Back to Journals » ImmunoTargets and Therapy » Volume 10
Cannabis and Autoimmunity: Possible Mechanisms of Action
Authors Giorgi V , Marotto D, Batticciotto A, Atzeni F, Bongiovanni S, Sarzi-Puttini P
Received 26 February 2021
Accepted for publication 25 June 2021
Published 21 July 2021 Volume 2021:10 Pages 261—271
DOI https://doi.org/10.2147/ITT.S267905
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Michael Shurin
Valeria Giorgi1 1, Daniela Marotto2 2, Alberto Batticciotto3 3, Fabiola Atzeni4 4, Sara Bongiovanni1 1, Piercarlo Sarzi-Puttini1 1
1Rheumatology Unit, Internal Medicine Department, ASST Fatebenefratelli-Sacco, Milan University School of Medicine, Milan, Italy; 2Rheumatology Unit, ATS Sardegna, P. Dettori Hospital, Tempio Pausania, Italy; 3Rheumatology Unit, Internal Medicine Department, ASST Settelaghi, Ospedale Di Circolo - Fondazione Macchi, Varese, Italy; 4Rheumatology Unit, Department of Clinical and Experimental Medicine, University of Messina, Messina, Italy
Correspondence: Valeria Giorgi Email [email protected]
Abstract: Medical cannabis (MC) describes the usually inhaled or ingested use of a cannabis plant or cannabis extract for medicinal purposes. The action of whole cannabis plants is extremely complex because their large number of active compounds not only bind to a plethora of different receptors but also interact with each other both synergistically and otherwise. Renewed interest in the medicinal properties of cannabis has led to increasing research into the practical uses of cannabis derivatives, and it has been found that the endocannabinoid system (particularly CB2 receptor activation) is a possible target for the treatment of inflammatory and the autoimmune diseases related to immune cell activation. However, in vivo findings still lack, creating difficulties in applying translational cannabinoid research to human immune functions. In this review, we summarized the main mechanisms of action of medical cannabis plant especially regarding the immune system and the endocannabinoid system, looking at preliminary clinical data in three most important autoimmune diseases of three different specialities: rheumatoid arthritis, multiple sclerosis and inflammatory bowel disease.
Keywords: cannabis, cannabis derivatives, cannabidiol, tetrahydrocannabinol, terpenes, autoimmunity, auto-antibodies
Introduction
Cannabis is one of the oldest of cultivated plants, and has been used as a raw material, food and medicinal drug for thousands of years.1–4 It contains 538 chemical compounds, of which just 100 are natural phytocannabinoids (PCs),5 which are usually divided into the 10 subclasses of delta 9-tetrahydrocannabinol (THC), D8THC, cannabigerol (CBG), cannabichromene (CBC), cannabidiol (CBD), cannabinodiol, cannabielsoin, cannabicyclol, cannabinol (CBN), cannabitriol, and miscellaneous. The CBD and D9THC subclasses have so far received the most scientific attention: Gaoni and Mechoulam were the first to isolate THC and CBD in order to determine their structure and stereochemistry, and they subsequently synthesised them in the 1960s.6 PCs are mainly secreted by the trichomes of female plants (the glandular protuberances found on the leaves and stems) in the form of a resin whose wealth of PCs (mainly THC) and terpenoids (eg, pinene, limonene, caryophyllene) give the plants their characteristic smell.
In this paper, we provided a brief description of cannabis as a medicinal remedy, synthesized the main sites of action of the cannabis plant with a special regard to the immune system, and tried to explain whether medical cannabis action on the endocannabinoid system may be of help for patients with autoimmune diseases. In conclusion, we summarized preliminary clinical data about medical cannabis in the three most important autoimmune diseases of three different specialities: rheumatoid arthritis, multiple sclerosis and inflammatory bowel disease.
Medical Cannabis
Medical cannabis (MC) refers to the plant or an extract (usually with a specific relative amount of THC and CBD) used for medical purposes. The preparations may be administered by means of vaporisation and inhalation, ingestion, or topical applications; the use of vaginal, rectal or sublingual administrations is less frequent.7 Oral cannabis formulations take effect 30–90 minutes after ingestion, and their effect peaks after 2–4 hours; and the approximate half-life of THC is eight days in fat,8,9 since PCs are highly lipophilic.10 Furthermore, it seems that the bio-distribution of orally ingested CBD and THC is greater in the lymphoid tissues of the intestinal lymphatic system than in the larger lymphatic tissue of the central compartment,11 which may be particularly useful when treating chronic intestinal conditions. Conversely, its delivery to the lungs aids rapid absorption and leads to an early onset of action (after only one minute) that peaks after 30 minutes at most. This suggests that this route of administration is best in the case of more acute conditions, such as multiple sclerosis-associated spasticity. The drawback is that the faster THC reaches the brain, the more likely the occurrence of side effects.12 Mild, dose-dependent, acute adverse events are well documented: the most frequent are drowsiness, dry mouth, dizziness, vertigo, and nausea, but others include blurred vision, tachycardia, gastrointestinal disturbances (diarrhea/stipsis, lost/increased appetite, dyspepsia), and muscle spasms.13–16 THC-related side effects (fatigue, tachycardia and dizziness) can be avoided by very slow dose titration, which also promotes tolerance to its psychoactive side effects.16
Pharmacodynamics and the Endocannabinoid System
THC binds to PPARγ receptors, GPR55, GPR18, TRPA1 (or capsaicin receptors, which are predominantly expressed in the nociceptive neurons of the peripheral nervous system but are also found in the central nervous system), TRPV2, TRPV3, TRPV4, several glutamate receptors, glycine receptors, and adenosine receptors, and acts as an antagonist of 5-hydroxytryptamine (HT)3 receptors and TRPM8. It has recently been discovered that GPR55, which is coupled to a G-alpha protein and was once considered an orphan receptor, is activated not only by THC but also by CBD, certain synthetic cannabinoids, AEA and 2-AG, and its activation increases intracellular calcium levels.17
CBD acts as an agonist of TRPA1, TRPV1, TRPV2, TRPV3, PPARγ, 5-HT1A, A2 and A1 adenosine, an antagonist of GPR55, GPR18, and 5-HT3A, and an inverse agonist of GPR3, GPR6, and GPR12,18 and can also down-regulate the enzymes FAAH and 5-LOX.19–21
In the last decade, most of the attention has been drawn on the endocannabinoid system. The endocannabinoid system is a human biological system that consists of cannabinoid (CB) receptors and their endogenous ligands (endocannabinoids) and modulating enzymes. An increasing number of endocannabinoids have recently been discovered, including arachidonoyl ethanolamide, also called anandamide (AEA), 2-arachidonoyl glycerol (2-AG),22,23 2-arachidonoyl glyceryl ether (noladin ether, 2-AGE), O-arachidonoylethanolamine (virodhamine), N-arachidonoyldopamine (NADA), and oleic acid amide (oleamide, OA).24 Endocannabinoid-degrading enzymes include fatty acid amide hydrolases (FAAH) and monoacylglycerol lipase (MAGL). Anandamide is mainly degraded by FAAH, but also by cyclooxygenase-2 (COX-2) and lipoxygenases (LOXs); 2-AG is degraded by MAGL, and sometimes by COX-2, LOXs and minor enzymes such as α/β hydrolase-6 and α/β hydrolase-1225. Endocannabinoids are lipophilic and it is believed that, like eicosanoids, they are produced and released on demand. In addition to their interactions with CB receptors, their interactions with other molecular targets make them highly flexible, which accounts for the complexity of the system as a whole and the biological action of the individual cannabinoids.26
CB1 receptors are mainly present in the central nervous system (CNS), but also found peripherally in hepatic, intestinal and adipose tissue,27 the eye, cardiovascular system, pancreas, immune system, bone, skin, and skeletal muscle, thus suggesting a potentially enormous periphery/brain network of connections.28 CB2 receptors are often called peripheral not only because they are mainly found in the immune system,29 but also because their activation is largely devoid of psychotropic effects.30 They are also present in microglial cells,31 and on chondrocytes, osteocytes, and fibroblasts – all cells that take part in the inflammation of the autoimmune diseases discussed in this article. Within the immune system, CB2 expression is higher in lymph nodes and spleen than in peripheral blood cells, and varies in different cell sub-populations (B cells > NK cells > monocytes > neutrophils > CD8+ T cells > CD4+ T cells).32,33
Much of CBD biological activity is independent of CB receptors, as has been demonstrated by its suppression of cytokine production in CB1 and CB2 receptor knock-out mice.34 Some data suggest that CBD can indirectly activate CB1 and CB2 by increasing AEA and 2-AG levels.35 Indirect targeting of the CB system is actually a well-known pharmacological technique: the most widely used analgesic drug is the decades-old paracetamol, which acts by producing AM404 and thus interfering with the reuptake of anandamide.36
THC has quite a high affinity for both CB receptors, but its efficacy depends on receptor density and coupling efficiency:37 ie, it mainly acts as an antagonist of endocannabinoids in tissues characterised by lower CB receptor density. CBD has less affinity for CB receptors and, more particularly, is a negative allosteric modulator of CB1, thus probably making it an antagonist of some of the effects of THC when they are concomitantly administered.38–40 However, a recent in vivo study did not confirm this hypothesis.41
Medical Cannabis and the Immune System
The activities of the large number of compounds contained in whole cannabis plants is extremely complex because the compounds themselves not only bind to a plethora of different receptors but also interact with each other both synergistically and otherwise:42 for example, pinene is an acetylcholinesterase inhibitor that may decrease the short-term memory impairment induced by THC.16 What follows is a brief summary of the activity of other PCs and terpenes (which have been more extensively reviewed elsewhere).43
CBG is an AEA reuptake inhibitor which, when combined with CBD, has anti-inflammatory activity as it reduces the expression of tumour necrosis factor (TNF) and up-regulates interleukin (IL)-10 and IL-37 levels.44 CBC is also an agonist of CB receptor 2,45 and CBN inhibits COX, LOX, and P450 cytochrome enzymes.
Terpenes are very promising compounds as they have a variety of positive (analgesic, anti-depressant, anti-oxidant, and anti-bacterial) functions,42 but the possible contribution of adding terpenoids to cannabinoids is uncertain.43 It has been reported that caryophyllenes are anti-microbial, anti-proliferative, anti-fungal, anti-oxidant and anti-inflammatory acetylcholinesterase inhibitors;46 D-limonene may be anti-inflammatory as it mediates the inhibition of pro-inflammatory mediators, leukocyte migration, and vascular permeability.47 Gamma-terpinene has an effect on the pro- and anti-inflammatory macrophage production of cytokines, particularly through the IL-10 axis.48
CB receptors are G protein-coupled receptors whose activation inhibits adenylate cyclase. By reducing cAMP levels, they decrease protein kinase A inhibition of K+ channels, thus decreasing cell K+ levels. Other signalling routes are the activation of mitogen-activated protein kinase and phosphoinositide-3-kinase.49 CB receptor activation also activates Kir channels and inhibits N- or P/Q-type Ca2+ channels. A post-synaptic increase in neuronal calcium can trigger the biosynthesis and release of endocannabinoids, which then bind pre-synaptically to CB1 and inhibit neurotransmitter release in the synaptic cleft in a process known as retrograde signalling.50
The final effect clearly depends on the location of the CB1 receptors. It has been demonstrated that in vivo THC administration increases the release of acetylcholine, dopamine and glutamate in certain rat brain areas, probably as a result of a CB1-mediated decrease in inhibitory neurotransmitters51,52 It has also been demonstrated that CB1 receptor activation may play a neuroprotective role in some of the modulatory systems involved in neurodegeneration53,54 and neuroinflammation.55,56 The mechanism of action of CB2 receptors is similar.
CB receptor binding has many effects on immune cells, but it mainly decreases immune system activation and immune cell migration by inhibiting immune cell mobilisation, inducing apoptosis, and suppressing transcription factors and cytokine release. Table 1 summarises these functions by type of immune cell.
 |
Table 1 Cannabinoid Mechanisms of Immune Suppression |
B cells are the immune cells with the highest levels of CB1 and CB2 receptors. Cannabinoids can decrease the production of antibodies in animals and humans,76–78 but their effect on B cell proliferation is still unclear. In T cells, cannabinoids clearly regulate T cell proliferation as T cell apoptosis is increased by AEA and CBD and decreased in CB2-deficient mice, and both AEA and THC decrease T cell proliferation79–81 (although some studies suggest that CBD has a more potent anti-proliferative effect than THC).11
Macrophages also express CB receptors:82,83 the activation of CB2 receptors blocks monocyte migration,84,85 but induces phagocytosis and chemokine release.86 THC increases macrophage apoptosis and inhibits the differentiation of human monocytes into antigen-presenting dendritic cells.80,87
Immunomodulation is apparently mainly regulated by inhibiting immune cell mobilisation and increasing apoptosis: endocannabinoids decrease neutrophil and macrophage mobilisation to sites of inflammation,85,88 and dendritic cell migration.89 CB1 receptor agonists down-regulate mast cell activation and relieve the inflammatory symptoms mediated by hypersensitivity reactions.90 On the other hand, CBD can decrease the number of neutrophils and compromise myeloperoxidase activity.59
Another mechanism by means of which cannabinoids control immune function is the induction of regulatory cells. CBD can induce myeloid-derived suppressor cells in a mouse model of hepatitis,91 and CBD, THC and CB2-selective cannabinoids can induce a regulatory phenotype by shifting the balance between inflammatory Th17 and regulatory T cells,59,67,80,92,93 which is mirrored by the induction of anti-inflammatory cytokines and reduction in pro-inflammatory cytokines. CBD can suppress many transcription factors, including NFAT, AP-1 and NF-kB, and this probably accounts for its widespread suppression of many cytokines.59 It also prevents NLRP3/inflammasome pathway activation and suppresses the gene expression of downstream proteins, thus decreasing the production of IL-1β.94
The selective activation of CB2 receptors induces IL-10 by increasing its production by macrophages,95 downregulates NF-κB92 (it is known that IL-10 production is increased in cannabis users),96 and reduces the LPS-induced up-regulation of the genes associated with inflammation in macrophages.85
THC inhibits interferon (IFN)-gamma secretion, which is also inhibited by CBD11,59 both directly (by means of a transcriptional mechanism) and indirectly by suppressing the expression of IFN-gamma receptors and increasing the IFN-gamma-induced genes that subsequently attenuate other immune targets. Consistently, CBD decreases IL-2 production in T cells,97 and the engagement of both CB receptors reduces IL-2 synthesis.98 CBD and THC also attenuate the expression of TNF-α.11
Finally, various studies have shown that both THC and CBD decrease the production and release of IL-6,99 and the action of CBD has been replicated in animal models of inflammation.59
These findings are particularly important in the context of autoimmune diseases, but it is always necessary to remember the difficulties of applying translational cannabinoid research to human immune functions. Some in vitro and animal data suggest that the immunomodulatory activity of THC and CB2 agonists can decrease resistance to infectious agents, which is comparable with the immunosuppressive effect of the drugs used to treat autoimmune diseases,9 whereas other data suggest that chronic THC exposure may actually help in the case of some viral diseases.100 Another example is the higher expression of a translocator protein in long-term cannabis users, which is a marker of microglial activation and inflammation.101 Furthermore, although CB receptor binding is important in the immunomodulatory action of the cannabis plant, it has to be reminded that some compounds, mainly CBD, have many CB receptor independent activities.
Medical Cannabis and Autoimmune Diseases
Cannabinoids immunomodulating traits span on a variety of cell, from lymphocytes and macrophages to chondrocytes. These cells, all take place in the pathogenesis of various autoimmune disease. As each autoimmune disease differ in symptoms, it also differs in the “culpable” immune cells and cytokines inciting the disease. Hence, declaring that cannabis treatment is beneficial for autoimmune diseases does not suffice, and treatment options should be tailored for each disease. For instance, RA is characterized by elevated levels of TNF-ɑ, IL-1 and IL-6 while IL-10 has an attenuating effect on the inflammation. Focusing on specific cannabinoids reveals that CBD, THC and CBG all reduce the levels of TNF-ɑ, a known therapeutic target in the case of RA. Moreover, CBD also reduces IL-6 levels as mentioned above, pinpointing him as possibly the most suitable treatment for RA. While TNF-ɑ is also a valid target in the treatment of MS, and naturally there is an overlap in cytokine profile of various autoimmune disease, MS is also driven by the presence of IFN-γ, thus highlighting THC as a likely candidate for therapeutic options. The vast diversity of cannabinoids and their wide range of action on immune cells advocates a tailored approach. Moreover, some autoimmune diseases such as juvenile idiopathic arthritis have been shown to associate with a variation in CB2 receptor, suggesting that cannabis or cannabinoid treatment should possibly be tailored not only per disease but also per person.
The endocannabinoid system, particularly CB2 receptor activation, is a possible target for the treatment of diseases characterised by immune cell activation, such as inflammatory and autoimmune diseases,18,102–104 on the basis of the mechanisms described above and shown in Figure 1. The data concerning the efficacy and side effects of the different MC preparations are still limited, and many physicians prefer to avoid suggesting their use;18,105 however, there has been abundant pre-clinical research in this area, as can be seen from the following examples.
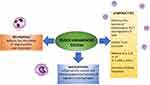 |
Figure 1 The endocannabinoid system is a key regulator of the immune system and therefore may be used as a target for the treatment of autoimmune diseases. |
Rheumatoid Arthritis (RA)
Cannabinoids interrelation with RA is implied by CB receptors expression in synovia of RA patients along with relatively high levels of endocannabinoids.106 The expression of CB receptors in the inflamed joint advocates the application of cannabinoids as therapeutic agents in RA due to their immunomodulating properties as mentioned in the previous sections.
It has been shown in vitro that non-selective synthetic cannabinoids reduce the production of IL-6 and IL-8 by RA fibroblast-like synovial cells,67 and that CBD increases intracellular calcium levels and reduces the viability and IL-6/IL-8/MMP-3 production of RA synovial fibroblasts.107 This is particularly interesting as IL-6 is considered a key player not only in RA inflammation but also in the pain, fatigue and mood disorders of RA patients.108,109 In mouse models, CBD (and particularly CB2 receptor activation) decreases synovitis and attenuates the progression of arthritis by reducing inflammatory cell infiltration, bone destruction, the production of anti-collagen type II, IgG1 and IFN-gamma, and the release of TNF.70,110 The anti-inflammatory effects of endocannabinoids on synovial fibroblasts may also be achieved indirectly using FAAH inhibitors.68 Interestingly, in vitro culture in the presence of CBD significantly increased Th17 cell differentiation in CD4+ T cells from the peripheral blood of patients with RA.111
There are currently no randomised clinical trials investigating the use of cannabis in the treatment of RA, partly because of the availability of effective biological anti-inflammatory agents in the therapeutic armamentarium.112 However, one preliminary randomised, placebo-controlled study has assessed the efficacy, tolerability and safety of five weeks’ treatment with a synthetic TCH analogue in 58 RA patients, and found that pain was significantly reduced and disease activity significantly suppressed.113
Multiple Sclerosis (MS)
In mouse models of MS, which are created using mice with experimental autoimmune encephalomyelitis (EAE), the crucial role of the endocannabinoid system in attenuating disease activity is highlighted by the fact that clinical remission is observed in mice with lower FAAH levels114 and that CB2 receptor knock-out mice have worse clinical EAE scores than wild-type mice. CB2-negative T cells are characterised by reduced levels of apoptosis, higher proliferation rates, and increased inflammatory cytokine production79 and, in line with this, CB2 receptor engagement in EAE models reduces many markers of inflammation (including iNOS, COX-2, TNF, and IL-1b).115 CB1 receptors may also be involved given their abundance in the central nervous system.116 MC and cannabinoid-based medicines are currently being investigated and used to treat MS-associated spasticity,117–119 but whether they can modify the neuroinflammation that causes progressive disability is still an open question: however, even if they cannot induce robust immunosuppression, they could still help by inhibiting the glial responses that facilitate neurodegeneration.120
Inflammatory Bowel Disease (IBD)
The use of MC to treat inflammatory bowel disease is supported by the findings of preclinical studies showing that the endocannabinoid system plays a role in regulating intestinal inflammation.121 In many animal models of colitis, the administration of CB1 or CB2 receptor agonists122–124 and the inhibition of FAAH has prevented or reduced colonic inflammation,125,126 and it has been found that CB1 and CB2 knockout mice are more susceptible to chemically induced colitis.123,127
Furthermore, immunohistochemical studies of humans have shown that the expression of CB2 receptors is amplified during inflammatory flares, thus suggesting that they are a potential therapeutic target.128,129 It is possible that the use of oral formulations may be more successful as it has been discovered that the THC and CBD they contain can efficiently reach the intestinal lymphatic system.11 MC can also have beneficial effects on the permeability of the gastrointestinal tract.130
Surveys and cross-sectional studies of IBD patients have shown that many of them smoke usually illegally obtained cannabis in order to alleviate their symptoms. The symptoms that are reported to improve the most are abdominal pain and cramps, appetite,131,132 and nausea.133 One 2014 study132 found that the use of cannabis correlated with a need for surgery, but a more recent study134 showed that the prevalence of partial or total colectomy was lower in cannabis users than non-users, and that there was also a trend towards a lower prevalence of bowel obstruction. A recent Australian survey found that clinical severity ratings were not different between cannabis users and non-users, but the former reported more hospitalisations, less engagement with specialist services, and less adherence to prescribed medications; the IBD that were positively affected by cannabis included abdominal pain, stress, sleep, cramping, and anxiety.135
The first retrospective study of the clinical use of MC dates back to 2011136 and, since then, researchers have concentrated more on the different compositions of the MC preparations used to treat IBD. The findings of one study suggest that CBD-only MC preparations are not effective for Crohn’s disease,137 and a study of a very small patient cohort by the same group found that a more THC-rich preparation induced significant, steroid-free clinical benefits.138 Furthermore, the use of MC capsules with a high CBD/THC ratio has led to improvements in the partial Mayo score, the patients’ subjective global impression of disease activity, and physicians global assessments of disease severity,139 although there were many dropouts due to THC-related adverse events. Finally, a recent small study found that inhaled THC did not improve disease markers or remission rates in a cohort of 127 ulcerative colitis patients, but did improve disease activity scores.140
Conclusions
The complexity of PCs and their wide range of interactions with multiple potential therapeutic targets support the legalisation of the medicinal use of the world’s oldest and best-known illicit drug, especially on the basis of observational studies,141 and emerging knowledge of autoimmunity and the high density of CB2 receptors on immune cells makes the endocannabinoid system a natural target for the treatment of inflammatory and autoimmune diseases.142 In vitro and animal studies of multiple sclerosis have provided convincing evidence of the immunomodulatory properties of cannabis, although there is still a lack of clinical evidence from randomised and controlled clinical trials. However, given our limited knowledge of the long-term safety and efficacy of cannabis and its possible drug–drug interactions, it is still too early to make any definite recommendations concerning the cannabinoid treatment of autoimmune diseases, and this should only prompt further research in order to fill these gaps.
Acknowledgment
We would like to thank Daphna Katz, Itai Katz and Prof. Yehuda Shoenfeld for having revised the manuscript and having added a crucial part about cannabis applications for autoimmune diseases.
Disclosure
All of the authors declare that they have no conflict of interest in relation to this paper.
References
1. Bonini SA, Premoli M, Tambaro S, et al. Cannabis sativa: a comprehensive ethnopharmacological review of a medicinal plant with a long history. J Ethnopharmacol. 2018;227:300–315. doi:10.1016/j.jep.2018.09.004
2. Touw M. The religious and medicinal uses of cannabis in China, India and Tibet. J Psychoactive Drugs. 1981;13:23–34. doi:10.1080/02791072.1981.10471447
3. Chopra IC, Chopra RN. The use of the cannabis drugs in India. Bull Narc. 1957;9:4–29.
4. Hall W. The Indian Hemp Drugs Commission 1893-1894. Addiction. 2019;114(9):1679–1682.
5. Hanuš LO, Meyer SM, Muñoz E, Taglialatela-Scafati O, Appendino G. Phytocannabinoids: a unified critical inventory. Nat Prod Rep. 2016;33:1357–1392. doi:10.1039/C6NP00074F
6. Gaoni Y, Mechoulam R. Isolation, structure, and partial synthesis of an active constituent of Hashish. J Am Chem Soc. 1964;86:1646–1647. doi:10.1021/ja01062a046
7. Russell C, Rueda S, Room R, Tyndall M, Fischer B. Routes of administration for cannabis use - basic prevalence and related health outcomes: a scoping review and synthesis. Int J Drug Policy. 2018;52:87–96. doi:10.1016/j.drugpo.2017.11.008
8. Mechoulam R, Fride E, Di Marzo V. Endocannabinoids. Eur J Pharmacol. 1998;359:1–18. doi:10.1016/S0014-2999(98)00649-9
9. Cabral GA, Rogers TJ, Lichtman AH. Turning over a new leaf: cannabinoid and endocannabinoid modulation of immune function. J Neuroimmune Pharmacol. 2015;10:193–203. doi:10.1007/s11481-015-9615-z
10. Pertwee RG. Cannabinoid pharmacology: the first 66 years. Br J Pharmacol. 2006;147:S163–S171. doi:10.1038/sj.bjp.0706406
11. Zgair A, Lee JB, Wong JCM, et al. Oral administration of cannabis with lipids leads to high levels of cannabinoids in the intestinal lymphatic system and prominent immunomodulation. Sci Rep. 2017;7:1–12. doi:10.1038/s41598-017-15026-z
12. Gould J. 4 Big questions. Nature. 2015;527:4. doi:10.1038/nj7577-265a
13. Wang T, Collet J-P, Shapiro S, Ware MA. Adverse effects of medical cannabinoids: a systematic review. CMAJ. 2008;178:1669–1678. doi:10.1503/cmaj.071178
14. Fitzcharles MA, Baerwald C, Ablin J, Häuser W. Efficacy, tolerability and safety of cannabinoids in chronic pain associated with rheumatic diseases (fibromyalgia syndrome, back pain, osteoarthritis, rheumatoid arthritis): a systematic review of randomized controlled trials. Schmerz. 2016;30:47–61. doi:10.1007/s00482-015-0084-3
15. Aviram J, Samuelly-Leichtag G. Efficacy of cannabis-based medicines for pain management: a Systematic Review and Meta- Analysis of Randomized Controlled Trials. Pain Physician. 2017;6:E755–E796. doi:10.36076/ppj.20.5.E755
16. Maccallum CA, Russo EB. Practical considerations in medical cannabis administration and dosing. Eur J Intern Med. 2018;49:12–19. doi:10.1016/j.ejim.2018.01.004
17. Ryberg E, Larsson N, Sjögren S, et al. The orphan receptor GPR55 is a novel cannabinoid receptor. Br J Pharmacol. 2007;152:1092–1101. doi:10.1038/sj.bjp.0707460
18. Almogi-Hazan O, Or R. Cannabis, the endocannabinoid system and immunity—the journey from the bedside to the bench and back. Int J Mol Sci. 2020;21:1–17. doi:10.3390/ijms21124448
19. Capasso R, Borrelli F, Aviello G, et al. Cannabidiol, extracted from cannabis sativa, selectively inhibits inflammatory hypermotility in mice. Br J Pharmacol. 2008;154:1001–1008. doi:10.1038/bjp.2008.177
20. Massi P, Valenti M, Vaccani A, et al. 5-Lipoxygenase and anandamide hydrolase (FAAH) mediate the antitumor activity of cannabidiol, a non-psychoactive cannabinoid. J Neurochem. 2008;104:1091–1100. doi:10.1111/j.1471-4159.2007.05073.x
21. Oláh A, Szekanecz Z, Bíró T. Targeting cannabinoid signaling in the immune system: “High”-ly exciting questions, possibilities, and challenges. Front Immunol. 2017;8:1. doi:10.3389/fimmu.2017.01487
22. Devane WA, Hanus L, Breuer A, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi:10.1126/science.1470919
23. Sugiura T, Kondo S, Sukagawa A, et al. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun. 1995;215:89–97.
24. Laezza C, Pagano C, Navarra G, et al. The endocannabinoid system: a target for cancer treatment. Int J Mol Sci. 2020;21. doi:10.3390/ijms21030747
25. Perisetti A, Rimu AH, Khan SA, Bansal P, Goyal H. Role of cannabis in inflammatory bowel diseases. Ann Gastroenterol. 2020;33:134–144. doi:10.20524/aog.2020.0452
26. Di Marzo V, De Petrocellis L. Why do cannabinoid receptors have more than one endogenous ligand? Philos Trans R Soc B Biol Sci. 2012;367:3216–3228.
27. Jourdan T, Djaouti L, Demizieux L, Gresti J, Vergès B, Degrace P. CB1 antagonism exerts specific molecular effects on visceral and subcutaneous fat and reverses liver steatosis in diet-induced obese mice. Diabetes. 2010;59:926–934. doi:10.2337/db09-1482
28. Busquets Garcia A, Soria-Gomez E, Bellocchio L, Marsicano G. Cannabinoid receptor type-1: breaking the dogmas. F1000Research. 2016;5:1–9. doi:10.12688/f1000research.8245.1
29. Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi:10.1038/365061a0
30. Kinsey SG, Mahadevan A, Zhao B, et al. The CB2 cannabinoid receptor-selective agonist O-3223 reduces pain and inflammation without apparent cannabinoid behavioral effects. Neuropharmacology. 2011;60:244–251. doi:10.1016/j.neuropharm.2010.09.004
31. Núñez E, Benito C, Pazos MR, et al. Cannabinoid CB2 receptors are expressed by perivascular microglial cells in the human brain: an immunohistochemical study. Synapse. 2004;53:208–213.
32. Galiègue S, Mary S, Marchand J, et al. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur J Biochem. 1995;232:54–61. doi:10.1111/j.1432-1033.1995.tb20780.x
33. Parolaro D. Presence and functional regulation of cannabinoid receptors in immune cells. Life Sci. 1999;65:637–644. doi:10.1016/S0024-3205(99)00286-6
34. Kaplan BLF, Springs AEB, Kaminski NE. The profile of immune modulation by cannabidiol (CBD) involves deregulation of nuclear factor of activated T cells (NFAT). Biochem Pharmacol. 2008;76:726–737. doi:10.1016/j.bcp.2008.06.022
35. Ligresti A, De Petrocellis L, Di Marzo V. From phytocannabinoids to cannabinoid receptors and endocannabinoids: pleiotropic physiological and pathological roles through complex pharmacology. Physiol Rev. 2016;96:1593–1659. doi:10.1152/physrev.00002.2016
36. Sharma CV, Long JH, Shah S, et al. First evidence of the conversion of paracetamol to AM404 in human cerebrospinal fluid. J Pain Res. 2017;10:2703–2709. doi:10.2147/JPR.S143500
37. Pertwee RG. The diverse CB 1 and CB 2 receptor pharmacology of three plant cannabinoids: Δ 9-tetrahydrocannabinol, cannabidiol and Δ 9-tetrahydrocannabivarin. Br J Pharmacol. 2008;153:199–215. doi:10.1038/sj.bjp.0707442
38. Pisanti S, Malfitano AM, Ciaglia E, et al. Cannabidiol: state of the art and new challenges for therapeutic applications. Pharmacol Ther. 2017;175:133–150. doi:10.1016/j.pharmthera.2017.02.041
39. Laprairie RB, Bagher AM, Kelly MEM, Denovan-Wright EM. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br J Pharmacol. 2015;172:4790–4805. doi:10.1111/bph.13250
40. Morgan CJA, Schafer G, Freeman TP, Curran HV. Impact of cannabidiol on the acute memory and psychotomimetic effects of smoked cannabis: naturalistic study. Br J Psychiatry. 2010;197:285–290. doi:10.1192/bjp.bp.110.077503
41. Woelfl T, Rohleder C, Mueller JK, et al. Effects of cannabidiol and delta-9-tetrahydrocannabinol on emotion, cognition, and attention: a Double-Blind, Placebo-Controlled, Randomized Experimental Trial in Healthy Volunteers. Front Psychiatry. 2020;11. doi:10.3389/fpsyt.2020.576877
42. Russo EB, Marcu J. Cannabis pharmacology: the usual suspects and a few promising leads. Adv Pharmacol. 2017;80:67–134.
43. Gonçalves ECD, Baldasso GM, Bicca MA, Paes RS, Capasso R, Dutra RC. Terpenoids, cannabimimetic ligands, beyond the cannabis plant. Molecules. 2020;25:1567. doi:10.3390/molecules25071567
44. Mammana S, Cavalli E, Gugliandolo A, et al. Could the combination of two non-psychotropic cannabinoids counteract neuroinflammation? Effectiveness of cannabidiol associated with cannabigerol. Med. 2019;55. doi:10.3390/medicina55110747
45. Udoh M, Santiago M, Devenish S, McGregor IS, Connor M. Cannabichromene is a cannabinoid CB2 receptor agonist. Br J Pharmacol. 2019;176:4537–4547. doi:10.1111/bph.14815
46. Bento AF, Marcon R, Dutra RC, et al. β-caryophyllene inhibits dextran sulfate sodium-induced colitis in mice through CB2 receptor activation and PPARγ pathway. Am J Pathol. 2011;178:1153–1166. doi:10.1016/j.ajpath.2010.11.052
47. de Almeida AAC, Silva RO, Nicolau LAD, et al. Physio-pharmacological investigations about the anti-inflammatory and antinociceptive efficacy of (+)-limonene epoxide. Inflammation. 2017;40:511–522. doi:10.1007/s10753-016-0496-y
48. Ramalho TR, Filgueiras LR, De Oliveira MTP, et al. Gamma-Terpinene modulation of LPS-stimulated macrophages is dependent on the PGE2/IL-10 Axis. Planta Med. 2016;82:1341–1345. doi:10.1055/s-0042-107799
49. Pertwee RG. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol Ther. 1997;74:129–180. doi:10.1016/S0163-7258(97)82001-3
50. Vaughan CW, Christie MJ. Retrograde signalling by endocannabinoids. Handb Exp Pharmacol. 2005;(168):367–83. doi:10.1007/3-540-26573-2_12
51. Pisanu A, Acquas E, Fenu S, Di Chiara G. Modulation of Δ9-THC-induced increase of cortical and hippocampal acetylcholine release by μ opioid and D1 dopamine receptors. Neuropharmacology. 2006;50:661–670. doi:10.1016/j.neuropharm.2005.11.023
52. Pistis M, Ferraro L, Pira L, et al. Delta(9)-tetrahydrocannabinol decreases extracellular GABA and increases extracellular glutamate and dopamine levels in the rat prefrontal cortex: an in vivo microdialysis study. Brain Res. 2002;948:155–158. doi:10.1016/S0006-8993(02)03055-X
53. Vrechi TA, Crunfli F, Costa AP, Torrão AS. Cannabinoid receptor Type 1 agonist ACEA protects neurons from death and attenuates endoplasmic reticulum stress-related apoptotic pathway signaling. Neurotox Res. 2018;33:846–855. doi:10.1007/s12640-017-9839-1
54. Xu C, Hermes DJ, Nwanguma B, et al. Endocannabinoids exert CB1 receptor-mediated neuroprotective effects in models of neuronal damage induced by HIV-1 Tat protein. Mol Cell Neurosci. 2017;83:92–102. doi:10.1016/j.mcn.2017.07.003
55. Saliba SW, Marcotegui AR, Fortwängler E, et al. Correction: AM404, paracetamol metabolite, prevents prostaglandin synthesis in activated microglia by inhibiting COX activity. J Neuroinflammation. 2018;15:34. doi:10.1186/s12974-018-1072-1
56. Lowin T, Apitz M, Anders S, Straub RH. Anti-inflammatory effects of N-acylethanolamines in rheumatoid arthritis synovial cells are mediated by TRPV1 and TRPA1 in a COX-2 dependent manner. Arthritis Res Ther. 2015;17. doi:10.1186/s13075-015-0845-5
57. Watzl B, Scuderi P, Watson RR. Marijuana components stimulate human peripheral blood mononuclear cell secretion of interferon-gamma and suppress interleukin-1 alpha in vitro. Int J Immunopharmacol. 1991;13:1091–1097. doi:10.1016/0192-0561(91)90160-9
58. Watzl B, Scuderi P, Watson RR. Influence of marijuana components (THC and CBD) on human mononuclear cell cytokine secretion in vitro. Adv Exp Med Biol. 1991:63–70.
59. Nichols JM, Kaplan BLF. Immune responses regulated by cannabidiol. Cannabis Cannabinoid Res. 2020;5:12–31. doi:10.1089/can.2018.0073
60. Wu HY, Chang AC, Wang CC, et al. Cannabidiol induced a contrasting pro-apoptotic effect between freshly isolated and precultured human monocytes. Toxicol Appl Pharmacol. 2010;246:141–147. doi:10.1016/j.taap.2010.05.003
61. Jenny M, Santer E, Pirich E, Schennach H, Fuchs D. Δ9-Tetrahydrocannabinol and cannabidiol modulate mitogen-induced tryptophan degradation and neopterin formation in peripheral blood mononuclear cells in vitro. J Neuroimmunol. 2009;207:75–82. doi:10.1016/j.jneuroim.2008.12.004
62. Kozela E, Lev N, Kaushansky N, et al. Cannabidiol inhibits pathogenic T cells, decreases spinal microglial activation and ameliorates multiple sclerosis-like disease in C57BL/6 mice. Br J Pharmacol. 2011;163:1507–1519. doi:10.1111/j.1476-5381.2011.01379.x
63. Jan TR, Kaminski NE. Role of mitogen-activated protein kinases in the differential regulation of interleukin-2 by cannabinol. J Leukoc Biol. 2001;69:841–849.
64. Chen W, Kaplan BLF, Pike ST, et al. Magnitude of stimulation dictates the cannabinoid-mediated differential T cell response to HIV gp120. J Leukoc Biol. 2012;92:1093–1102. doi:10.1189/jlb.0212082
65. Devinsky O, Patel AD, Thiele EA, et al. Randomized, dose-ranging safety trial of cannabidiol in Dravet syndrome. Neurology. 2018;90:e1204–e1211. doi:10.1212/WNL.0000000000005254
66. Elliott DM, Singh N, Nagarkatti M, Nagarkatti PS. Cannabidiol attenuates experimental autoimmune encephalomyelitis model of multiple sclerosis through induction of myeloid-derived suppressor cells. Front Immunol. 2018;9. doi:10.3389/fimmu.2018.01782
67. Selvi E, Lorenzini S, Garcia-Gonzalez E, et al. Inhibitory effect of synthetic cannabinoids on cytokine production in rheumatoid fibroblast-like synoviocytes. Clin Exp Rheumatol. 2008;26:574–581.
68. Kinsey SG, Naidu PS, Cravatt BF, Dudley DT, Lichtman AH. Fatty acid amide hydrolase blockade attenuates the development of collagen-induced arthritis and related thermal hyperalgesia in mice. Pharmacol Biochem Behav. 2011;99:718–725. doi:10.1016/j.pbb.2011.06.022
69. Gentili M, Ronchetti S, Ricci E, et al. Selective CB2 inverse agonist JTE907 drives T cell differentiation towards a Treg cell phenotype and ameliorates inflammation in a mouse model of inflammatory bowel disease. Pharmacol Res. 2019;141:21–31. doi:10.1016/j.phrs.2018.12.005
70. Malfait AM, Gallily R, Sumariwalla PF, et al. The nonpsychoactive cannabis constituent cannabidiol is an oral anti-arthritic therapeutic in murine collagen-induced arthritis. Proc Natl Acad Sci U S A. 2000;97:9561–9566. doi:10.1073/pnas.160105897
71. Shapiro CM, Orlina AR, Unger P, Billings AA. Antibody response to cannabis. JAMA. 1974;230:81–82. doi:10.1001/jama.1974.03240010049030
72. McHugh D, Tanner C, Mechoulam R, Pertwee RG, Ross RA. Inhibition of human neutrophil chemotaxis by endogenous cannabinoids and phytocannabinoids: evidence for a site distinct from CB1 and CB 2. Mol Pharmacol. 2008;73:441–450. doi:10.1124/mol.107.041863
73. Walter L, Franklin A, Witting A, et al. Nonpsychotropic cannabinoid receptors regulate microglial cell migration. J Neurosci. 2003;23:1398–1405. doi:10.1523/JNEUROSCI.23-04-01398.2003
74. Giudice ED, Rinaldi L, Passarotto M, et al. Cannabidiol, unlike synthetic cannabinoids, triggers activation of RBL-2H3 mast cells. J Leukoc Biol. 2007;81:1512–1522. doi:10.1189/jlb.1206738
75. Cassol OJ
76. El-Gohary M, Eid MA. Effect of cannabinoid ingestion (in the form of bhang) on the immune system of high school and university students. Hum Exp Toxicol. 2004;23:149–156. doi:10.1191/0960327104ht426oa
77. Jan TR, Su ST, Wu HY, Liao MH. Suppressive effects of cannabidiol on antigen-specific antibody production and functional activity of splenocytes in ovalbumin-sensitized BALB/c mice. Int Immunopharmacol. 2007;7:773–780. doi:10.1016/j.intimp.2007.01.015
78. Dotsey E, Ushach I, Pone E, et al. Transient cannabinoid receptor 2 blockade during immunization heightens intensity and breadth of antigen-specific antibody responses in young and aged mice. Sci Rep. 2017;7. doi:10.1038/srep42584
79. Maresz K, Pryce G, Ponomarev ED, et al. Direct suppression of CNS autoimmune inflammation via the cannabinoid receptor CB1 on neurons and CB2 on autoreactive T cells. Nat Med. 2007;13:492–497. doi:10.1038/nm1561
80. Rieder SA, Chauhan A, Singh U, Nagarkatti M, Nagarkatti P. Cannabinoid-induced apoptosis in immune cells as a pathway to immunosuppression. Immunobiology. 2010;215:598–605. doi:10.1016/j.imbio.2009.04.001
81. Schwarz H, Blanco FJ, Lotz M. Anadamide, an endogenous cannabinoid receptor agonist inhibits lymphocyte proliferation and induces apoptosis. J Neuroimmunol. 1994;55:107–115. doi:10.1016/0165-5728(94)90152-X
82. Chiurchiù V, Lanuti M, Catanzaro G, Fezza F, Rapino C, Maccarrone M. Detailed characterization of the endocannabinoid system in human macrophages and foam cells, and anti-inflammatory role of type-2 cannabinoid receptor. Atherosclerosis. 2014;233:55–63. doi:10.1016/j.atherosclerosis.2013.12.042
83. Staiano RI, Loffredo S, Borriello F, et al. Human lung-resident macrophages express CB 1 and CB 2 receptors whose activation inhibits the release of angiogenic and lymphangiogenic factors. J Leukoc Biol. 2016;99:531–540. doi:10.1189/jlb.3HI1214-584R
84. Lee WS, Erdelyi K, Matyas C, et al. Cannabidiol limits T cell–mediated chronic autoimmune myocarditis: implications to autoimmune disorders and organ transplantation. Mol Med. 2016;22:136–146. doi:10.2119/molmed.2016.00007
85. Persidsky Y, Fan S, Dykstra H, Reichenbach NL, Rom S, Ramirez SH. Activation of cannabinoid type two receptors (CB2) diminish inflammatory responses in macrophages and brain endothelium. J Neuroimmune Pharmacol. 2015;10:302–308. doi:10.1007/s11481-015-9591-3
86. Kishimoto S, Kobayashi Y, Oka S, Gokoh M, Waku K, Sugiura T. 2-Arachidonoylglycerol, an endogenous cannabinoid receptor ligand, induces accelerated production of chemokines in HL-60 cells. J Biochem. 2004;135:517–524. doi:10.1093/jb/mvh063
87. Roth MD, Castaneda JT, Kiertscher SM. Exposure to Δ9-tetrahydrocannabinol impairs the differentiation of human monocyte-derived dendritic cells and their capacity for T cell activation. J Neuroimmune Pharmacol. 2015;10:333–343. doi:10.1007/s11481-015-9587-z
88. Kapellos TS, Taylor L, Feuerborn A, et al. Cannabinoid receptor 2 deficiency exacerbates inflammation and neutrophil recruitment. FASEB J. 2019;33:6154–6167. doi:10.1096/fj.201802524R
89. Adhikary S, Kocieda VP, Yen JH, Tuma RF, Ganea D. Signaling through cannabinoid receptor 2 suppresses murine dendritic cell migration by inhibiting matrix metalloproteinase 9 expression. Blood. 2012;120:3741–3749. doi:10.1182/blood-2012-06-435362
90. Nam G, Jeong SK, Park BM, et al. Selective cannabinoid receptor-1 agonists regulate mast cell activation in an oxazolone-induced atopic dermatitis model. Ann Dermatol. 2016;28:22–29. doi:10.5021/ad.2016.28.1.22
91. Hegde VL, Singh UP, Nagarkatti PS, Nagarkatti M. Critical role of mast cells and peroxisome proliferator–activated receptor γ in the induction of myeloid-derived suppressor cells by marijuana cannabidiol in vivo. J Immunol. 2015;194:5211–5222. doi:10.4049/jimmunol.1401844
92. Robinson RH, Meissler JJ, Fan X, Yu D, Adler MW, Eisenstein TK. A CB2-selective cannabinoid suppresses T-cell activities and increases Tregs and IL-10. J Neuroimmune Pharmacol. 2015;10(2):318–332. doi:10.1007/s11481-015-9611-3
93. Stebulis JA, Johnson DR, Rossetti RG, Burstein SH, Zurier RB. Ajulemic acid, a synthetic cannabinoid acid, induces an antiinflammatory profile of eicosanoids in human synovial cells. Life Sci. 2008;83:666–670. doi:10.1016/j.lfs.2008.09.004
94. Peyravian N, Deo S, Daunert S, Jimenez JJ. Cannabidiol as a novel therapeutic for immune modulation. ImmunoTargets Ther. 2020;9:131–140. doi:10.2147/ITT.S263690
95. Correa F, Mestre L, Docagne F, Guaza C. Activation of cannabinoid CB 2 receptor negatively regulates IL-12p40 production in murine macrophages: role of IL-10 and ERK1/2 kinase signaling. Br J Pharmacol. 2005;145:441–448. doi:10.1038/sj.bjp.0706215
96. Abo-Elnazar S, Moaaz M, Ghoneim H, Molokhia T, El-Korany W. Th17/Treg imbalance in opioids and cannabinoids addiction: relationship to NF-κB activation in CD4+ T cells. Egypt J Immunol. 2014;21:33–47.
97. Castillo PE, Younts TJ, Chávez AE, Hashimotodani Y. Endocannabinoid signaling and synaptic function. Neuron. 2012;76:70–81. doi:10.1016/j.neuron.2012.09.020
98. Börner C, Smida M, Höllt V, Schraven B, Kraus J. Cannabinoid receptor type 1- and 2-mediated increase in cyclic AMP inhibits T cell receptor-triggered signaling. J Biol Chem. 2009;284:35450–35460. doi:10.1074/jbc.M109.006338
99. Katz-Talmor D, Katz I, Porat-Katz BS, Shoenfeld Y. Cannabinoids for the treatment of rheumatic diseases — where do we stand? Nat Rev Rheumatol. 2018;14:488–498. doi:10.1038/s41584-018-0025-5
100. Molina PE, Amedee AM, Winsauer P, Nelson S, Bagby G, Simon L. Behavioral, metabolic, and immune consequences of chronic alcohol or cannabinoids on HIV/AIDs: studies in the non-human primate SIV model. J Neuroimmune Pharmacol. 2015;10:217–232. doi:10.1007/s11481-015-9599-8
101. Da silva T, Hafizi S, Watts JJ, et al. In vivo imaging of translocator protein in long-term cannabis users. JAMA Psychiatry. 2019;76:1305–1313. doi:10.1001/jamapsychiatry.2019.2516
102. Di Marzo V. New approaches and challenges to targeting the endocannabinoid system. Nat Rev Drug Discov. 2018;17:623–639.
103. Śledziński P, Nowak-Terpiłowska A, Zeyland J. Cannabinoids in medicine: cancer, immunity, and microbial diseases. Int J Mol Sci. 2021;22:1–22.
104. Sarzi-Puttini P, Batticciotto A, Atzeni F, et al. Medical cannabis and cannabinoids in rheumatology: where are we now? Expert Rev Clin Immunol. 2019;15:1019–1032. doi:10.1080/1744666X.2019.1665997
105. Fitzcharles M-A, Ste-Marie PA, Clauw DJ, et al. Rheumatologists lack confidence in their knowledge of cannabinoids pertaining to the management of rheumatic complaints. BMC Musculoskelet Disord. 2014;15:258. doi:10.1186/1471-2474-15-258
106. Richardson D, Pearson RG, Kurian N, et al. Characterisation of the cannabinoid receptor system in synovial tissue and fluid in patients with osteoarthritis and rheumatoid arthritis. Arthritis Res Ther. 2008;10:R43. doi:10.1186/ar2401
107. Lowin T, Tingting R, Zurmahr J, Classen T, Schneider M, Pongratz G. Cannabidiol (CBD): a killer for inflammatory rheumatoid arthritis synovial fibroblasts. Cell Death Dis. 2020;11:1–11. doi:10.1038/s41419-020-02892-1
108. Atzeni F, Nucera V, Masala IF, Sarzi-Puttini P, Bonitta G. Il-6 Involvement in pain, fatigue and mood disorders in rheumatoid arthritis and the effects of Il-6 inhibitor sarilumab. Pharmacol Res. 2019;149:104402. doi:10.1016/j.phrs.2019.104402
109. Fonseca JE, Santos MJ, Canhão H, Choy E. Interleukin-6 as a key player in systemic inflammation and joint destruction. Autoimmun Rev. 2009;8:538–542. doi:10.1016/j.autrev.2009.01.012
110. Gui H, Liu X, Liu LR, Su DF, Dai SM. Activation of cannabinoid receptor 2 attenuates synovitis and joint distruction in collagen-induced arthritis. Immunobiology. 2015;220:817–822. doi:10.1016/j.imbio.2014.12.012
111. Kotschenreuther K, Waqué I, Yan S, et al. Cannabinoids drive Th17 cell differentiation in patients with rheumatic autoimmune diseases. Cell Mol Immunol. 2021;18:764–766. doi:10.1038/s41423-020-0437-4
112. Sarzi-Puttini P, Ablin J, Trabelsi A, Fitzcharles MA, Marotto D, Häuser W. Cannabinoids in the treatment of rheumatic diseases: pros and cons. Autoimmun Rev. 2019;18:102409. doi:10.1016/j.autrev.2019.102409
113. Blake DR, Robson P, Ho M, Jubb RW, McCabe CS. Preliminary assessment of the efficacy, tolerability and safety of a cannabis-based medicine (Sativex) in the treatment of pain caused by rheumatoid arthritis. Rheumatology. 2006;45:50–52. doi:10.1093/rheumatology/kei183
114. Webb M, Luo L, Ma JY, Tham CS. Genetic deletion of Fatty Acid Amide Hydrolase results in improved long-term outcome in chronic autoimmune encephalitis. Neurosci Lett. 2008;439:106–110. doi:10.1016/j.neulet.2008.04.090
115. Wen J, Ribeiro R, Tanaka M, Zhang Y. Activation of CB2 receptor is required for the therapeutic effect of ABHD6 inhibition in experimental autoimmune encephalomyelitis. Neuropharmacology. 2015;99:196–209. doi:10.1016/j.neuropharm.2015.07.010
116. De Lago E, Moreno-Martet M, Cabranes A, Ramos JA, Fernández-Ruiz J. Cannabinoids ameliorate disease progression in a model of multiple sclerosis in mice, acting preferentially through CB1 receptor-mediated anti-inflammatory effects. Neuropharmacology. 2012;62:2299–2308. doi:10.1016/j.neuropharm.2012.01.030
117. Paolicelli D, Direnzo V, Manni A, et al. Long-term data of efficacy, safety, and tolerability in a real-life setting of THC/CBD oromucosal spray-treated multiple sclerosis patients. J Clin Pharmacol. 2016;56:845–851. doi:10.1002/jcph.670
118. Celius EG, Vila C. The influence of THC:CBD oromucosal spray on driving ability in patients with multiple sclerosis-related spasticity. Brain Behav. 2018;8(5):e00962. doi:10.1002/brb3.962
119. Zettl UK, Rommer P, Hipp P, Patejdl R. Evidence for the efficacy and effectiveness of THC-CBD oromucosal spray in symptom management of patients with spasticity due to multiple sclerosis. Ther Adv Neurol Disord. 2016;9:9–30. doi:10.1177/1756285615612659
120. Baker D, Jackson SJ, Pryce G. Cannabinoid control of neuroinflammation related to multiple sclerosis. Br J Pharmacol. 2007;152:649–654. doi:10.1038/sj.bjp.0707458
121. Pesce M, D’Alessandro A, Borrelli O, et al. Endocannabinoid-related compounds in gastrointestinal diseases. J Cell Mol Med. 2018;22:706–715. doi:10.1111/jcmm.13359
122. Storr MA, Keenan CM, Zhang H, Patel KD, Makriyannis A, Sharkey KA. Activation of the cannabinoid 2 receptor (CB2) protects against experimental colitis. Inflamm Bowel Dis. 2009;15:1678–1685. doi:10.1002/ibd.20960
123. Massa F, Marsicano G, Hermann H, et al. The endogenous cannabinoid system protects against colonic inflammation. J Clin Invest. 2004;113:1202–1209. doi:10.1172/JCI200419465
124. D’Argenio G, Valenti M, Scaglione G, Cosenza V, Sorrentini I, Di Marzo V. Up-regulation of anandamide levels as an endogenous mechanism and a pharmacological strategy to limit colon inflammation. FASEB J. 2006;20:568–570. doi:10.1096/fj.05-4943fje
125. Sałaga M, Mokrowiecka A, Zakrzewski PK, et al. Experimental colitis in mice is attenuated by changes in the levels of endocannabinoid metabolites induced by selective inhibition of fatty acid amide hydrolase (FAAH). J Crohns Colitis. 2014;8:998–1009. doi:10.1016/j.crohns.2014.01.025
126. Alhouayek M, Lambert DM, Delzenne NM, Cani PD, Muccioli GG. Increasing endogenous 2-arachidonoylglycerol levels counteracts colitis and related systemic inflammation. FASEB J. 2011;25:2711–2721. doi:10.1096/fj.10-176602
127. Engel MA, Kellermann CA, Burnat G, Hahn EG, Rau T, Konturek PC. Mice lacking cannabinoid CB1-, CB2-receptors or both receptors show increased susceptibility to trinitrobenzene sulfonic acid [TNBS]-induced colitis. J Physiol Pharmacol. 2010;61:89–97.
128. Marquéz L, Suárez J, Iglesias M, Bermudez-Silva FJ, Rodríguez de Fonseca F, Andreu M. Ulcerative colitis induces changes on the expression of the endocannabinoid system in the human colonic tissue. PLoS One. 2009;4:e6893. doi:10.1371/journal.pone.0006893
129. Duncan M, Mouihate A, Mackie K, et al. Cannabinoid CB2 receptors in the enteric nervous system modulate gastrointestinal contractility in lipopolysaccharide-treated rats. Am J Physiol Gastrointest Liver Physiol. 2008;295:G78–G87. doi:10.1152/ajpgi.90285.2008
130. Couch DG, Cook H, Ortori C, Barrett D, Lund JN, O’Sullivan SE. Palmitoylethanolamide and cannabidiol prevent inflammation-induced hyperpermeability of the human gut in vitro and in vivo-a Randomized, Placebo-controlled, Double-blind Controlled Trial. Inflamm Bowel Dis. 2019;25:1006–1018. doi:10.1093/ibd/izz017
131. Lal S, Prasad N, Ryan M, et al. Cannabis use amongst patients with inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2011;23:891–896. doi:10.1097/MEG.0b013e328349bb4c
132. Storr M, Devlin S, Kaplan GG, Panaccione R, Andrews CN. Cannabis use provides symptom relief in patients with inflammatory bowel disease but is associated with worse disease prognosis in patients with Crohn’s disease. Inflamm Bowel Dis. 2014;20:472–480. doi:10.1097/01.MIB.0000440982.79036.d6
133. Allegretti JR, Courtwright A, Lucci M, Korzenik JR, Levine J. Marijuana use patterns among patients with inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:2809–2814. doi:10.1097/01.MIB.0000435851.94391.37
134. Mbachi C, Attar B, Oyenubi O, et al. Association between cannabis use and complications related to ulcerative colitis in hospitalized patients: a propensity matched retrospective cohort study. Med (United States). 2019;98. doi:10.1097/MD.0000000000016551
135. Benson MJ, Abelev SV, Connor SJ, et al. Medicinal cannabis for inflammatory bowel disease: a survey of perspectives, experiences, and current use in Australian patients. Crohns Colitis. 2020;2:1–15.
136. Naftali T, Lev LB, Yablekovitz D, Half E, Konikoff FM. Treatment of Crohn’s disease with cannabis: an observational study. Isr Med Assoc J. 2011;13:455–458.
137. Naftali T, Mechulam R, Marii A, et al. Low-dose cannabidiol is safe but not effective in the treatment for Crohn’s disease, a Randomized Controlled Trial. Dig Dis Sci. 2017;62:1615–1620. doi:10.1007/s10620-017-4540-z
138. Naftali T, Bar-Lev Schleider L, Dotan I, Lansky EP, Sklerovsky Benjaminov F, Konikoff FM. Cannabis induces a clinical response in patients with crohn’s disease: a prospective placebo-controlled study. Clin Gastroenterol Hepatol. 2013;11(10):1276–1280.e1. doi:10.1016/j.cgh.2013.04.034
139. Irving PM, Iqbal T, Nwokolo C, et al. A Randomized, Double-blind, Placebo-controlled, Parallel-group, Pilot Study of Cannabidiol-rich Botanical Extract in the Symptomatic Treatment of Ulcerative Colitis. Inflamm Bowel Dis. 2018;24:714–724. doi:10.1093/ibd/izy002
140. Naftali T, Bar-Lev Schleider L, Sklerovsky Benjaminov F, Lish I, Konikoff FM, Ringel Y. Medical cannabis for inflammatory bowel disease: real-life experience of mode of consumption and assessment of side-effects. Eur J Gastroenterol Hepatol. 2019;31:1376–1381. doi:10.1097/MEG.0000000000001565
141. Giorgi V, Bongiovanni S, Atzeni F, Marotto D, Salaffi F, Sarzi-Puttini P. Adding medical cannabis to standard analgesic treatment for fibromyalgia: a prospective observational study. Clin Exp Rheumatol. 2020;38:53–59.
142. Katz D, Katz I, Porat-Katz BS, Shoenfeld Y. Medical cannabis: another piece in the mosaic of autoimmunity? Clin Pharmacol Ther. 2017;101:230–238. doi:10.1002/cpt.568
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
