Back to Journals » Journal of Pain Research » Volume 13
CBD Effects on TRPV1 Signaling Pathways in Cultured DRG Neurons
Authors Anand U , Jones B, Korchev Y, Bloom SR, Pacchetti B, Anand P, Sodergren MH
Received 21 April 2020
Accepted for publication 11 August 2020
Published 11 September 2020 Volume 2020:13 Pages 2269—2278
DOI https://doi.org/10.2147/JPR.S258433
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Michael A Ueberall
Uma Anand,1 Ben Jones,2 Yuri Korchev,3 Stephen R Bloom,2 Barbara Pacchetti,4 Praveen Anand,5 Mikael Hans Sodergren1
1Department of Surgery and Cancer, Imperial College London, Hammersmith Hospital, London W12 ONN, UK; 2Department of Metabolism, Digestion and Reproduction, Imperial College London, Hammersmith Hospital, London W12 ONN, UK; 3Nanomedicine Laboratory, BN5 Commonwealth Building, Imperial College London, Hammersmith Hospital, London W12 ONN, UK; 4EMMAC Life Sciences Ltd, London EC2A 2EW, UK; 5Peripheral Neuropathy Unit, Department of Brain Sciences, Imperial College London, Hammersmith Hospital, London W12 ONN, UK
Correspondence: Uma Anand Email [email protected]
Introduction: Cannabidiol (CBD) is reported to produce pain relief, but the clinically relevant cellular and molecular mechanisms remain uncertain. The TRPV1 receptor integrates noxious stimuli and plays a key role in pain signaling. Hence, we conducted in vitro studies, to elucidate the efficacy and mechanisms of CBD for inhibiting neuronal hypersensitivity in cultured rat sensory neurons, following activation of TRPV1.
Methods: Adult rat dorsal root ganglion (DRG) neurons were cultured and supplemented with the neurotrophic factors NGF and GDNF, in an established model of neuronal hypersensitivity. Neurons were stimulated with CBD (Adven 150, EMMAC Life Sciences) at 1, 10, 100 nMol/L and 1, 10 and 50 μMol/L, 48 h after plating. In separate experiments, DRG neurons were also stimulated with capsaicin with or without CBD (1 nMol/L to10 μMol/L), in a functional calcium imaging assay. The effects of the adenylyl cyclase activator forskolin and the calcineurin inhibitor cyclosporin were determined. We also measured forskolin-stimulated cAMP levels, without and after treatment with CBD, using a homogenous time-resolved fluorescence (HTRF) assay. The results were analysed using Mann-Whitney test.
Results: DRG neurons treated with 10 and 50 μMol/L CBD showed calcium influx, but not at lower doses. Neurons treated with capsaicin demonstrated robust calcium influx, which was dose-dependently reduced in the presence of low dose CBD (IC50 = 100 nMol/L). The inhibition or desensitization by CBD was reversed in the presence of forskolin and cyclosporin. Forskolin-stimulated cAMP levels were significantly reduced in CBD treated neurons.
Conclusion: CBD at low doses corresponding to plasma concentrations observed physiologically inhibits or desensitizes neuronal TRPV1 signalling by inhibiting the adenylyl cyclase – cAMP pathway, which is essential for maintaining TRPV1 phosphorylation and sensitization. CBD also facilitated calcineurin-mediated TRPV1 inhibition. These mechanisms may underlie nociceptor desensitization and the therapeutic effect of CBD in animal models and patients with acute and chronic pain.
Keywords: cannabidiol, CBD, chronic pain, DRG neurons, cAMP, calcium imaging, desensitization
Introduction
Several cannabinoid preparations have been developed for treating conditions such as epilepsy, chemotherapy-associated nausea, and chronic pain.1,2 Significant dose-related pain relief has been reported in clinical studies of cannabinoids for postoperative pain,3 and for chronic neuropathic pain following brachial plexus injury.4
Cannabidiol (CBD), the non-psychoactive component of Cannabis sativa, has been reported to exert anti-convulsive, anti-inflammatory, neuroprotective and analgesic effects, and to be well tolerated in humans.5 A number of preclinical and clinical studies have examined the molecular basis and therapeutic effects of CBD, in experimental models of neurological disorders and in clinical trials.6 The efficacy of CBD in attenuating seizures and social deficits was demonstrated in a mouse model of Dravet syndrome.7 Clinical trials have also shown that CBD is well tolerated in children and young adults with Dravet syndrome and Lennox-Gastaut syndrome, with reduced incidence of convulsive seizures.8–10 CBD is a major constituent in Sativex, combined with Δ9-THC, and used for treating the limb spasticity and pain associated with multiple sclerosis.11
Cannabinoids exert their effects mainly via the cannabinoid receptors CB1 and CB2.12 For CBD, further receptor targets are also implicated, including the orphan G-protein coupled receptor GPR55, the transient receptor potential of vanilloid subtype 1 (TRPV1), the 5-HT1a receptor and the α3 and α1 glycine receptors.13–15
In contrast to Δ9-THC, CBD has the advantage of not being psychoactive5,6 but is reported to potentiate the beneficial effects of Δ9-THC, to enhance its tolerability and to widen its therapeutic window.16 The presence of CBD in cannabis preparations containing a high ratio of CBD:Δ9-THC may protect against the development of psychotic symptoms, compared with preparations with low CBD:Δ9-THC ratios.17 The beneficial effects of CBD have been described in several experimental animal models of neurological disorders, especially epilepsy, via neuronal inhibition. In a model of paclitaxel-induced neuropathy in mice, treatment with CBD alleviated the development of allodynia.18
CBD has been reported to produce pain relief, but the clinically relevant cellular and molecular mechanisms remain uncertain. The TRPV1 receptor is a potential target for some of the pharmacological effects of CBD and its analogues, via calcium influx in TRPV1 receptor-overexpressing HEK cells, with similar efficacy to capsaicin.19 Recent studies also indicate that CBD and cannabidivarin (CBDV) stimulate and then desensitize TRPV1, TRPV2 and TRPA1 receptors in transfected HEK cells, and diminish epileptiform activity in hippocampal slices.20 CBD is reported to induce calcium influx in TRPA1- and TRPM8-expressing DRG neurons, though at very high concentrations (100 µMol/L) in mustard oil sensitive neonatal DRG neurons.21 The relevance of CBD effects on TRP channels other than TRPV1 is uncertain, since they were observed at high micromolar concentrations.22
Pharmacokinetic studies have provided estimates of systemic plasma concentrations following CBD exposure. Healthy adults administered a single oral dose of 750 mg purified CBD had estimated blood maximum CBD concentration (Cmax) of 1000 ng/mL (3.3 µMol/L).23 Cmax of 3.0 ± 3.1 µg/L serum CBD levels (equivalent to 10 nMol/L), and maximum time (Tmax) of 2.8 ± 1.3 h were also reported in healthy volunteers after a single dose of nabiximols, containing 10 mg each of CBD and THC.24 Similarly, a Phase 1 study of purified CBD reported the maximum plasma concentration of CBD in healthy volunteers administered 1500 mg CBD was 1385 ng/mL (4.6 μMol/L), with an average Tmax of 4 h.25 These studies raise questions about the relevance of in vitro studies using significantly higher ligand concentrations.
We have now examined the effect of CBD, at physiologically relevant concentrations, in pain signaling via TRPV1 in cultured adult rat DRG neurons. TRPV1 is a non-selective transmembrane cation channel expressed by polymodal nociceptors and integrates a number of noxious exogenous and endogenous stimuli, including capsaicin, heat, low pH, and inflammatory ligands,26 to mediate thermal hyperalgesia.27 The neurotrophic factors (NTFs) NGF and GDNF are increased in tissues in painful conditions28 and have a sensitizing effect on sensory neurons via TRPV1.29–31 In this study, DRG neurons were cultured with NTFs to generate a model of neuronal hypersensitivity as previously described,30 and the effects of CBD on capsaicin responses were studied in this model.
Materials and Methods
Neuronal Cultures
Bilateral DRG from all levels were micro-dissected from freshly euthanized adult female Wistar rats (Charles River UK Ltd, Margate, Kent, UK) (with approvals from the Animal Welfare Ethical Review Body, Imperial College, following UK Home Office approved procedures, and in keeping with the 3Rs ARRIVE guidelines). DRG were collected in Ham’s F12 medium and enzyme digested in Ham’s F12 nutrient medium containing 0.2% collagenase and 0.5% dispase for 3 hours at 37°C, as previously described.32,33 Enzyme digested tissue was triturated in modified BSF2 medium [containing 2% HIFCS, 0.1 mg/mL transferrin, 60 ng/mL progesterone, 0.16 μg/mL sodium selenite, 3 mg/mL bovine serum albumin (BSA), penicillin/streptomycin 100 μg/mL each, 16 μg/mL putrescine, 10 μg/mL insulin], soybean trypsin inhibitor and DNAse to obtain a neuronal suspension. One rat was used for each experiment, and the neuron suspension was plated on several poly-l-lysine and laminin (20 μg/mL each) coated glass-bottom MatTek dishes (MatTek Corp, USA), at 5000 neurons per dish for calcium imaging studies. Two-milliliter BSF2 medium supplemented with 100 ng/mL of NGF and 50 ng/mL GDNF were added to all culture dishes and incubated at 37°C in a humidified environment of 5% CO2 in air, for at least 48 hours before further study.
Functional Studies
Calcium imaging was used to determine the effect of acute CBD application on the capsaicin sensitivity of DRG neurons. Studies were conducted in HEPES buffered phenol-red free Hanks Balanced Salt Solution (HBSS) containing 0.1% BSA, as previously described.32,33 Experiments were conducted at 37°C in a humidified environment on an inverted Nikon microscope (Diaphot 300; Nikon, UK Ltd, Kingston upon Thames, Surrey, UK) and cultures were alternately excited at 340 and 380 nm λex wavelengths. Responses to paired capsaicin stimuli were measured as the maximum change in the 340/380 λex nm ratio from baseline in neurons loaded with 2 µMol/L Fura2 AM (Life Technologies, Paisley, UK), before, during and after addition. Images of 15 −20 neurons in each experiment were captured every 2 seconds in each of three channels – brightfield, 340 and 380 nm λex/510 λem, and recordings of mean intracellular changes in bound/unbound Ca2+ ratio were obtained before, during and after the addition of capsaicin. This provided baseline recordings as well as intracellular changes in Ca2+ levels in response to added capsaicin. Cells were uniformly loaded with the dye and no intracellular compartmentalisation of the loaded dye was observed. Images were acquired with a Hamamatsu Orca CCD Camera and analysed with AQM Advance Kinetic imaging software. Individual cells under study were highlighted as regions of interest for calculating the mean ratios of bound to unbound calcium within the area of interest.
In each experiment, neurons were exposed to capsaicin for a maximum of two applications, first to identify capsaicin sensitive neurons (using 200 nmol/L capsaicin for 15 seconds), followed by washout and rest period of 45 minutes. The second stimulus of 1 μMol/L capsaicin was used to test the effect of the added CBD or vehicle (0.2% ethanol), after the washout period. Only neurons responding to 200 nmol/L capsaicin with a rapid and sustained increase in 340/380 ratio of more than 20% from the baseline were selected for the study. The second capsaicin stimulus, of 1 μMol/L, was applied after the baseline had returned to normal. Neurons in which the baseline remained elevated were considered to be desensitized and excluded from the analysis.
Statistical Analysis
In each experiment, for each neuron, the second response to capsaicin was expressed as a percentage of the first, with or without added drugs. Percent responses for each experiment were normalized to the vehicle-treated control. Averages were calculated for each group, and the non-parametric Mann–Whitney test was used to compare between groups, using Graphpad Prism software. Data are presented as mean ± s.e.m., *P<0.05 was considered statistically significant, **P<0.01, and ***P<0.001. “n” indicates the number of animals used for each group.
Solutions
CBD (Adven 150, EMMAC Life Sciences) was dissolved in DMSO to obtain a 100 mMol/L stock solution, and capsaicin was dissolved in ethanol at 20 mMol/L concentration. CBD and capsaicin stocks were aliquoted and stored at −20°C, and fresh aliquots were made up to 500x final concentration in ethanol prior to use. All chemicals were obtained from Sigma-Aldrich UK unless otherwise stated.
cAMP Assay
For each experiment, DRG neurons were plated in poly-l-lysine (20 µg/mL) and laminin (20 µg/mL) coated 24 well plates, in duplicate, at 8000 neurons/well, in BSF2 medium plus NGF (100 ng/mL) and GDNF (50 ng/mL) and incubated at 37°C in a humidified incubator containing 5% CO2. After 48 hours, the medium was aspirated from each well and replaced with 250 µL of HEPES buffered HBSS containing 10 µMol/L IBMX (3-isobutyl-1-methylxanthine), followed by 1 µMol/L CBD and stimulated with 1 µMol/L Forskolin, at 37°C. Lysis buffer (100 µL) was added to each well after 10 minutes. Cells and medium were aspirated and cAMP levels were assayed using the cAMP Dynamic 2 assay (Cisbio) and read using a Spectramax i3x multimode plate reader with HTRF module installed, according to the manufacturer’s instructions.
Results
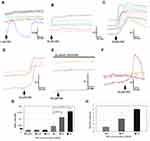
We used calcium imaging to indicate neuronal activation in response to the application of CBD at 1, 10, 100 nMol/L, and 1, 10 and 50 µMol/L, and observed calcium influx consistently at 10 and 50 µMol/L (Figure 1). Only 340/380 ratio changes of 0.02 or more from the baseline were considered to constitute a response. The responses to CBD at 10 µMol/L (0.11 ± 0.02, n=7, *P=0.03), and 50 µMol/L (0.15 ± 0.02, n=5, *P=0.01), were significantly higher than responses to 1 µMol/L CBD (0.04 ± 0.02, n=6). The proportion of neurons responding was dose dependent, with increasing numbers responding at higher concentration. The proportion of neurons responding with calcium influx to 1 µMol/L CBD was 1.25 ± 0.25%, to 10 µMol/L CBD was 3.4 ± 1.6%, and the maximum of 6 ± 0.5% to 50 µMol/L CBD (data from n=4 rats, from a field of 200 neurons per experiment). CBD-mediated calcium influx was prevented in the presence of the TRPV1 antagonist SB705498. Application of CBD was followed by desensitization to the subsequent application (Figure 1). The proportion of neurons responding with calcium influx to CBD application was far less than those responding to 200 nMol/L capsaicin (~50%).
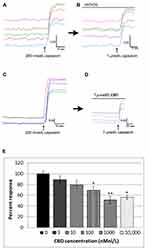
We also used calcium imaging to examine the effect of CBD on capsaicin responses, in a model of neuronal hypersensitivity. As TRPV1 expression is restricted to a subset of DRG neurons, this required the identification of individual capsaicin-sensitive neurons. In control experiments, TRPV1 expressing neurons were identified by a 15-second application of 200 nmol/L capsaicin (Figure 2A), followed by washout of medium and a rest period of 45 minutes to allow the intracellular 340/380 (bound/unbound calcium) ratio to return to the baseline. A response was characterized by a rapid and sustained increase in intracellular calcium indicated by a rise in the 340/380 ratio, more than 0.02. The second stimulus of 1 µMol/L capsaicin in the presence of vehicle resulted in a slightly reduced response compared with the first response (74.4 ± 5% of the first response), due to tachyphylaxis (Figure 2B). Further, dose-related reduction of the second response was observed in the presence of CBD (Figure 2C and D). Responses in the presence of CBD at different doses were normalized to vehicle-treated neurons (Figure 2E, Table 1).
 |
Table 1 CBD Dose-Related Effects on Capsaicin Responses Normalized to Control |
In order to identify the mechanism underlying CBD-mediated desensitization, we examined the effect of forskolin in our functional assay. Forskolin is known to activate adenylyl cyclase, leading to the formation of cAMP. The availability of cAMP plays an important role in TRPV1 sensitization, by phosphorylation via activation of protein kinase A, and TRPV1 is desensitized when dephosphorylated. Capsaicin responses were maintained in the presence of 20 µMol/L forskolin (95.9 ± 6.12%, n=6), compared with CBD-mediated desensitization of capsaicin responses, that were reduced (51.37 ± 6.5%, n=6). In the combined presence of 20 µMol/L forskolin and 1 µMol/L CBD, desensitization was abolished (average capsaicin response 96 ± 11% of control, n=5) (Table 2, Figure 3A), indicating that the inhibitory effect of CBD was reversed in the presence of cAMP induced by the activity of forskolin.
 |
Table 2 Effect of Forskolin on CBD-Mediated TRPV1 Desensitization |
We also examined the effect of the calcineurin inhibitor cyclosporin, on CBD-mediated desensitization of capsaicin responses. Calcineurin (also known as protein phosphatase PP2B) is responsible for protein dephosphorylation, and TRPV1 desensitization. Capsaicin responses were maintained in the presence of 100 nMol/L cyclosporin (90.5 ± 5.6%, n=5), and the presence of cyclosporin reversed the CBD-induced desensitization, significantly enhancing capsaicin responses to 102.4 ± 14.8% of control (n=5, *P<0.05) (Table 3, Figure 3B).
 |
Table 3 Effect of Cyclosporin on CBD-Mediated TRPV1 Desensitization |
To confirm the role of cAMP in our functional assay, we quantified cAMP levels biochemically by homogenous time-resolved fluorescence (HTRF), which showed that basal levels of cAMP were similar in CBD treated neurons to those treated with vehicle alone. Elevation of cAMP levels by 1 µMol/L forskolin was significantly diminished in the presence of 1 µMol/L CBD (Figure 4). This suggests that CBD is able to prevent the activation of adenylyl cyclase by forskolin, presumably through activation of a Gαi-coupled GPCR.
Discussion
The effects of CBD have been widely reported, including pain relief, but the underlying mechanisms remain undetermined, and are now of great interest globally.
This study shows that CBD stimulated calcium influx in DRG neurons at concentrations of 10 and 50 µMol/L as previously reported,19,20 in agreement with the findings of Iannotti et al. Similar to their findings, we have observed increased numbers of neurons responding with calcium influx to high dose CBD application, even though the percentage of neurons responding was low. These stimulatory effects were proposed to define CBD as a TRPV1 agonist with calcium-dependent effects of subsequent desensitization. As the calcium influx following the application of CBD was abolished in the presence of the TRPV1 antagonist SB705498, we conclude that CBD interacts with TRPV1 in DRG neurons. We also observed dose-related desensitization with CBD concentrations from 1 nMol/L to 1 µMol/L, which do not generate calcium influx. At 1 µMol/L CBD, 1.25 ± 0.25% of neurons showed calcium influx just above the detection threshold, which is far less than the TRPV1 responding neurons. Thus, calcium influx-mediated desensitization is unlikely to apply to our observations in this study, unless the low dose CBD-mediated calcium influx that was below the sensitivity of detection of our system. The concentrations used in our study seek to determine the effects of CBD at concentrations within the range (Cmax 1–3 µMol/L), observed in the plasma of healthy volunteers given purified CBD in clinical studies.24,25
The CBD-induced desensitization was significantly greater than the expected reduction in neuronal responsiveness due to repeat capsaicin stimulation (i.e. tachyphylaxis).34,35 Our study showed that maximum desensitization was observed with 1 µMol/L CBD; the higher concentration tested, 10 µMol/L, resulted in less desensitization. This observation suggests that higher micromolar concentrations may have a predominantly stimulatory acute effect, as observed in our study and as reported in previous studies.19,20
Having established the desensitization effect of CBD at “physiological” doses on capsaicin-evoked responses in cultured DRG neurons, we investigated the underlying mechanisms. Calcium imaging also showed that CBD-mediated TRPV1 inhibition/desensitization was completely reversed in the presence of forskolin, which is known to activate adenylyl cyclase, leading to the elevation of cAMP.36 Capsaicin responses in the presence of forskolin alone were similar to vehicle controls. The increased availability of cAMP leads to the phosphorylation and sensitization of TRPV1. This was prevented in the presence of CBD, when TRPV1 was desensitized, and cAMP levels were significantly reduced compared with forskolin. In the combined presence of CBD and forskolin, cAMP levels were reduced compared to those with forskolin alone (Figure 4). Thus, CBD appears to prevent the activation of adenylyl cyclase by forskolin, resulting in reduced cAMP and consequent TRPV1 desensitization.
TRPV1 desensitization by CBD was also reversed by the calcineurin inhibitor cyclosporin, which resulted in significant sensitization compared to vehicle-treated neurons. Calcineurin is the protein phosphatase PP2B, responsible for protein dephosphorylation, and consequent TRPV1 desensitization.34–36 The calcineurin inhibitor cyclosporin reversed CBD-mediated TRPV1 inhibition and significantly sensitized capsaicin responses, similar to cyclosporin controls alone. Calcineurin acts by dephosphorylating proteins, here TRPV1, and its inhibition would lead to the maintenance of the phosphorylated form of TRPV1, with consequent sensitization. The enhanced responses suggest that calcineurin may exert a basal inhibitory role in regulating TRPV1 sensitivity. Similar cannabinoid inhibition of TRPV1 has previously been reported, following calcium influx and calcineurin activation.14 The effects of cyclosporin and forskolin suggest there may be two potentially synergistic pathways for TRPV1 phosphorylation and sensitization which are blocked by CBD.
TRPV1 is modulated by several different signaling pathways,37 including binding to Phosphatidylinositol-4,5-bisphosphate,31,38,39 phosphorylation by protein kinase A,40 protein kinase C,41 protein kinase D,42 and the calcium-calmodulin dependent protein kinase II.43 The phosphorylation state of TRPV1 depends on the balance between kinase activity and phosphatase activity, with the protein phosphatase 2B calcineurin playing an important part in TRPV1 desensitization.34,36,37
As adenylyl cyclase inhibition leading to reduced cAMP is a hallmark of cannabinoid action,44 we examined this mechanism in the inhibition of TRPV1 by CBD, by measuring cAMP levels. While cAMP levels were similar in CBD treated neurons to vehicle, they were significantly elevated in the presence of forskolin, as expected. Forskolin-stimulated cAMP levels were significantly diminished by CBD, suggesting that CBD mediates its inhibitory effects via a Gi pathway, the main pathway in cannabinoid signaling.44 cAMP levels depleted by CBD are likely to prevent TRPV1 phosphorylation, resulting in diminished capsaicin responses, while pre-incubation with forskolin abolished the CBD inhibitory effect, and restored capsaicin responses. These findings are in accord with our previous study, which showed TRPV1 inhibition in cultured human DRG neurons by cannabinoid receptor subtype 2 (CB2R) agonists, which was reversed by 8-bromo-cAMP, via a Gi pathway.32
The present study shows that CBD activated calcium influx in DRG neurons at 10 and 50 µMol/L, but between 1 nMol/L to 1 µMol/L concentrations, CBD inhibited capsaicin responses by blocking the adenylyl cyclase – cAMP signaling pathway, which is essential for maintaining TRPV1 sensitization. Increased cAMP levels in neurons are generally associated with increased nociception, whereas agents that decrease cAMP synthesis have analgesic effects. Forskolin stimulation leads to activation of adenylyl cyclase with the formation of cAMP, which plays an important role in maintaining TRPV1 sensitization, as TRPV1 was shown to be sensitized when phosphorylated by PKA, and desensitized when dephosphorylated.35,41 Similarly, cAMP generation in response to inflammatory mediators such as prostaglandins, and the direct activation of PKA with cAMP analogues, is known to cause behavioural hypersensitivity.45,46
Apart from the effects of CBD signaling via a range of receptors including CB1, CB2, GPR55, TRPA1, TRPM8, 5-HT1a and the α3 and α1 glycine receptors,13–15 other effects of CBD have been described recently. These include non-selective inhibition of voltage-dependent sodium currents,47 diminished action potential firing frequency via G-protein-coupled receptors, and restoration of the excitability of inhibitory interneurons in a mouse model of Dravet syndrome.7 Inhibition of voltage-gated T-type channels in mouse trigeminal neurons by CBD, with similar potency and efficacy as in our study (by about 45% at 1 µMol/L), has also been reported.48 TRPV1 signaling pathways were identified as a target of CBD, as carrageenan-induced thermal hyperalgesia in rats was abolished by CBD and capsazepine, but not by CB1 or CB2 antagonists.13 Another study found no direct effect of CBD on TRPV1, which mediated vasorespiratory effects induced by capsaicin and anandamide but not CBD in anaesthetized rats.49 CBD was also reported to block the progression of collagen-induced arthritis in mice, suppress lymphocyte proliferation, and diminish lipopolysaccharide-induced TNF secretion.50 It should be emphasized that further studies with selective antagonists are required to identify the GPCR(s) mediating the inhibitory effects of CBD observed in our study, as well as CBD effects in human vs. rat sensory neurons.
In conclusion, our study shows that at physiological concentrations, CBD inhibits TRPV1 signaling by a dual mechanism: the first by inhibiting the adenylyl cyclase – cAMP pathway, which is essential for maintaining TRPV1 sensitization. The second pathway likely involves calcineurin-mediated TRPV1 inhibition. Both mechanisms may underlie nociceptor desensitization and the therapeutic effect of CBD in animal models and patients with acute and chronic pain.
Disclosure
Barbara Pacchetti reports personal fees from EMMAC Life Sciences, outside the submitted work. Mikael Sodergren reports consultancy for Emmac Life Sciences, outside the submitted work. The authors report no other potential conflict of interest in this work.
References
1. Russo EB. Cannabinoids in the management of difficult to treat pain. Ther Clin Risk Manag. 2008;4(1):245–259. doi:10.2147/TCRM.S1928
2. Anand P, Whiteside G, Fowler CJ, Hohmann AG. Targeting CB2 receptors and the endocannabinoid system for the treatment of pain. Brain Res Rev. 2009;60(1):255–266. doi:10.1016/j.brainresrev.2008.12.003
3. Holdcroft A, Maze M, Doré C, Tebbs S, Thompson S. A multicenter dose-escalation study of the analgesic and adverse effects of an oral cannabis extract (Cannador) for postoperative pain management. Anesthesiol. 2006;104(5):1040–1046. doi:10.1097/00000542-200605000-00021
4. Berman JS, Symonds C, Birch R. Efficacy of two cannabis based medicinal extracts for relief of central neuropathic pain from brachial plexus avulsion: results of a randomised controlled trial. Pain. 2004;112(3):299–306. doi:10.1016/j.pain.2004.09.013
5. Pertwee RG. The pharmacology and therapeutic potential of cannabidiol. In: Di Marzo V, editor. Cannabinoids. New York: Kluwer Academic/Plenum Publishers; 2004:32–83.
6. Devinsky O, Cilio MR, Cross H, et al. Cannabidiol: pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders. Epilepsia. 2014;55(6):791–802. doi:10.1111/epi.12631
7. Kaplan JS, Stella N, Caterall WA, Westenbroek RE. Cannabidiol attenuates seizures and social deficits in a mouse model of dravet syndrome. PNAS. 2017;114(42):11229–11234. doi:10.1073/pnas.1711351114
8. Devinsky O, Patel AD, Thiele EA, et al. Randomized, dose-ranging safety trial of cannabidiol in dravet syndrome. Neurology. 2018a;90(14):e1204–e1211. doi:10.1212/WNL.0000000000005254
9. Szaflarski JP, Bebin EM, Comi AM, et al. Long‐term safety and treatment effects of cannabidiol in children and adults with treatment‐resistant epilepsies: expanded access program results. Epilepsia. 2018;59(8):1540–1548. doi:10.1111/epi.14477
10. Devinsky O, Marsh E, Friedman D, et al. Cannabidiol in patients with treatment-resistant epilepsy: an open-label interventional trial. Lancet Neurol. 2016;15(3):270–278. doi:10.1016/S1474-4422(15)00379-8
11. Wade DT, Makela P, Robson P, House H, Bateman C. Do cannabis-based medicinal extracts have general or specific effects on symptoms in multiple sclerosis? A double-blind, randomized, placebocontrolled study on 160 patients. Multiple Sclerosis. 2004;10:434–441. doi:10.1191/1352458504ms1082oa
12. Pertwee RG. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol Ther. 1997;74(2):129–180. doi:10.1016/S0163-7258(97)82001-3
13. Costa B, Giagnoni G, Franke C, Trovato AE, Colleoni M. Vanilloid TRPV1 receptor mediates the antihyperalgesic effect of the nonpsychoactive cannabinoid, cannabidiol, in a rat model of acute inflammation. Br J Pharmacol. 2004;143(2):247–250. doi:10.1038/sj.bjp.0705920
14. Patwardhan AM, Jeske NA, Price TJ, Gamper N, Akopian AN, Hargreaves KM. The cannabinoid WIN 55,212-2 inhibits transient receptor potential vanilloid 1 (TRPV1) and evokes peripheral antihyperalgesia via calcineurin. PNAS. 2006;103(30):11393–11398. doi:10.1073/pnas.0603861103
15. Lowin T, Schneider M, Pongratz G. Joints for joints: cannabinoids in the treatment of rheumatoid arthritis. Curr Opin Rheumatol. 2018;2019(31):271–278.
16. Karniol I, Carlini E. Pharmacological interaction between cannabidiol and Δ9-tetrahydrocannabinol. Psychopharmacologia. 1973;33:53–70. doi:10.1007/BF00428793
17. Schubart CD, Sommer IEC, van Gastel WA, et al. Cannabis with high cannabidiol content is associated with fewer psychotic experiences. Schizophr Res. 2011;130(1–3):216–221. doi:10.1016/j.schres.2011.04.017
18. Ward SJ, Ramirez MD, Neelakantan H, Walker EA. Cannabidiol prevents the development of cold and mechanical allodynia in paclitaxel-treated female C57Bl6 mice. Anesth Analg. 2011;113(4):947–950. doi:10.1213/ANE.0b013e3182283486
19. Bisogno T, Hanus L, De Petrocellis L, et al. Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br J Pharmacol. 2001;134(4):845–852. doi:10.1038/sj.bjp.0704327
20. Iannotti FA, Hill CL, Leo A, et al. Nonpsychotropic plant Cannabinoids, Cannabidivarin (CBDV) and Cannabidiol (CBD), activate and desensitize Transient Receptor Potential Vanilloid 1 (TRPV1) channels in vitro: potential for the treatment of neuronal hyperexcitability. ACS Chem Neurosci. 2014;5(11):1131–1141. doi:10.1021/cn5000524
21. De Petrocellis L, Vellani V, Schiano-Moriello A, et al. Plant-derived cannabinoids modulate the activity of transient receptor potential channels of ankyrin type-1 and melastatin type-8. J Pharmacol Exp Ther. 2008;325(3):1007–1015. doi:10.1124/jpet.107.134809
22. Bih CI, Chen T, Nunn AV, Bazelot M, Dallas M, Whalley BJ. Molecular targets of cannabidiol in neurological targets. Neurotherapeutics. 2015;12(4):699–730.
23. Crockett J, Critchley D, Tayo B, Berwaerts J, Morrison G. A phase 1, randomized, pharmacokinetic trial of the effect of different meal compositions, whole milk, and alcohol on cannabidiol exposure and safety in healthy subjects. Epilepsia. 2020;61(2):267–277. doi:10.1111/epi.16419
24. Guy G, Robson P. A Phase I, open label, four-way crossover study to compare the pharmacokinetic profiles of a single dose of 20 mg of a cannabis based medicine extract (CBME) administered on 3 different areas of the buccal mucosa and to investigate the pharmacokinetics of CBME per oral in healthy male and female volunteers (GWPK0112). J Cannabis Ther. 2004;3:79–120.
25. Taylor L, Gidal B, Blakey G, Tayo B, Morrison G. A phase I, randomized, double-blind, placebo-controlled, single ascending dose, multiple dose, and food effect trial of the safety, tolerability and pharmacokinetics of highly purified cannabidiol in healthy subjects. CNS Drugs. 2018;32:1053–1067. doi:10.1007/s40263-018-0578-5
26. Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi:10.1038/39807
27. Davis JB, Gray J, Gunthorpe MJ, et al. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405(6783):183–187. doi:10.1038/35012076
28. Anand P. Neurotrophic factors and their receptors in human sensory neuropathies. Prog Brain Res. 2004;146:477–492.
29. Shu X, Mendell LM. Nerve growth factor acutely sensitizes the response of adult rat sensory neurons to capsaicin. Neurosci Lett. 1999;274(3):159–162. doi:10.1016/S0304-3940(99)00701-6
30. Anand U, Otto WR, Casula MA, et al. The effect of neurotrophic factors on morphology, TRPV1 expression and capsaicin responses of cultured human DRG sensory neurons. Neurosci Lett. 2006;399(1–2):51–56. doi:10.1016/j.neulet.2006.01.046
31. Malin SA, Molliver DC, Koerber HR, et al. Glial cell line-derived neurotrophic factor family members sensitize nociceptors in vitro and produce thermal hyperalgesia in vivo. J Neurosci. 2006;26(33):8588–8599. doi:10.1523/JNEUROSCI.1726-06.2006
32. Anand U, Otto WR, Sanchez-Herrera D, et al. Cannabinoid receptor CB2 localisation and agonist-mediated inhibition of capsaicin responses in human sensory neurons. Pain. 2008;138(3):667–680. doi:10.1016/j.pain.2008.06.007
33. Anand U, Otto WR, Anand P. Sensitization of capsaicin and icilin responses in oxaliplatin treated adult rat DRG neurons. Mol Pain. 2010;6:82. doi:10.1186/1744-8069-6-82
34. Docherty RJ, Yeats JC, Bevan S, Boddeke HW. Inhibition of calcineurin inhibits the desensitization of capsaicin-evoked currents in cultured dorsal root ganglion neurons from adult rats. Pflueg Arch Eur J Physiol. 1996;431:828–837. doi:10.1007/s004240050074
35. Mohapatra DP, Nau C. Desensitization of capsaicin-activated currents in the vanilloid receptor TRPV1 is decreased by the cyclic AMP-dependent protein kinase pathway. J Biol Chem. 2003;278(50):50080–50090. doi:10.1074/jbc.M306619200
36. Mohapatra DP, Nau C. Regulation of Ca 2 +-dependent desensitization in the vanilloid receptor TRPV1 by calcineurin and cAMP-dependent protein kinase. J Biol Chem. 2005;280(14):13424–13432. doi:10.1074/jbc.M410917200
37. Cortright DN, Szallasi A. Biochemical pharmacology of the vanilloid receptor TRPV1. An update. Eur J Biochem. 2004;271(10):1814–1819. doi:10.1111/j.1432-1033.2004.04082.x
38. Chuang H-H, Prescott ED, Kong H, et al. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature. 2001;411(6840):957–962. doi:10.1038/35082088
39. Prescott ED, Julius D. A modular PIP2 binding site as a determinant of capsaicin receptor sensitivity. Science. 2003;300(5623):1284–1288. doi:10.1126/science.1083646
40. Bhave G, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RWIV. c-AMP dependent protein kinase regulates desensitization of the capsaicin receptor (VR1) by direct phosphorylation. Neuron. 2002;35(4):721–731. doi:10.1016/S0896-6273(02)00802-4
41. Bhave G, Hu HJ, Glauner KS, et al. Protein Kinase C phosphorylation sensitizes but does not activate capsaicin receptor transient potential receptor vanilloid 1 (TRPV1). PNAS. 2003;100:12480–12485. doi:10.1073/pnas.2032100100
42. Wang Y, Kedei N, Wang M, et al. Interaction between protein kinase Cmu and the vanilloid receptor type 1. J Biol Chem. 2004;279:53674–53682. doi:10.1074/jbc.M410331200
43. Jung J, Shin JS, Lee SY, et al. Phosphorylation of vanilloid receptor 1 by Ca2+/calmodulin-dependent kinase II regulates its vanilloid binding. J Biol Chem. 2004;279:7048–7054. doi:10.1074/jbc.M311448200
44. Rhee M-H, Bayewitch M, Avidor-Reiss A, Levy R, Vogel Z. Cannabinoid receptor activation differentially regulates the various adenylyl cyclase isozymes. J Neurochem. 1998;71:1525–1534. doi:10.1046/j.1471-4159.1998.71041525.x
45. Taiwo YO, Bjerknes LK, Goetzl EJ, Levine JD. Mediation of primary afferent peripheral hyperalgesia by the cAMP second messenger system. Neuroscience. 1989;32(3):577–580. doi:10.1016/0306-4522(89)90280-7
46. Taiwo YO, Levine JD. Further confirmation of the role of adenyl cyclase and of cAMP-dependent protein kinase in primary afferent hyperalgesia. Neuroscience. 1991;44(1):131–135. doi:10.1016/0306-4522(91)90255-M
47. Ghovanloo MR, Shuart NG, Mezeyova J, Dean RA, Ruben PC, Goodchild SJ. Inhibitory effects of cannabidiol on voltage-dependent sodium currents. J Biol Chem. 2018;293(43):16546–16558. doi:10.1074/jbc.RA118.004929
48. Ross HR, Napier I, Connor M. Inhibition of recombinant human T-type calcium channels by Δ9 -tetrahydrocannabinol and cannabidiol. J Biol Chem. 2007;283(23):16124–16134. doi:10.1074/jbc.M707104200
49. McQueen DS, Bond SM, Smith PJW, Balali-Mood K, Smart D. Cannabidiol lacks the vanilloid VR1-mediated vasorespiratory effects of capsaicin and anandamide in anaesthetized rats. Eur J Pharmacol. 2004;491(2–3):181–189. doi:10.1016/j.ejphar.2004.03.045
50. Malfait AM, Gallily R, Sumariwall PF, et al. The nonpsychoactive cannabis constituent cannabidiol is an oral anti-arthritic therapeutic in murine collagen-induced arthritis. PNAS. 2000;97(17):9561–9566. doi:10.1073/pnas.160105897
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.