides were rinsed and incubated with PBS for 3 minutes. Then, the cells were hybridizated with centromere probe 8 (CEP8) (Abbott Laboratories, Chicago, IL, USA) for 4 hours. S500 StatSpinThermoBrite Slide Hybridization/Denaturation System (Abbott Molecular, Abbott Park, IL, USA) was used to identify aneuploid tumor cells. Samples were subsequently incubated with the indicated post-fluorescence labeled monoclonal antibodies (1:200 dilution), including Alexa Fluor (AF) 594-CD45 (ATCC, Manassas, VA, USA, Clone 9.4), CD44 (MiltenyiBiotec, San Diego, CA, USA), Cy5-CD31 (Abcam, Burlingame, CA, USA, Catalog No. EP3095), and Cy7-vimentin (Abcam, Catalog No. EPR3776) for 20 min in the dark.25 After washing, we use mounting media with DAPI (Vector Laboratories, Burlingame, CA, USA) to mount the samples, then scanned the images of CTCs/CTECs for analysis.
Image Scanning and Cell Counting
Metafer-iFISH system (Carl Zeiss, MetaSystems, and Cytelligen5) was used to scan images and analyze CTCs and CTECs. CTCs were defined as DAPI+/CD45-/CD31-/CD44 (+ or -)/vimentin (+ or -) with aneuploid CEP8, CTECs were defined as DAPI+/CD45-/CD31+/CD44 (+ or -)/vimentin (+ or -) with aneuploid CEP8. Circulating tumor microemboli (CTMs) were defined as multiple CTCs (≥3) aggregated into clusters. Circulating tumor endothelial microemboli (CTEMs) were defined as multiple CTECs (≥3) aggregated into clusters. Automated CTC classification and statistical analysis were applied upon cell size, cell cluster, and chromosome ploidy.
Statistical Analyses
Categorical variables were expressed as a percentage and compared using χ2 test or Fisher’s exact test. Continuous variables were expressed as the mean ± standard deviation or the median (interquartile range) and compared using Student’s t-test or Mann–Whitney U-test. Kaplan–Meier method and comparison using a Log rank test were applied to estimate survival data. Prognostic factors for disease-free survival (DFS) or overall survival (OS) were identified using univariate and multivariate Cox proportional hazards models. In univariate Cox regression analysis, all variables with P value <0.10 were included in multivariate Cox model with backward selection. Unless otherwise specified, 2-sided statistical tests were used and a P value less than 0.05 was considered statistically significant. The hazard ratios (HR) and their 95% confidence intervals (95% CI) were calculated to show the variation. Spearman correlation coefficients were used to evaluate the correlation between pathological characteristics and CTEC levels. The optimal cutoff values of independent prognostic factors were assessed by the X-Tile software (version 3.6.1).26 SPSS version 22.0 (IBM, Armonk, NY, USA) was used for statistical analysis.
Results
Detection of CTECs and CTCs by SE-iFISH
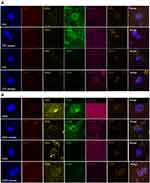
CTECs and CTCs were enriched and identified in the peripheral blood of 73 patients by applying the sorting method. CTECs and CTCs can be classified by the platform with the detection of aneuploid CEP8. The CTCs identification criteria were as follows (Figure 1A): nuclear DAPI+, CD45-, CD31-, aneuploid CEP8 positive, and CTCs tumor marker (CD44 and vimentin) positive or negative. CTECs with the same aneuploidy of CEP8 were also found under the fluorescence microscope. The definition of CTECs was similar to CTCs except the endothelial cell marker CD31 was strongly positive (Figure 1B).
Participant Characteristics and Categorical Analysis of CTECs and CTCs
During the study period, we evaluated 125 patients whose preoperative diagnosis were considered PDAC. Fifty-two cases did not meet the inclusion criteria: 29 patients had unresectable tumors, 19 patients’ postoperative pathological diagnosis were not PDAC, 3 patients died from postoperative complication within 1 month, 1 patient was lost to follow-up. A total of 73 cases were enrolled into the study. The flow diagram is presented in Figure 2. The median (range) follow-up duration was 10.8 (1.2–31.8) months. Patients’ demographic characteristics, surgical details and pathological data are summarized in Table 1.
 |
Table 1 Demographic Characteristics, Surgical Details and Pathological Details |
 |
Figure 2 Flow diagram of patient enrollment. |
Table 2 and Figure 3 describe the dynamics of CA 19–9, CTCs and CTECs in different phases. The mean total CA19-9 level, numbers of vimentin+ CTCs and numbers of vimentin+ CTECs showed a decreasing trend at POD7 and then increased at POM1, respectively. The mean total numbers of CTCs, CD44+ CTCs, CTECs and CD44+ CTECs increased at POD7 and then decreased at POM1, respectively. We divided the patients into early recurrence (ER) group (DFS<6 months) and late recurrence (LR) group (DFS≥6 months) according to the DFS. We compared the level of CTCs and CTECs between the ER group and the LR group. The mean preoperative CD44+ CTECs level was significantly higher in the ER group than it in the LR group (3.00 vs 0.56, P = 0.023). The mean preoperative vimentin+ CTC levels (0.06 vs 0.63, P = 0.012) and POD7 vimentin+ CTC levels (0 vs 0.21, P = 0.022) showed significantly lower in the ER group than it in the LR group. The POM1 CA19-9 level was significantly higher in the ER group than it in the LR group (415.77U/mL vs 28.52U/mL, P = 0.002).
 |
Table 2 CTCs and CTECs Details |
Detection of CD44+ CTECs Associated with Poor DFS in Enrolled Patients
Univariate Cox regression analysis was applied to investigate the association between DFS or OS, as well as demographic characteristics, pathological data, perioperative details, CTCs and CTECs levels. History of smoke (P = 0.030), history of diabetes (P = 0.011), neoadjuvant chemotherapy (P = 0.020), type of operation (total pancreatectomy, P = 0.001), differentiation of tumor (poor, P = 0.042), positive lymph nodes numbers (P < 0.001), preoperative CD44+ CTECs level (P = 0.001), POD7 CD44+ CTCs level (P = 0.011), POM1 CA19-9 level (P < 0.001) were identified as statistically significant influential factors for DFS (Tables 3 and 4). History of diabetes (P = 0.002), neoadjuvant chemotherapy (P = 0.009), type of operation (total pancreatectomy, P = 0.001), preoperative CD44+ CTECs level (P = 0.010), POD7 CD44+ CTCs level (P = 0.011), POM1 CA19-9 level (P = 0.007) was identified as statistically significant influential factors for OS (Tables 5 and 6).
 |
Table 3 Univariate and Multivariate Cox Regression Analyses of Prognosis Factors (Clinicopathological Factors) for Disease Free Survival |
 |
Table 4 Univariate and Multivariate Cox Regression Analyses of Prognosis Factors (CA19-9, CTCs and CTECs) for Disease Free Survival |
 |
Table 5 Univariate and Multivariate Cox Regression Analyses of Prognosis Factors (Clinicopathological Factors) for Overall Survival |
 |
Table 6 Univariate and Multivariate Cox Regression Analyses of Prognosis Factors (CA19-9, CTCs and CTECs) for Overall Survival |
According to the pre-specified criteria, potential prognostic factors with P values < 0.10 in the univariate analysis were included in the multivariate Cox regression model. History of diabetes [HR 2.656 (1.194–5.908), P = 0.017], positive lymph nodes number [HR 1.871 (1.388–2.522), P < 0.001], preoperative CD44+ CTECs level [HR 1.216 (1.064–1.390), P = 0.004] and POM1 CA19-9 level [HR 1.002 (1.001–1.002), P < 0.001] were identified as independent prognostic factors for DFS (Table 3). We determined the optimal cutoff value of the 3 independent prognostic factors for DFS. The cutoff value for positive lymph nodes number was 2 (Figure S1A). The cutoff value for preoperative CD44+ CTECs number was 3 (Figure S1B), and it for POM1 CA19-9 level was 89.6 U/mL (Figure S1C). History of diabetes [HR 7.227 (1.916–27.265), P = 0.004] and POM1 CA19-9 level [HR 1.001 (1.000–1.002), P = 0.026] were identified as independent prognostic factors for OS (Tables 5 and 6). The cutoff value for POM1 CA19-9 level was 131.9U/mL (Figure S1D). Then, we estimated incidence of disease recurrence or death in different risk-stratified subgroups using Kaplan–Meier method. History of diabetes (11.2 months vs 5.8 months, P = 0.009, Figure 4A), positive lymph nodes>2 (11.3 months vs 5.3 months, P < 0.001, Figure 4B), preoperative CD44+ CTECs>3 (11.1 months vs 5.1 months, P = 0.002, Figure 4C), POM1 CA19-9>89.6 U/mL (11.3 months vs 4.2 months, P < 0.001, Figure 4D) were significantly associated with increased risk of tumor recurrence. History of diabetes (16.6 months vs 11.7 months, P = 0.001, Figure 4E) and POM1 CA19-9 > 131.9U/mL (15.4 months vs 7.6 months, P < 0.001, Figure 4F) were significantly associated with increased risk of death. Spearman correlation analysis showed no significant correlation between preoperative CD44+CTECs and pathological characteristics (Supplemental Material Table S1)
Discussion
In the present pilot study, we identified both CTECs and CTCs in patients with PDAC, and investigated their potential clinical impact. We found that CD44+ CTECs might be related to early recurrence and poor prognosis in patients with PDAC after radical surgery. Many potential factors may affect the prognosis of pancreatic cancer, including patient factors (CA19-9 level, lymph nodes, history of diabetes, etc) and treatment-related factors (surgical margin status, postoperative adjuvant therapy, etc).27 Our study identified 3 independent risk factors (history of diabetes, positive lymph nodes and POM1 CA19-9 level) associated with DFS and 1 independent risk factor (history of diabetes) associated with OS, which is agreement with the previous findings. Meanwhile, various studies reported that CTCs and CTECs were associated with the prognosis of pancreatic cancer.12–15 The present study demonstrated preoperative CD44+ CTECs number was significantly higher in patients whose DFS<6 months and could be an independent factor for shorter DFS. CTECs are cells that originate from tumor endothelial cells (TECs) of blood vessels and are shed into peripheral blood. As indicated in some studies, elevated CTECs count may be a prognostic factor in adults with malignant diseases, such as breast cancer13 and lung cancer.14,15 Some other studies explored the role of specific phenotype CTECs in clinical utilities, such as drug therapy effect evaluation. PD-L1+CTECs were found to be associated with a shorter progression-free survival in NSCLC patients receiving PD-L1 immunotherapy, and PD-L1 therapy would facilitate the karyotype shifting of CTECs.28 The levels of aneuploid CTEC may be influenced by neoadjuvant chemotherapy in patients with locally advanced breast cancer.29 CTECs expressing stemness marker of CD44 in PDAC patients remain to be investigated.10 Poruk KE et al proved that CTCs labeled with stemness marker such as CD44 are independent predictors of decreased disease-free and overall survival.20 The CD44+ CTECs might also present a characteristics of stem cells or tumor initiating cells (TIC), suggest a possible mechanism for metastatic spread. Our finding based on clinical data provide fundamental evidence for the role of CD44+ CTECs in PDAC.
In the process from TECs to CTECs, the inducible endothelial-to-mesenchymal transition (Endo-MT) may occur, similar to the epithelial-to-mesenchymal transition (EMT) for CTCs.30 Cao et al proved that CTECs could bind to metastatic cancer cells and inhibit apoptosis of tumor cells.31 CTECs has also shown its potential in the treatment of malignant tumors. Previous reports indicated that CTEC levels were associated with the clinical outcome in patients of breast tumor under chemotherapy combined with anti-VEGF treatment.32 The conclusion also supports that CTEC levels may be associated with prognosis of PDAC patients receiving gemcitabine chemotherapy.33 However, Kindler et al proved that gemcitabine combined bevacizumab34 or axitinib (a VEGF inhibitor)35 does not improve advanced pancreatic cancer patients’ survival through Phase III clinical trial. Based on the present study, we speculate that the conclusion of these clinical trials may be related to the fact that CTECs and their various subtypes were not identified in the past. Subgroup analysis based on CTEC may lead to a different conclusion. Some studies36,37 also proposed that tumor cells may become less sensitive to anti-angiogenic agents in hypoxia or nutrient deprivation. Signals from the stromal compartment may play a major role in refractoriness of anti-angiogenic therapy and could potentially acquired resistance to VEGF inhibitors.38 We hypothesized that CTECs especially CD44+CTECs may lead to distant metastases, cross-talk with the stromal cells in tumor microenvironment (TME), then facilitate tumor growth, angiogenesis and drug resistance. Recent studies showed that the inhibition of CD44 signaling could lead to effective therapeutic responses in PDAC models.39 Detection and characterization of CD44+ CTECs show potential to assist in evaluating the efficacy of the classic chemotherapy regimen combining angiogenesis inhibitors and anti-CD44 therapy.
It is controversial whether CTCs and CTECs can be driven into the blood to disseminate tumor cells by surgical manipulation.40 In present study, most phenotype of CTCs and CTECs showed an increasing trend at POD7 and then decreased at POM1. But CTCs and CTECs at POD7 or POM1 did not correlate with prognosis. We speculate that the postoperative elevation may be due to an inflammatory response or decreased immunity of patients. Moreover, in the ER subgroup, the CD44+ CTECs level was significantly higher preoperatively while the CA 19–9 levels significantly increased to a high level in POM1. CD44+ CTEC may have potential in detecting pre-existing micrometastatic foci.
Several limitations should be taken into account when interpreting these results in present study. Patients’ follow-up may be further extended to observe significant association between CTEC levels and OS. Considering the convenience and invasiveness for the patients in this study, the blood samples were collected from peripheral vein instead of portal vein. As a result, the count of CTCs and CTECs might be diminished by the percolatory function of the lung capillary bed. The present study was a retrospective study, a selection bias may exist. High volume multicenter study should be designed to further validate the present conclusion. Future efforts need to be focused on the origin of CTECs and its role in tumor progression using the technique of single-cell sequencing.
Conclusion
In summary, our preliminary study demonstrated that preoperative CD44+ CTECs could be an independent factor for shorter DFS in patients with PDAC. We speculate that CD44+ CTECs may have association with greater angiogenic ability, resulting in greater invasive and metastasis ability of the tumor, leading to a worse prognosis.
Data Sharing Statement
The datasets analyzed during the current study would be available from the corresponding author on reasonable request.
Ethics Approval
All the patients signed informed consent for the publication of this study, following the regulations outlined in the Declaration of Helsinki. Ethical approval of this study was obtained by the ethics committee of Peking Union Medical College Hospital.
Acknowledgment
We thank all of the staff from Peking Union Medical College Hospital.
Funding
This study was supported by the project of National Natural Science Foundation of China (82073238& 81903150), the project of CAMS Innovation Fund for Medical Sciences (2016-I2M-3-005), the project of National Major Research and Development Programs of the Ministry of Science and Technology of China (2017YFC1308602) and the project of Beijing Natural Science Foundation Program (7194305).
Disclosure
The authors declare no conflicts of interest.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi:10.3322/caac.21590
2. Wong MCS, Jiang JY, Liang M, Fang Y, Yeung MS, Sung JJY. Global temporal patterns of pancreatic cancer and association with socioeconomic development. Sci Rep. 2017;7(1):3165. doi:10.1038/s41598-017-02997-2
3. Noone AM, Krapcho M, Miller D, et al.. SEER cancer statistics review, 1975–2015, based on November 2017 SEER data submission, posted to the SEER web site. Bethesda, MD: National Cancer Institute; 2018 cited Apr.
4. Lin PP. Aneuploid CTC and CEC. Diagnostics (Basel). 2018;8(2):26. doi:10.3390/diagnostics8020026
5. Lin PP, Gires O, Wang DD, Li L, Wang H. Comprehensive in situ co-detection of aneuploid circulating endothelial and tumor cells. Sci Rep. 2017;7(1):9789. doi:10.1038/s41598-017-10763-7
6. Akino T, Hida K, Hida Y, et al. Cytogenetic abnormalities of tumor-associated endothelial cells in human malignant tumors. Am J Pathol. 2009;175(6):2657–2667. doi:10.2353/ajpath.2009.090202
7. Hida K, Klagsbrun M. A new perspective on tumor endothelial cells: unexpected chromosome and centrosome abnormalities. Cancer Res. 2005;65(7):2507–2510. doi:10.1158/0008-5472.CAN-05-0002
8. Dudley AC. Tumor endothelial cells. Cold Spring Harb Perspect Med. 2012;2(3):a006536. doi:10.1101/cshperspect.a006536
9. Hida K, Maishi N, Annan DA, Hida Y. Contribution of tumor endothelial cells in cancer progression. Int J Mol Sci. 2018;19(5):1272. doi:10.3390/ijms19051272
10. Zhao Y, Li J, Li D, et al. Tumor biology and multidisciplinary strategies of oligometastasis in gastrointestinal cancers. Semin Cancer Biol. 2020;60:334–343. doi:10.1016/j.semcancer.2019.08.026
11. Bertolini F, Shaked Y, Mancuso P, Kerbel RS. The multifaceted circulating endothelial cell in cancer: towards marker and target identification. Nat Rev Cancer. 2006;6(11):835–845. doi:10.1038/nrc1971
12. Cima I, Kong SL, Sengupta D, et al. Tumor-derived circulating endothelial cell clusters in colorectal cancer. Sci Transl Med. 2016;8(345):345ra89. doi:10.1126/scitranslmed.aad7369
13. Bidard FC, Mathiot C, Degeorges A, et al. Clinical value of circulating endothelial cells and circulating tumor cells in metastatic breast cancer patients treated first line with bevacizumab and chemotherapy. Ann Oncol. 2010;21(9):1765–1771. doi:10.1093/annonc/mdq052
14. Wang J, Xiao J, Wei X, et al. Circulating endothelial cells and tumor blood volume as predictors in lung cancer. Cancer Sci. 2013;104(4):445–452. doi:10.1111/cas.12097
15. Lei Y, Sun N, Zhang G, et al. Combined detection of aneuploid circulating tumor-derived endothelial cells and circulating tumor cells may improve diagnosis of early stage non-small-cell lung cancer. Clin Transl Med. 2020;10(3):e128. doi:10.1002/ctm2.128
16. Grover PK, Cummins AG, Price TJ, et al. Circulating tumor cells: the evolving concept and the inadequacy of their enrichment by EpCAM-based methodology for basic and clinical cancer research. Ann Oncol. 2014;25:1506–1516. doi:10.1093/annonc/mdu018
17. Aktas B, Tewes M, Fehm T, et al. Stem cell and epithelial-mesenchymal transition markers are frequently overexpressed in circulating tumor cells of metastatic breast cancer patients. Breast Cancer Res. 2009;11(4):R46. doi:10.1186/bcr2333
18. Fan CW, Chen T, Shang YN, et al. Cancer-initiating cells derived from human rectal adenocarcinoma tissues carry mesenchymal phenotypes and resist drug therapies. Cell Death Dis. 2013;4(10):e828. doi:10.1038/cddis.2013.337
19. Li M, Zhang B, Zhang Z, et al. Stem cell-like circulating tumor cells indicate poor prognosis in gastric cancer. Biomed Res Int. 2014;2014:981261.
20. Poruk KE, Blackford AL, Weiss MJ, et al. Circulating tumor cells expressing markers of tumor-initiating cells predict poor survival and cancer recurrence in patients with pancreatic ductal adenocarcinoma. Clin Cancer Res. 2017;23(11):2681–2690. doi:10.1158/1078-0432.CCR-16-1467
21. Yu M, Bardia A, Wittner BS, et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science. 2013;339(6119):580–584. doi:10.1126/science.1228522
22. Huber MA, Kraut N, Beug H. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr Opin Cell Biol. 2005;17(5):548–558. doi:10.1016/j.ceb.2005.08.001
23. Poruk KE, Valero V
24. Wu G, Zhu R, Li Y, Zhao Y, Dai M. Prognostic significance of circulating tumor microemboli in patients with pancreatic ductal adenocarcinoma. Oncol Lett. 2018;15(5):7376–7382. doi:10.3892/ol.2018.8264
25. Lin P, Fischer T, Weiss T, Farquhar MG. Calnuc, an EF-hand Ca(2+) binding protein, specifically interacts with the C-terminal alpha5-helix of G(alpha)i3. Proc Natl Acad Sci U S A. 2000;97(2):674–679. doi:10.1073/pnas.97.2.674
26. Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10(21):7252–7259. doi:10.1158/1078-0432.CCR-04-0713
27. Barhli A, Cros J, Bartholin L, Neuzillet C. Prognostic stratification of resected pancreatic ductal adenocarcinoma: past, present, and future. Dig Liver Dis. 2018;50(10):979–990. doi:10.1016/j.dld.2018.08.009
28. Zhang L, Zhang X, Liu Y, et al. PD-L1+ aneuploid circulating tumor endothelial cells (CTECs) exhibit resistance to the checkpoint blockade immunotherapy in advanced NSCLC patients. Cancer Lett. 2020;469:355–366. doi:10.1016/j.canlet.2019.10.041
29. Ma G, Jiang Y, Liang M, et al. Dynamic monitoring of CD45-/CD31+/DAPI+ circulating endothelial cells aneuploid for chromosome 8 during neoadjuvant chemotherapy in locally advanced breast cancer. Ther Adv Med Oncol. 2020;12:1758835920918470. doi:10.1177/1758835920918470
30. Piera-Velazquez S, Mendoza FA, Jimenez SA. Endothelial to mesenchymal transition (EndoMT) in the pathogenesis of human fibrotic diseases. J Clin Med. 2016;5(4):45. doi:10.3390/jcm5040045
31. Cao Z, Livas T, Kyprianou N. Anoikis and EMT: lethal “Liaisons” during cancer progression. Crit Rev Oncog. 2016;21(3–4):155–168. doi:10.1615/CritRevOncog.2016016955
32. Calleri A, Bono A, Bagnardi V, et al. Predictive potential of angiogenic growth factors and circulating endothelial cells in breast cancer patients receiving metronomic chemotherapy plus bevacizumab. Clin Cancer Res. 2009;15(24):7652–7657. doi:10.1158/1078-0432.CCR-09-1493
33. Kondo S, Ueno H, Hashimoto J, et al. Circulating endothelial cells and other angiogenesis factors in pancreatic carcinoma patients receiving gemcitabine chemotherapy. BMC Cancer. 2012;12:268. doi:10.1186/1471-2407-12-268
34. Kindler HL, Niedzwiecki D, Hollis D, et al. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the Cancer and Leukemia Group B (CALGB 80303). J Clin Oncol. 2010;28(22):3617–3622. doi:10.1200/JCO.2010.28.1386
35. Kindler HL, Ioka T, Richel DJ, et al. Axitinib plus gemcitabine versus placebo plus gemcitabine in patients with advanced pancreatic adenocarcinoma: a double-blind randomised Phase 3 study. Lancet Oncol. 2011;12(3):256–262. doi:10.1016/S1470-2045(11)70004-3
36. Crawford Y, Ferrara N. Tumor and stromal pathways mediating refractoriness/resistance to anti-angiogenic therapies. Trends Pharmacol Sci. 2009;30(12):624–630. doi:10.1016/j.tips.2009.09.004
37. Allen E, Miéville P, Warren CM, et al. Metabolic symbiosis enables adaptive resistance to anti-angiogenic therapy that is dependent on mTOR signaling. Cell Rep. 2016;15(6):1144–1160. doi:10.1016/j.celrep.2016.04.029
38. Huijbers EJ, van Beijnum JR, Thijssen VL, Sabrkhany S, Nowak-Sliwinska P, Griffioen AW. Role of the tumor stroma in resistance to anti-angiogenic therapy. Drug Resist Updat. 2016;25:26–37. doi:10.1016/j.drup.2016.02.002
39. Molejon MI, Tellechea JI, Moutardier V, et al. Targeting CD44 as a novel therapeutic approach for treating pancreatic cancer recurrence. Oncoscience. 2015;2(6):572–575. doi:10.18632/oncoscience.172
40. Sergeant G, Roskams T, van Pelt J, Houtmeyers F, Aerts R, Topal B. Perioperative cancer cell dissemination detected with a real-time RT-PCR assay for EpCAM is not associated with worse prognosis in pancreatic ductal adenocarcinoma. BMC Cancer. 2011;11:47. doi:10.1186/1471-2407-11-47
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.