Back to Journals » Breast Cancer: Targets and Therapy » Volume 15
CD24 May Serve as an Immunotherapy Target in Triple-Negative Breast Cancer by Regulating the Expression of PD-L1
Authors Zhu X , Yu J, Ai F, Wang Y, Lv W, Yu G, Cao X, Lin J
Received 19 April 2023
Accepted for publication 21 December 2023
Published 28 December 2023 Volume 2023:15 Pages 967—984
DOI https://doi.org/10.2147/BCTT.S409054
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Pranela Rameshwar
Xudong Zhu,1,2 Jiahui Yu,3 Fulu Ai,1 Yue Wang,1 Wu Lv,1 Guilin Yu,1 Xiankui Cao,1 Jie Lin1
1Department of General Surgery, Cancer Hospital of China Medical University, Cancer Hospital of Dalian University of Technology, Liaoning Cancer Hospital and Institute, Shenyang, Liaoning, 110042, People’s Republic of China; 2Department of Oncology, Shengjing Hospital of China Medical University, Shenyang, Liaoning, 110004, People’s Republic of China; 3Department of Ultrasound, Shengjing Hospital of China Medical University, Shenyang, Liaoning, 110004, People’s Republic of China
Correspondence: Jie Lin; Xudong Zhu, Department of General Surgery, Cancer Hospital of China Medical University, Cancer Hospital of Dalian University of Technology, Liaoning Cancer Hospital and Institute, Shenyang, Liaoning, 110042, People’s Republic of China, Tel +86 13940081800 ; +86 13354204706, Email [email protected]; [email protected]
Purpose: CD24 mediates a “don’t eat me” signal to escape the immune environment. However, the correlation between CD24 and PD-L1 is unclear. This study aimed to assess if CD24 can serve as a target for immunotherapy of triple-negative breast cancer (TNBC).
Methods: Data on CD24 expression in breast cancer were acquired using the Oncomine and UALCAN tools. The role of CD24 expression on the prognosis of patients with TNBC was assessed using Kaplan–Meier analyses. Subsequently, STRING and TISIDB databases were used to construct protein–protein interaction networks and to explore immune-related molecules regulated by CD24. Immunofluorescence and immunohistochemistry assays were conducted to validate CD24 and PD-L1 expression and tumor infiltration lymphocyte (TIL) level. Survival analysis was also performed to explore the effect of CD24 and PD-L1 expression and TIL level in patients with TNBC. ShRNA was also used to explore the regulation role of CD24 on PD-L1 expression.
Results: CD24 expression was significantly higher in breast cancer than in normal tissues, with high expression being significantly associated with a worse prognosis. CD24 was found to be significantly regulated by chemokines, immunoinhibitors, immunostimulators and TILs. Furthermore, CD24 expression showed a significant positive correlation with PD-L1 expression and a negative correlation with TIL level. In association with PD-L1, CD24 was found to positively regulate lymphocyte costimulation, T cell costimulation, and leukocyte activation. Furthermore, CD24 and PD-L1 co-expression contributed to worse survival outcomes. In addition, CD24 expression was found to attenuate the positive effects of high-level TILs on the prognosis of patients with TNBC. CD24 can also regulate the expression of PD-L1 in TNBC cells.
Conclusion: CD24 may attenuate the positive effects of high TIL levels on survival and may facilitate the immune escape of TNBC by regulating PD-L1 expression. Thus, it is a potential target for immunotherapy in TNBC.
Keywords: triple negative breast cancer, CD24, PD-L1, prognosis, immunotherapy
Introduction
Breast cancer is associated with the highest morbidity among all types of cancers, thus greatly threatening women’s health.1–3 By 2020, an estimated 276,480 women in the United States may be diagnosed with breast cancer, with mortality occurring in 42,170 of them.2,4,5 Among all molecular subtypes of breast cancer, triple-negative breast cancer (TNBC) is correlated with the highest malignancy and heterogeneity; however, data on effective therapeutic targets are limited.6–8 Although immunotherapy has considerably improved the prognosis of patients with TNBC, its less-than-expected efficacy caused by tumor immune escape is still a major problem for clinicians and oncologists.9,10
CD24, also known as heat-stable antigen, is a heavily glycosylated, mucin-type, cell-surface protein.11,12 Reportedly, CD24 is associated with cancer histological grade, cancer stage, and shorter survival in patients with breast cancer.13 Furthermore, Barkal et al have reported that CD24 can bind to sialic-acid-binding Ig-like lectin 10 (Siglec-10), the inhibitory receptor expressed in tumor-associated macrophages. It thus contributes to the immune escape of breast cancer cells, eliciting a “don’t eat me” signal. CD24 may act as the dominant innate immune checkpoint in breast cancer and serve as a promising immunotherapy target.14
Programmed death ligand 1 (PD-L1) is another important target in tumor immunotherapy.15–17 It can effectively promote tumor immune escape by binding with programmed cell death protein 1.10 For PD-L1 positive patients with metastatic TNBC, immune checkpoint inhibitor (ICI) based on PD-L1 has been approved in combination strategies with corresponding chemotherapy drugs. Furthermore, the combination strategies could significantly decrease the rate of early death compared with ICI alone.18–21 Few studies have explored the correlations between PD-L1 and CD24, particularly in TNBC. However, it is still unclear whether CD24 can act along with PD-L1 to regulate the immune escape of TNBC. Accordingly, the present study explored the expression and prognostic effect of CD24 in patients with TNBC. In addition, the study evaluated the regulation of immune-related molecules by CD24 and the correlations between CD24 expression, PD-L1 expression, and tumor infiltration lymphocyte (TIL) level in clinical TNBC specimens. Furthermore, the effect of CD24 high expression and co-expression of CD24 and PD-L1 on the survival outcomes of TNBC was also explored. Lastly, the study explored the mechanism underlying the regulation of CD24 expression. The findings of this study suggested that besides Siglec-10, CD24 may in association with PD-L1 and other immune-related molecules to regulated the immune escape of tumor cells in patients with TNBC.
Materials and Methods
Sangerbox
Sangerbox (http://vip.sangerbox.com/home.html) tool was applied for assessing the following: CD24 expression across TCGA pan-tumors and the prognostic effect of CD24 on survival, including progression-free interval (PFI), disease-specific survival (DSS), overall survival (OS), and disease-free interval (DFI) across TCGA pan-tumors. For the survival analysis, statistical method Cox-regression analysis was applied in Sangerbox tool. Sangerbox is a kind of online tool. As long as we submit the gene name, we can get the corresponding results ultimately.
Oncomine analysis
Oncomine next-generation sequencing (http://www.oncomine.org) was performed to analyze CD24 mRNA levels in different tumor types.22 In addition, the level of CD24 mRNA transcripts was analyzed in breast cancer and normal tissues. Just like the Sangerbox, Oncomine is also a kind of online tool. As long as we submit the gene name, we can get the corresponding results ultimately.
UALCAN Analysis
The UALCAN platform (http://ualcan.path.uab.edu/index.html) was conducted to assess the level of CD24 mRNA transcripts in patients with breast cancer according to sample type, cancer stage, patient’s age, major subclasses (within TNBC types), menopause status, histologic subtypes, lymph nodal metastasis status, and TP53 mutation status.23 UALCAN is also a kind of online tool. As long as we submit the gene name, we can get the corresponding results ultimately.
GEPIA Analysis
Using data from the TCGA database, the GEPIA platform (http://gepia.cancer-pku.cn/) was used to determine the prognostic effect of high CD24 expression on the OS of patients with TNBC. In addition, the correlations between CD24 and its co-expressed genes as well as CD24 and m6A methylation-related genes were analyzed.24
Kaplan–Meier analysis
Kaplan–Meier analysis (http://kmplot.com) was conducted to assess the prognostic effect of high CD24 expression on the distant metastasis-free survival (DMFS), OS, post-progression survival (PPS), and relapse-free survival (RFS) at the mRNA level and OS at the pan-cancer RNA-seq level.25,26
Patients with TNBC and Specimens
This research included patients who were diagnosed with TNBC from February 2008 to October 2011 at the China Medical University Hospital. In all patients, the diagnosis of TNBC was established by histology, and all patients underwent modified radical mastectomy and received standard post-operative chemotherapy. Additional inclusion criteria were similar to those reported previously.27,28 Patients with the following criteria were excluded: those without complete clinicopathological information, those receiving neoadjuvant chemotherapy, and those with unknown survivance conditions. The definition of disease-free survival (DFS) and OS were similar to that reported previously.27,28 The study was approved by the institutional review board of the China Medical University Hospital (2020PS282K). The informed consent has also obtained from the study participants prior to study commencement. Also, our study complied with the Declaration of Helsinki. The cut-off value for the Ki67 index was 20%.29 The molecular type of TNBC was determined using the NCCN guidelines.30
Immunofluorescence Analysis
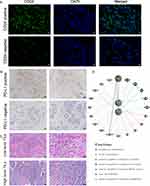
The process of 176 TNBC specimens’ fixation and dehydration is consistent with previous studies28,31 Subsequently, the specimens were blocked with 5% bovine serum albumin (Solarbio, SW3015) for 40 min. They were then incubated with anti-CD24 (1:100; Abcam, ab202073) at 4°C overnight. Next day, these specimens were washed with tris-buffered saline (TBS) and incubated with goat anti-rabbit IgG (H&L) (Alexa Fluor 488) (1:200; Abcam, ab150077) for 4 h at 37°C. These specimens were then washed again with TBS and incubated with DAPI (1:100; Solarbio company, C0060) for 5 min. Fluorescent images of the specimens were obtained using Nikon E800 upright microscope. All images were then processed by the NIS-Elements F3.0 software and analyzed using the ImageJ software. Positive stained tumor cells were quantified by ImageJ software and expressed as the average percentage of positive stained tumor cells ± SD in 10 fields. The analytical method of immunofluorescence was as per Kryczek I’ method.32
Immunohistochemistry Analysis
PD-L1 expression and TIL levels were determined as previously described.31 The score systems of PD-L1 expression and TIL levels were also the same as our previous study.31
GeneMANIA Analysis
The GeneMANIA platform (http://www.genemania.org) was used to construct a CD24–CD274 interaction network based on physical interactions, co-expression, predictions, and genetic interaction as well as to evaluate functions.33
Breast Cancer Cell and Cell Culture
Human breast cancer cell lines ZR-75-1, MCF7, JIMT1, MDA-MB-468, MDA-MB-231 and SKBR3 were purchased from Procell (https://www.procell.com.cn), and were cultured by the medium supplied by Procell. All these breast cancer cells were incubated at an atmosphere of 37°C and 5% CO2.
Western Blot
Breast cancer cell lysates were separated by 10% sodium dodecyl sulfate polyacrylamide gel and proteins were transferred to 0.45um PVDF membranes. After that, these PVDF membranes were blocked in 5% fat-free milk for 2 hours. Then, these membranes were incubated with a mouse anti-human CD24 antibody (1:5000, Proteintech, 67627-1-Ig), a rabbit anti-human PD-L1 antibody (1:500, Sangon Biotech, D164446), a mouse anti-human HSP90 antibody (1:5000, Proteintech, 60318-1-Ig) and a mouse anti-human GAPDH antibody (1:50000, Proteintech, 60004-1-Ig) overnight at 4°C. The second day, these membranes were incubated with a goat anti-mouse IgG or a goat anti-rabbit IgG (Zhong Shan Jin Qiao) for one hour. An enhanced chemiluminescent reagent was used to visualize these immunoreactive bands.
Plasmid Transfection
The plasmid transfection assays were performed per the standard: 4uL lipo3000, 5 uL p3000, 2.5μg CD24 silence plasmid and 2.5 μg negative control (NC) plasmid per well in a 6-well plate. Plasmid and p3000 was added to one tube, and lipo3000 was added to the other tube. Then, the contents were mixed well and incubated for 15 min. After that it was added to a 6-well plate overnight, and the medium was changed the second day to complete the process of plasmid transfection. The shRNA target sequence for CD24 was 5’-CTTCTGCATCTCTACTCTTAA-3’. The NC sequence was 5’- CCTAAGGTTAAGTCGCCCTCGC-3’.
STRING Database and Functional and Pathway Enrichment Analysis
Using the STRING database (https://string-db.org/), genes co-expressed with CD24 were determined to build protein-protein interaction (PPI) networks.34 Subsequently, functional and pathway enrichment analysis of genes co-expressed with CD24 was conducted by the DAVID database (https://david.ncifcrf.gov/).35
TISIDB Analysis
The correlations between CD24 and chemokines, immunomodulators, and lymphocytes were assessed by the TISIDB portal (http://cis.hku.hk/TISIDB).36 In addition, CD24 expression in different immune and molecular subgroups of breast cancer was analyzed. TISIDB is also a kind of online tool. As long as we submit the gene name, we can get the corresponding results ultimately.
Microarray Data
Microarray data were generated by downloading the gene expression profile matrix file37 from the Gene Expression Omnibus (GEO) database (GSE52327). The dataset included data from eight ALDH1A1-positive and -negative breast cancer samples each. The data processing method is consistent with previous researches.38,39
Data Analysis and Screening for Differentially Expressed Genes (DEGs)
The DEGs from the GEO datasets were processed using the limma R package,40 and the genes with P<0.05 and |log fold change| > 1 were considered as differentially expressed. The R packages ggpubr and ggthemes were used to create hierarchical plots of the dataset to visualize the DEGs. The R package pheatmap was applied to draw heatmaps and assess the expression of selected DEGs.
Statistical Analysis
GEO datasets were analyzed using the R (v.3.5.1) language. The relationships between CD24 expression, PD-L1 expression, and TIL level were analyzed by Pearson correlation. Moreover, the relationships between CD24 expression and age, histological grade, T grade, N grade, menopausal status, and Ki67 index were analyzed by Chi-square test. The Kaplan–Meier analysis was conducted to generate survival curves using the SPSS 25.0 software. All statistical analyses were conducted by the SPSS 25.0 software.
Results
Expression of CD24 and Its Prognostic Effect on TCGA Pan-Tumors
Among TCGA pan-tumors, kidney renal papillary cell carcinoma was correlated with the highest CD24 expression and uveal melanoma with the lowest expression. Besides, CD24 expression was higher in breast cancer than in a few other tumor types (Figure 1A). Next, CD24 expression was significantly higher in most TCGA pan-tumors than in normal tissues (Figure 1B), except in rectum adenocarcinoma and pancreatic adenocarcinoma. In addition, CD24 expression was significantly higher in breast cancer tissues than in normal tissues (P<0.001). In patients with breast cancer, high CD24 expression was related to significantly worse OS, DSS, DFI, and PFI (Figure 1C–F).
Bioinformatic Analysis of CD24 Expression in Breast Cancer and Normal Tissues
The CD24 mRNA level in 20 types of cancer was obtained by the Oncomine tool (Figure 2A). Particularly, the CD24 mRNA level was significantly higher in breast cancer than in normal tissues. In addition, CD24 mRNA levels were assessed in a few single-study subgroups. In all five subgroups, CD24 mRNA level was significantly higher in breast cancer than in normal tissues (Figure 2B–F). A meta-analysis of the five subgroups presented a significantly higher CD24 mRNA level in breast cancer than in normal tissues (Figure 2G).
The UALCAN database analysis presented a significantly higher CD24 mRNA level in breast cancer than in normal tissues (P<0.001, Figure 2H). With regard to the cancer stage, stages 2 and 4 were associated with the highest and lowest CD24 mRNA level, respectively, albeit without statistical significance (Figure 2I). Moreover, patients aged 61–80 years had the highest CD24 mRNA level and those aged 81–100 years had the lowest level. This difference was also not significant (Figure 2J). When assessed based on major TNBC subtypes, the highest CD24 mRNA level was noted in patients with TNBC basal-like 1 subtype and the lowest was noted in patients with TNBC mesenchymal stem-like subtype (both P<0.001, Figure 2K). Based on the menopause status, the CD24 mRNA level was the highest in post-menopausal patients and the lowest in pre-menopausal patients, albeit without significant difference (Figure 2L). Of all histologic subtypes, medullary and metaplastic carcinoma was associated with the highest and lowest CD24 mRNA level, respectively. Moreover, the difference was not statistically significant (Figure 2M). With regard to nodal metastasis, patients with the N3 stage had the highest CD24 mRNA level, while the N1 stage was associated with the lowest, both without statistical significance (Figure 2N). Moreover, the CD24 mRNA level was significantly higher in patients with TP53 mutation than those without the mutation (P<0.001, Figure 2O).
The Prognostic Effect of CD24 Expression on Patients with Breast Cancer Based on the Bioinformatic Analysis
Firstly, we explored the prognostic effect of CD24 expression by GEPIA database. Using the data from TCGA-breast cancer, GEPIA website showed that a high CD24 mRNA level was significantly correlated with a shorter OS (P=0.0083, Figure 3A). Then, we used Kaplan-Meier plotter database to explore the prognostic effect of CD24 expression. At the level of breast cancer-mRNA, a high level of CD24 mRNA expression was significantly correlated with a shorter DMFS (P=2.2e-07, Figure 3B), OS (P=4.7e-05, Figure 3C), RFS (P=4.1e-13, Figure 3D), and PPS (P=0.00034, Figure 3E). With regard to the level of pan-cancer RNA-seq, a high level of CD24 mRNA was also significantly correlated with a shorter OS on patients with breast cancer (P=0.0011, Figure 3F). All these results indicate that high CD24 expression may worsen prognosis in patients with breast cancer.
Correlation of CD24 Expression with PD-L1 Expression and TILs Level in Patients with TNBC
Reportedly, PD-L1 expression can attenuate the positive prognostic effect of high-level TILs on TNBC.31 Owing to the role of CD24 in regulating the cancer immune environment, the current study assessed whether CD24 expression was related to PD-L1 or TILs in TNBC. Using the TNBC specimens, CD24 expression, PD-L1 expression, and TIL level were evaluated (Figure 4A–C). The results revealed that CD24 was significantly positively related to PD-L1 expression in TNBC (P=0.011; Table 1). Besides, CD24 expression showed a significant negative correlation with TIL level in TNBC (P=0.015; Table 2). The gene–gene interaction network for CD24 and PD-L1 is depicted in Figure 4D. The results revealed that CD24, in association with PD-L1, can positively regulate the function of lymphocyte co-stimulation, T cell co-stimulation, leukocyte activation, lymphocyte activation, immune effector process, mast cell activation, and leukocyte proliferation.
 |
Table 1 The Correlation Analysis of CD24 and PD-L1 Expression in Patients with TNBC |
 |
Table 2 The Correlation Analysis of CD24 Expression and TILs in Patients with TNBC |
CD24 Attenuates the Positive Prognostic Effects of High-Level TILs in Patients with TNBC
The correlations between CD24 expression and clinicopathological characteristics of patients with TNBC are demonstrated in Table 3. CD24 expression was significantly correlated with higher histological grade (P=0.027) and N grade (P=0.034). Moreover, CD24 expression was significantly correlated with a shorter DFS (P<0.001; Figure 5A) and OS (P=0.010; Figure 5B) in patients with TNBC. Furthermore, PD-L1 expression was significantly related to a poor survival outcome. Thus, we further assessed if CD24 and PD-L1 co-expression contributed to a worse prognosis. The results revealed that CD24+/PD-L1+ patients had the shortest DFS (P<0.001; Figure 5C). Although there was no significant difference between CD24+/PD-L1+ patients and CD24+/PD-L1-, CD24-/PD-L1+ patients with regard to OS, the former had the shortest OS (Figure 5D). These results indicate that CD24 and PD-L1 co-expression can worsen the survival outcome of patients with TNBC.
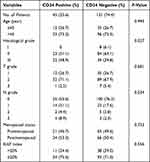 |
Table 3 The Correlations Between CD24 Expression and Clinicopathological Characteristics of Patients with TNBC |
High-level TILs can improve the survival outcomes of patients with TNBC.31,41 Notably, CD24 expression was significantly correlated with a shorter DFS (P=0.004; Figure 5E) and OS (P=0.006; Figure 5F) in patients with high TIL levels. Therefore, CD24 may attenuate the positive prognostic effects of high-level TILs on patients with TNBC.
CD24 Can Directly Regulate the Expression of PD-L1 in MDA-MB-231 TNBC Cell
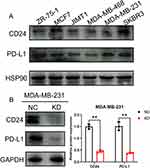
To explore the association between CD24 expression and PD-L1 expression. We firstly detected the expression of PD-L1 and CD24 on various breast cancer cell lines. In MDA-MB-231 cell, there is a relatively high expression level of both CD24 and PD-L1 (Figure 6A). Furthermore, in MDA-MB-231 cells, we silenced the expression of CD24 by shRNA, then we found that CD24 silence can significantly decrease the expression of PD-L1 (Figure 6B). All these results proved that CD24 can directly regulate the expression of PD-L1 in MDAMB-231 TNBC cell.
PPI Network and Functional and Pathway Enrichment Analysis
The PPI network of CD24 co-expressed genes constructed using the STRING database was shown in Figure 7A.
The GO analysis interprets data in three domains: biological process (BP), cellular component (CC), and molecular function (MF). The analysis revealed that CD24 co-expressed genes were primarily enriched in leukocyte adhesion (BP), plasma membrane (CC), and protein complex binding (MF). Detailed results of GO analysis are shown in Figure 7B–D. The KEGG pathway enrichment demonstrated that the CD24 co-expressed genes were enriched in cancer-related pathways such as leukocyte transendothelial migration, T-cell receptor signaling pathway, natural killer (NK) cell-mediated cytotoxicity, and B-cell receptor signaling pathway (Figure 7E).
Regulation of Immune-Related Molecules by CD24
Barkal et al suggested that CD24 contributes to immune escape by binding to the inhibitory receptor Siglec-10.8 Moreover, the KEGG analysis presented that CD24 and its co-expressed genes may regulate immune-related pathways. Thus, this study further explored the regulation of immune-related molecules by CD24 using TISIDB database. The relationship between CD24 expression and chemokines was presented in Figure 8A. In patients with breast cancer, CD24 showed the greatest association with the chemokines CCL7, CCL14, CCL20, and CXCL14. Moreover, the chemokine receptors that CD24 showed the greatest association with were CCR10, CX3CR1, CXCR2, and CXCR4 (Figure 8B). Immunomodulators were classified into immunoinhibitors, immunostimulators, and major histocompatibility complex (MHC) molecules. Figure 8C showed the correlations between CD24 expression in breast cancer and immunoinhibitors. CD24 showed the greatest association with the immunoinhibitors IDO1, TGFB1, TGFBR1, and VTCN1. Figure 8D presented the relationship between CD24 expression and immunostimulators. The immunostimulators that CD24 was highly associated with were ICOSLG, TNFRSF14, TNFSF13, and ULBP1. With regard to MHC molecules, CD24 was highly associated with HLA-DOA, HLA-DOB, HLA-DPA1, and HLADPB1 MHC molecules (Figure 8E). Figure 8F showed the relationships between CD24 expression and TILs. CD24 was highly associated with the following TILs: eosinophils, neutrophils, NK cells, and pDC.
Mechanism Underlying the Regulation of CD24 Expression in Breast Cancer
Reportedly, CD24 is highly correlated with breast cancer stem cell biomarker ALDH1A1.42 In line with this, we assessed the GEO dataset GSE52327 which included 8 ALDH1A1+ and 8 ALDH1A1- breast cancer samples. We used this dataset to identify DEGs and explore if CD24 expression is influenced by ALDH1A1. However, no changes were noted in CD24 expression in this dataset (Supplementary Figure 1A).
Because CD24 and its binding proteins form an interaction network, each protein in the network may regulate each other to play a role. Therefore, we further used this dataset to analyze whether ALDH1A1 expression affected the expression of other CD24 binding proteins. As a result, to indirectly regulate CD24 expression, volcano plots were plotted to assess DEGs from the dataset (Supplementary Figure 1B). Using a Venn diagram, common DEGs were screened from this dataset along with CD24-binding proteins (Supplementary Figure 1C). These screened proteins are shown in Supplementary Figure 1A. We further explored the correlations between these screened proteins and CD24 using the GEPIA dataset. GEPIA dataset also showed that the expression of ALDH1A1 was not significantly correlated with CD24 in breast cancer (Supplementary Figure 1D). Other results revealed that only CD44 showed a significant positive correlation with CD24 (P=0.017; Supplementary Figure 1E), and SELPLG showed a significant negative correlation with CD24 (P=0.049; Supplementary Figure 1F). No other proteins were significantly related to CD24 expression (Supplementary Figure 1G). Therefore, CD44 and SELPLG may regulate CD24 expression. Besides, ALDH1A1 was negatively correlated with CD44 and SELPLG expression (Supplementary Figure 1A). However, CD44 was positively correlated with CD24 expression, and SELPLG was negatively correlated with CD24 expression. This may preliminarily explain why ALDH1A1 does not affect the expression of CD24.
Methylation of N6 adenosine (m6A) is one of the most common types of RNA modification that significantly contributes to the invasion and metastasis of TNBC.43–46 In addition, m6A methylation regulates the expression of key oncogenes.47 Therefore, this study explored the relationship between 20 primary regulators of m6A RNA methylation (11 readers, 7 writers, and 2 erasers) and CD24 expression.48 For readers, HNRNPA2B1, IGF2BP3, RBMX, YTHDF2, and YTHDF3 showed significant positive correlation with CD24. For writers, METTL3 showed a significant negative correlation and RBM15 and WTAP showed a significant positive correlation with CD24 (Supplementary Figure 2A). None of the erasers were significantly correlated with CD24. No other regulators of m6A RNA methylation were significantly correlated with CD24 (Supplementary Figure 2B).
Discussion
Only a few studies have explored the role of CD24 gene in the development of overall breast cancer preliminarily. Zhang P et al found that CD24 gene amplification significantly contributed to the overexpression of CD24 mRNA, and was significantly related to a worse OS in overall patients with breast cancer.49 However, they did not explore the effect of CD24 gene amplification on the expression of CD24 protein and prove the effect of CD24 protein overexpression using their own solid data. Also, they only found that CD24 gene amplification was mostly enriched in breast cancer of basal-like subtype by online tools. However, they did not validate this by clinical TNBC specimens, their results were not very convincing. This point guided the further research direction for CD24: the role of CD24 in TNBC. Our study exactly explored this important issue. In this study, we firstly validated the high expression and adverse prognostic effect of CD24 on patients with TNBC using clinical TNBC specimens. Then, we further explored the co-expression of CD24 and PD-L1 may contribute to a worse survival outcome for patients with TNBC. At last, we found that CD24 high expression can attenuate the positive effects of high-level TILs on the prognosis of patients with TNBC. All these results were firstly found by our group, and predicted that CD24 may serve as a potential therapy target for patients with TNBC.
Another previous study aimed to evaluate the regulatory role of CD24 in patients with TNBC.50 The findings demonstrated that patients with CD24-positive TNBC had poor DFS and OS after taxane-based treatment. Moreover, high CD24 expression was associated with increased resistance to docetaxel, and CD24-knockdown cells were more sensitive to docetaxel. Furthermore, CD24 regulates the chemosensitivity of TNBC by Bcl-2 and TGF-βR1 signaling via the ATM-NDRG2 pathway.50 Moreover, it has been found that CD24 knockdown can reduce lymph node and lung metastases as well as the number of blood and lymphatic vessels in the tumor microenvironment. In addition, CD24 silencing can impair EGFR/Met-mediated signaling and decrease the expression of lymphangiogenesis- and angiogenesis-related factors, including vascular endothelial growth factors A and C. This occurs via the promotion of EGFR and Met protein instability via the lysosomal degradation pathway in TNBC cells.51 To further support this, in TNBC, CD24 was been shown to bind with Siglec-10, thus emitting a “Don’t eat me” signal.14 Irrespective, there are no studies assessing the role of CD24 in regulating the cancer immune environment and the relationship between CD24 and PD-L1 in TNBC.
Previous reported studies on CD24 in breast cancer have primarily focused on its role in stem cells, such as CD44+/CD24− cells.37,52–54 However, in breast stem cells, CD24 was negative. Studies evaluating the role of CD24 in the prognosis of patients with breast cancer have found that high CD24 expression is correlated with worse survival outcomes.13,55,56 These results indicate that the function of CD24 in TNBC is complex. Accordingly, our study primarily explored the regulation of immune-related molecules by CD24 and the correlations between CD24 expression, PD-L1 expression, and TIL level. In addition, the study evaluated CD24 expression in breast cancer and normal tissues, the relationship between CD24 expression and clinicopathological characteristics, and the role of CD24 expression in the prognosis of patients. The study found that CD24 could regulate certain chemokines, such as CCL7, CCL14, CCL20, and CXCL14; immunoinhibitors, such as IDO1, TGFB1, TGFBR1, and VTCN1; immunostimulators, such as ICOSLG, TNFRSF14, TNFSF13, and ULBP1; MHC molecules, such as HLA-DOA, HLA-DOB, HLA-DPA1, and HLADPB1; and TILs, such as eosinophils, neutrophils, NK, and pDC. GeneMANIA database analysis revealed that CD24 may act in association with PD-L1 to participate in altering the cancer immune environment. Furthermore, we found CD24 expression was significantly positively related to PD-L1 expression and negatively associated with TIL levels. CD24 and PD-L1 co-expression contributed to worse survival outcomes. In addition, CD24 expression was found to attenuate the positive effects of high-level TILs on the prognosis of patients with TNBC by using clinical TNBC samples. Most importantly, we also found that CD24 can directly regulate the expression of PD-L1 in TNBC cells. All these results predicted that CD24 may contribute to breast cancer immune escape by interacting with PD-L1, in addition to Siglec-10. Thus, CD24 may serve as a target for immunotherapy of TNBC. We think our results may create a new approach to clinical immunotherapy for TNBC.
The present study additionally explored the mechanism underlying the regulatory role of CD24 in breast cancer using bioinformatic tools. Analysis of the GEO dataset GSE52327 suggested that ALDH1A1 (breast cancer stem cell marker)57,58 regulates CD24 expression by either CD44 or SELPLG. Notably, m6A in mRNA has become a crucial epi-transcriptomic modification that can control cancer cell self-renewal and fate, thus regulating breast cancer progression.45,59–61 Therefore, we further explored whether the regulators of m6A RNA methylation influence CD24 expression. GEPIA database analysis revealed that HNRNPA2B1, IGF2BP3, RBMX, YTHDF2, YTHDF3, METTL3, RBM15B, and WTAP may participate in regulating CD24 expression in breast cancer. However, this was a main limitation of our study. Future in vitro and in vivo studies are required to validate the exact molecular function and the regulatory mechanism underlying CD24 in patients with TNBC.
Conclusions
In conclusion, CD24 expression was significantly higher in breast cancer than in normal tissues and was associated with worse survival outcomes in patients with TNBC. Moreover, CD24 expression showed a significant positive correlation with PD-L1 expression and a negative correlation with TIL level. CD24 and PD-L1 co-expression contributed to worse survival outcomes. In addition, CD24 could further attenuate the positive effects of high TIL levels on the survival outcomes of patients with TNBC. CD24 can also regulate the expression of PD-L1 in TNBC cells. Finally, CD24 may act with PD-L1 to regulate cancer immune escape, to serve as a target for immunotherapy in TNBC.
Data Sharing Statement
These data and materials can be available from the corresponding author on reasonable request.
Ethics Approval and Informed Consent
The research was approved by the China Medical University Hospital institutional review board (2020PS282K). And the informed consent has obtained from the study participants prior to study commencement.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by Doctoral Start-up Foundation of Liaoning Province (2023-BS-048).
Disclosure
These authors have declared that they have no competing interests.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. doi:10.3322/caac.21442
2. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–48. doi:10.3322/caac.21763
3. Heer E, Harper A, Escandor N, Sung H, McCormack V, Fidler-Benaoudia MM. Global burden and trends in premenopausal and postmenopausal breast cancer: a population-based study. Lancet Glob Health. 2020;8(8):e1027–e1037. doi:10.1016/S2214-109X(20)30215-1
4. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. doi:10.3322/caac.21590
5. Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends--an update. Cancer Epidemiol Biomarkers Prev. 2016;25(1):16–27. doi:10.1158/1055-9965.EPI-15-0578
6. Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–752. doi:10.1038/35021093
7. Keenan TE, Tolaney SM. Role of immunotherapy in triple-negative breast cancer. J Natl Compr Canc Netw. 2020;18(4):479–489. doi:10.6004/jnccn.2020.7554
8. Bianchini G, De Angelis C, Licata L, Gianni L. Treatment landscape of triple-negative breast cancer - expanded options, evolving needs. Nat Rev Clin Oncol. 2022;19(2):91–113. doi:10.1038/s41571-021-00565-2
9. Micalizzi DS, Maheswaran S. On the trail of invasive cells in breast cancer. Nature. 2018;554(7692):308–309. doi:10.1038/d41586-018-01634-w
10. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. doi:10.1038/nrc3239
11. Pirruccello SJ, LeBien TW. The human B cell-associated antigen CD24 is a single chain sialoglycoprotein. J Immunol. 1986;136(10):3779–3784. doi:10.4049/jimmunol.136.10.3779
12. Ni YH, Zhao X, Wang W. CD24, A review of its role in tumor diagnosis, progression and therapy. Curr Gene Ther. 2020;20(2):109–126. doi:10.2174/1566523220666200623170738
13. Wang Z, Wang Q, Wang Q, Wang Y, Chen J. Prognostic significance of CD24 and CD44 in breast cancer: a meta-analysis. Int J Biol Markers. 2017;32(1):e75–e82. doi:10.5301/jbm.5000224
14. Barkal AA, Brewer RE, Markovic M, et al. CD24 signalling through macrophage Siglec-10 is a target for cancer immunotherapy. Nature. 2019;572(7769):392–396. doi:10.1038/s41586-019-1456-0
15. Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27(1):111–122. doi:10.1016/j.immuni.2007.05.016
16. Gou Q, Dong C, Xu H, et al. PD-L1 degradation pathway and immunotherapy for cancer. Cell Death Dis. 2020;11(11):955. doi:10.1038/s41419-020-03140-2
17. Li W, Wu F, Zhao S, Shi P, Wang S, Cui D. Correlation between PD-1/PD-L1 expression and polarization in tumor-associated macrophages: a key player in tumor immunotherapy. Cytokine Growth Factor Rev. 2022;67:49–57. doi:10.1016/j.cytogfr.2022.07.004
18. Rizzo A, Ricci AD, Lanotte L, et al. Immune-based combinations for metastatic triple negative breast cancer in clinical trials: current knowledge and therapeutic prospects. Expert Opin Investig Drugs. 2022;31(6):557–565. doi:10.1080/13543784.2022.2009456
19. Viscardi G, Tralongo AC, Massari F, et al. Comparative assessment of early mortality risk upon immune checkpoint inhibitors alone or in combination with other agents across solid malignancies: a systematic review and meta-analysis. Eur J Cancer. 2022;177:175–185. doi:10.1016/j.ejca.2022.09.031
20. Rizzo A, Ricci AD. Biomarkers for breast cancer immunotherapy: PD-L1, TILs, and beyond. Expert Opin Investig Drugs. 2022;31(6):549–555. doi:10.1080/13543784.2022.2008354
21. Rizzo A, Cusmai A, Acquafredda S, Rinaldi L, Palmiotti G. Ladiratuzumab vedotin for metastatic triple negative cancer: preliminary results, key challenges, and clinical potential. Expert Opin Investig Drugs. 2022;31(6):495–498. doi:10.1080/13543784.2022.2042252
22. Rhodes DR, Kalyana-Sundaram S, Mahavisno V, et al. Oncomine 3.0: genes, pathways, and networks in a collection of 18,000 cancer gene expression profiles. Neoplasia. 2007;9(2):166–180. doi:10.1593/neo.07112
23. Chandrashekar DS, Bashel B, Balasubramanya SAH, et al. UALCAN: a portal for facilitating tumor subgroup gene expression and survival analyses. Neoplasia. 2017;19(8):649–658. doi:10.1016/j.neo.2017.05.002
24. Tang Z, Li C, Kang B, Gao G, Li C, Zhang Z. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017;45(W1):W98–W102. doi:10.1093/nar/gkx247
25. Gyorffy B, Lanczky A, Eklund AC, et al. An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1809 patients. Breast Cancer Res Treat. 2010;123(3):725–731. doi:10.1007/s10549-009-0674-9
26. Nagy A, Lanczky A, Menyhart O, Gyorffy B. Validation of miRNA prognostic power in hepatocellular carcinoma using expression data of independent datasets. Sci Rep. 2018;8(1):9227. doi:10.1038/s41598-018-27521-y
27. Shen Y, Xue J, Yu J, et al. Comprehensive analysis of the expression, prognostic significance, and regulation pathway of G2E3 in breast cancer. World J Surg Oncol. 2022;20(1):398. doi:10.1186/s12957-022-02871-0
28. Zhu X, Zhang Y, Bai Y, et al. HCK can serve as novel prognostic biomarker and therapeutic target for breast cancer patients. Int J Med Sci. 2020;17(17):2773–2789. doi:10.7150/ijms.43161
29. Coates AS, Winer EP, Goldhirsch A, et al. Tailoring therapies--improving the management of early breast cancer: st gallen international expert consensus on the primary therapy of early breast cancer 2015. Ann Oncol. 2015;26(8):1533–1546. doi:10.1093/annonc/mdv221
30. Goetz MP, Gradishar WJ, Anderson BO, et al. NCCN guidelines insights: breast cancer, version 3.2018. J Natl Compr Canc Netw. 2019;17(2):118–126. doi:10.6004/jnccn.2019.0009
31. Zhu X, Zhang Q, Wang D, Liu C, Han B, Yang JM. Expression of PD-L1 attenuates the positive impacts of high-level tumor-infiltrating lymphocytes on prognosis of triple-negative breast cancer. Cancer Biol Ther. 2019;20(8):1105–1112. doi:10.1080/15384047.2019.1595282
32. Kryczek I, Zou L, Rodriguez P, et al. B7-H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma. J Exp Med. 2006;203(4):871–881. doi:10.1084/jem.20050930
33. Warde-Farley D, Donaldson SL, Comes O, et al. The genemania prediction server: biological network integration for gene prioritization and predicting gene function. Nucleic Acids Res. 2010;38:W214–W220. doi:10.1093/nar/gkq537
34. Szklarczyk D, Franceschini A, Wyder S, et al. STRING v10: protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015;43(Database issue):D447–D452. doi:10.1093/nar/gku1003
35. da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4(1):44–57. doi:10.1038/nprot.2008.211
36. Ru B, Wong CN, Tong Y, et al. TISIDB: an integrated repository portal for tumor-immune system interactions. Bioinformatics. 2019;35(20):4200–4202. doi:10.1093/bioinformatics/btz210
37. Liu S, Cong Y, Wang D, et al. Breast cancer stem cells transition between epithelial and mesenchymal states reflective of their normal counterparts. Stem Cell Rep. 2014;2(1):78–91. doi:10.1016/j.stemcr.2013.11.009
38. Zhang W, Gao L, Wang C, et al. Combining bioinformatics and experiments to identify and verify key genes with prognostic values in endometrial carcinoma. J Cancer. 2020;11(3):716–732. doi:10.7150/jca.35854
39. Zheng MJ, Gou R, Zhang WC, et al. Screening of prognostic biomarkers for endometrial carcinoma based on a ceRNA network. PeerJ. 2018;6:e6091. doi:10.7717/peerj.6091
40. Ritchie ME, Phipson B, Wu D, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43(7):e47. doi:10.1093/nar/gkv007
41. Araujo JM, De la cruz-ku G, Cornejo M, et al. Prognostic capability of TNBC 3-gene score among triple-negative breast cancer subtypes. Cancers. 2022;14(17):4286. doi:10.3390/cancers14174286
42. Wang Q, Jiang J, Ying G, et al. Tamoxifen enhances stemness and promotes metastasis of ERalpha36(+) breast cancer by upregulating ALDH1A1 in cancer cells. Cell Res. 2018;28(3):336–358. doi:10.1038/cr.2018.15
43. Yang Y, Hsu PJ, Chen YS, Yang YG. Dynamic transcriptomic m(6)A decoration: writers, erasers, readers and functions in RNA metabolism. Cell Res. 2018;28(6):616–624. doi:10.1038/s41422-018-0040-8
44. Yin H, Zhang X, Yang P, et al. RNA m6A methylation orchestrates cancer growth and metastasis via macrophage reprogramming. Nat Commun. 2021;12(1):1394. doi:10.1038/s41467-021-21514-8
45. Wu Y, Wang Z, Han L, et al. PRMT5 regulates RNA m6A demethylation for doxorubicin sensitivity in breast cancer. Mol Ther. 2022;30(7):2603–2617. doi:10.1016/j.ymthe.2022.03.003
46. Tan B, Zhou K, Liu W, et al. RNA N(6) -methyladenosine reader YTHDC1 is essential for TGF-beta-mediated metastasis of triple negative breast cancer. Theranostics. 2022;12(13):5727–5743. doi:10.7150/thno.71872
47. Roundtree IA, Evans ME, Pan T, He C. Dynamic RNA modifications in gene expression regulation. Cell. 2017;169(7):1187–1200. doi:10.1016/j.cell.2017.05.045
48. Li Y, Xiao J, Bai J, et al. Molecular characterization and clinical relevance of m(6)A regulators across 33 cancer types. Mol Cancer. 2019;18(1):137. doi:10.1186/s12943-019-1066-3
49. Zhang P, Zheng P, Liu Y. Amplification of the CD24 gene is an independent predictor for poor prognosis of breast cancer. Front Genet. 2019;10:560. doi:10.3389/fgene.2019.00560
50. Deng X, Apple S, Zhao H, et al. CD24 expression and differential resistance to chemotherapy in triple-negative breast cancer. Oncotarget. 2017;8(24):38294–38308. doi:10.18632/oncotarget.16203
51. Chan SH, Tsai KW, Chiu SY, et al. Identification of the novel role of CD24 as an oncogenesis regulator and therapeutic target for triple-negative breast cancer. Mol Cancer Ther. 2019;18(1):147–161. doi:10.1158/1535-7163.MCT-18-0292
52. O’Conor CJ, Chen T, Gonzalez I, Cao D, Peng Y. Cancer stem cells in triple-negative breast cancer: a potential target and prognostic marker. Biomarker Med. 2018;12(7):813–820. doi:10.2217/bmm-2017-0398
53. Sheridan C, Kishimoto H, Fuchs RK, et al. CD44+/CD24- breast cancer cells exhibit enhanced invasive properties: an early step necessary for metastasis. Breast Cancer Res. 2006;8(5):R59. doi:10.1186/bcr1610
54. Carrasco E, Alvarez PJ, Prados J, et al. Cancer stem cells and their implication in breast cancer. Eur J Clin Invest. 2014;44(7):678–687. doi:10.1111/eci.12276
55. Jing X, Cui X, Liang H, et al. CD24 is a potential biomarker for prognosis in human breast carcinoma. Cell Physiol Biochem. 2018;48(1):111–119. doi:10.1159/000491667
56. Strati A, Nikolaou M, Georgoulias V, Lianidou ES. Prognostic significance of TWIST1, CD24, CD44, and ALDH1 transcript quantification in epcam-positive circulating tumor cells from early stage breast cancer Patients. Cells. 2019;8(7):652. doi:10.3390/cells8070652
57. Zhao D, Mo Y, Li MT, et al. NOTCH-induced aldehyde dehydrogenase 1A1 deacetylation promotes breast cancer stem cells. J Clin Invest. 2014;124(12):5453–5465. doi:10.1172/JCI76611
58. Shi L, Tang X, Qian M, et al. A SIRT1-centered circuitry regulates breast cancer stemness and metastasis. Oncogene. 2018;37(49):6299–6315. doi:10.1038/s41388-018-0370-5
59. Zhang C, Samanta D, Lu H, et al. Hypoxia induces the breast cancer stem cell phenotype by HIF-dependent and ALKBH5-mediated m(6)A-demethylation of NANOG mRNA. Proc Natl Acad Sci U S A. 2016;113(14):E2047–E2056.
60. Wan W, Ao X, Chen Q, et al. METTL3/IGF2BP3 axis inhibits tumor immune surveillance by upregulating N(6)-methyladenosine modification of PD-L1 mRNA in breast cancer. Mol Cancer. 2022;21(1):60. doi:10.1186/s12943-021-01447-y
61. Zou Y, Zheng S, Xie X, et al. N6-methyladenosine regulated FGFR4 attenuates ferroptotic cell death in recalcitrant HER2-positive breast cancer. Nat Commun. 2022;13(1):2672. doi:10.1038/s41467-022-30217-7
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.