Back to Journals » Clinical Ophthalmology » Volume 17
Canaloplasty and Trabeculotomy with the OMNI System in Patients with Open-Angle Glaucoma: Two-Year Results from the ROMEO Study
Authors Williamson BK , Vold SD, Campbell A, Hirsch L, Selvadurai D, Aminlari AE, Cotliar J, Dickerson Jnr JE
Received 10 February 2023
Accepted for publication 28 March 2023
Published 6 April 2023 Volume 2023:17 Pages 1057—1066
DOI https://doi.org/10.2147/OPTH.S407918
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Blake K Williamson,1 Steven D Vold,2 Anita Campbell,3 Louis Hirsch,4 Deepan Selvadurai,5 Ardalan E Aminlari,6 Jeremy Cotliar,7 Jaime E Dickerson Jnr8,9
1The Williamson Eye Center, Baton Rouge, LA, USA; 2Vold Vision, Fayetteville, AR, USA; 3Grene Vision Group, Wichita, KS, USA; 4Mercy Eye Specialists, Springfield, MO, USA; 5Buffalo Ophthalmology, Williamsville, NY, USA; 6Morris Eye Group, Encinitas, CA, USA; 7Arthur M Cotliar Eye Care and Surgery, New York, NY, USA; 8North Texas Eye Research Institute, University of North Texas Health Science Center, Fort Worth, TX, USA; 9Sight Sciences, Inc., Menlo Park, CA, USA
Correspondence: Jaime E Dickerson Jnr, Email [email protected]
Purpose: To provide extended safety and effectiveness follow-up for eyes treated with circumferential canaloplasty and trabeculotomy (CP+TR) that were included in the 12-month ROMEO study.
Setting: Seven multi-subspecialty ophthalmology practices located in 6 states (Arkansas, California, Kansas, Louisiana, Missouri, and New York).
Design: Retrospective, multicenter, IRB approved.
Subjects: Eligible eyes had mild–moderate glaucoma and were treated with CP+TR with cataract surgery or as a standalone intervention.
Methods: Main outcome measures were mean IOP, mean number of ocular hypotensive medications, mean change in number of medications, proportion of patients with a ≥ 20% reduction in IOP or with IOP ≤ 18 mmHg, and proportion of patients medication free. Safety outcomes were adverse events and secondary surgical interventions (SSI).
Results: Eight surgeons at 7 centers contributed 72 patients stratified by pre-operative intraocular pressure (IOP); > 18 mmHg (Grp1), ≤ 18 mmHg (Grp2). Mean follow-up of 2.1 years (min 1.4, max 3.5). 2-year IOP (SD) was 15.6 mmHg (− 6.1 mmHg, − 28% from baseline) on 1.4 medications (− 0.9, − 39%) for Grp1 with cataract surgery; 14.7 mmHg (− 7.4 mmHg, − 33% from baseline) on 1.6 medications (− 0.7, − 15%) for Grp1 standalone, 13.7 mmHg (− 0.6 mmHg, − 4.2%) on 1.2 medications (− 0.8, − 35%) for Grp2 with cataract surgery, 13.3 mmHg (− 2.3 mmHg, − 14.7%) on 1.2 medications (− 1.0, − 46%) for Grp2 standalone. The proportion of patients at 2 years with either a ≥ 20% IOP reduction or IOP between 6 and 18 mmHg and no increase in medication or SSI was 75% (54 of 72, 95% CI 69.9%, 80.1%). One-third of patients (24 of 72) were medication free whereas 9 of 72 were pre-surgical. No device-related adverse events during extended follow-up; 6 eyes (8.3%) required additional surgical or laser intervention for IOP control after 12 months.
Conclusion: CP+TR provides effective IOP control that is sustained for 2 years or more.
Keywords: canaloplasty, trabeculotomy, MIGS, OMNI, glaucoma
Introduction
The ROMEO (Retrospective, Observational, Multicenter, Evaluation of OMNI) study was the first multicenter clinical evaluation of canaloplasty followed by trabeculotomy using the OMNI® Surgical System (OSS, Sight Sciences, Menlo Park, CA, USA) for treatment of mild to moderate open-angle glaucoma (OAG) as a standalone procedure in pseudophakic eyes or combined with phacoemulsification cataract surgery. The results from ROMEO were the basis for its FDA-cleared indication “to reduce intraocular pressure in adult patients with primary open‐angle glaucoma”. While the strengths of the ROMEO study included data collection at multiple investigative sites from multiple surgeons across several US states, and outcomes derived from actual day to day clinical usage in the treatment of glaucoma patients rather than study subjects, follow-up was limited to 12 months, a relatively short period considering that glaucoma is a life-long disease.
Longer follow-up is desirable, and indeed essential, to assess the durability of IOP-lowering effectiveness, and for the detection of potential delayed safety signals.1,2 Many micro-invasive glaucoma surgery (MIGS) device or procedure studies are limited to just 6–12 months of follow-up, although there are a few exceptions, notably the PMA trials (2-year) with post-approval safety extensions through 5 years required by the US Food and Drug Administration for the MIGS implants.3,4 The paucity of longer-term data is often cited as an important gap in the evidence available for MIGS.5
The aim of the present study was to return to several of the ROMEO investigative sites and collect additional follow-up on the ROMEO patients to assess durability of the treatment effect and monitor safety through 2 years post-surgery.
Methods
This was a multicenter, retrospective, stratified, observational study of all eyes meeting eligibility criteria. Seven of 11 original ROMEO study sites participated in this study, representing 86% of the original enrollment (111 of 129) in ROMEO. Patients were stratified into two groups: baseline (BL) IOP >18 mmHg (Group 1), and BL IOP ≤18 mmHg (Group 2). All surgeries took place between March 14, 2018 and June 20, 2019.
Design of the study, including datapoints, eligibility criteria, and 100% source data verification, was based on the FDA guidance document, Use of Real-World Evidence to Support Regulatory Decision-Making for Medical Devices, August 31, 2017. This study included all patients from the ROMEO study at the participating sites for whom a minimum of 18 months follow-up data was available, ie, not lost to follow-up.
Eligibility criteria for ROMEO have been described in detail elsewhere.6,7 In brief, these were: underwent canaloplasty and trabeculotomy with OSS; had a diagnosis of open-angle glaucoma including pigmentary (PG) and pseudoexfoliative glaucoma (PXG); visual field mean deviation (Humphrey) was not worse than −12 dB; cup to disc ratio was not worse than 0.9; on 0–4 topical ocular hypotensive medications at the pre-operative baseline (BL); and a pre-operative medicated IOP ≤36 mmHg.
Inclusion in the present extension study required a minimum of 18 months of follow-up unless there had been a secondary surgical intervention for IOP control (SSI) prior to 18 months; such patients were treated as failures for binary endpoints (eg ≥20% IOP reduction); IOP and medication use data subsequent to the SSI were not included in the endpoint analyses. Only one eye per patient could have been enrolled in ROMEO.
Additional data collected at 18 months or longer included IOP (Goldmann), ocular hypotensive medication use, best corrected visual acuity (Snellen), adverse events, and any secondary laser or surgical procedures for IOP control (SSI).
The study was reviewed by the WCG Institutional Review Board (Puyallup, WA, USA) and waiver of consent was granted due to the retrospective non-interventional nature of the study. All patient data was treated with confidentiality, in accordance with the Declaration of Helsinki. This study is not considered an “Applicable Clinical Trial” under 42 CFR 11.22(b) and was therefore not required to be listed on clinicaltrials.gov.
All surgeries for eyes with extended follow-up were performed by the authors (BW, SV, AC, LH, DS, AA, JC). The surgical technique for the OSS was described in detail previously.6,7 The procedure was performed via a temporal clear corneal incision (~2 mm) (the same incision as created for the cataract surgery for combined cases) The anterior chamber (AC) was irrigated with 2% lidocaine and deepened with viscoelastic. The OSS was introduced through the incision into the AC. A small <1 mm goniotomy was created with the cannula tip in the nasal trabecular meshwork allowing access to Schlemm’s canal. The microcatheter was advanced for 180° and, when retracted, viscoelastic was delivered dilating the canal and distal outflow pathway. This was repeated for the opposite hemisphere to viscodilate 360°, if desired. Advancing the microcatheter again and then withdrawing by removal of the OSS through the corneal incision unroofed Schlemm’s canal creating the trabeculotomy. The AC was then irrigated with BSS removing viscoelastic, and the AC was pressurized. Typical post-operative treatment included a topical steroid, and antibiotic.
Endpoints for this descriptive, extended follow-up study included mean IOP, median percent change in IOP from pre-operative BL, mean number of ocular hypotensive medications, mean change in the number of medications, proportion of patients with a ≥20% reduction from BL in IOP, proportion of patients with IOP ≤18 mmHg, and proportion of patients medication free. Endpoints were planned to be stratified by type of procedure (combined with cataract surgery or standalone in pseudophakic patients), and by BL IOP (>18 mmHg, Group 1; ≤18 mmHg, Group 2) to better assess effectiveness in these two IOP subgroups with different treatment goals. The selection of 18 mmHg as the boundary between these two strata was made a priori and based on the findings of AGIS 7 regarding the overall lack of progression, on average, when IOP was consistently below 18 mmHg.8 The proportion of subjects with IOP ≤18 mmHg and ≥6 mmHg OR with a ≥20% reduction from pre-operative BL IOP and on the same or fewer number of ocular hypotensive medications compared to the pre-operative BL and with no additional IOP-lowering surgery or laser (the primary endpoint from ROMEO) was also assessed for the extended follow-up period.
All ocular adverse events for the study eye were recorded, including clinically significant hyphema (layered >1mm, persistent for 1 week or more, and/or requiring intervention), loss of two or more lines of best corrected visual acuity at or after 3 months post-operative (BCVA, Snellen), IOP elevation ≥10 mmHg above pre-operative BL at >30 days post-operative, secondary surgical or laser intervention for IOP control (SSI) and slit lamp and fundus abnormalities.
All subjects were included in the analysis set. The study was designed as a descriptive study with no a priori hypotheses. SIgmaStat 4.0 was used for all analyses (Systat Software Inc., Palo Alto, CA, USA). Mean, median (where appropriate), standard deviation, maximum, and minimum are provided for continuous variables; percentage and 95% confidence intervals are provided for categorical variables. Comparisons between BL and follow-up time points were made using t-tests, or where equal variance (Brown–Forsythe) or normality (Shapiro–Wilk) tests failed, Mann–Whitney rank sum tests. Significance was set at 0.05 and adjusted using the Bonferroni method in the case of multiple comparisons, eg to P=0.00313 in the case of comparisons of IOP or medications at all 4 time points and for the 4 subgroups versus BL. For subjects that required an SSI, IOP measurements and medication usage data subsequent to the SSI were excluded from the analysis set; these patients were treatment failures for binary endpoints. Missing data due to a procedure not being done or a missed visit were treated as missing. No imputation was carried out.
Results
Longer-term follow-up data was available for 72 of the original 111 ROMEO patients from the participating centers (65%; 56% of all 129 original ROMEO patients), referred to herein as “consistent cohort” (CC). Average length of follow-up was 2.1±0.45 years. This extended follow-up data is therefore referred to as “2 year”. The attrition due to lost to follow-up was 35% and is due in part to the retrospective study design (patients are not scheduled for study visits or reminded to attend visits as they would be in a prospective clinical trial), and in part to the coincidence in time of post-year one follow-up and the first year of the COVID-19 pandemic, with many patients reluctant to make routine clinic visits. Patient demographics and BL characteristics (Table 1) were similar to the original full cohort from ROMEO: mean age was 72.1 for both, majority female in both (60% and 53%), primarily POAG (93.1% and 94.5%), and mostly white, 75% for both.
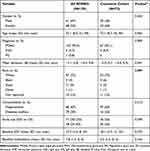 |
Table 1 Baseline Characteristics and Demographics |
Effectiveness
The proportion of patients at 2 years with either a ≥20% IOP reduction or an IOP between 6 and 18 mmHg and no increase in medication or SSI was 75% (54 of 72, 95% CI 69.9%, 80.1%), a decrease from the proportion for the same patients at 12 months (61 of 72, 84.7%, 95% CI 80.5%, 88.9%) but a modest decrease from the overall ROMEO population (100 of 129, 77.5%, 95% CI 70.1%, 84.9%).
The CC patients all came from 7 of the original 11 ROMEO sites, which were generally higher enrolling centers. Comparison of IOP outcomes between the CC and the full ROMEO dataset showed no significant differences at BL or any follow-up visit through Month 12 (Figure 1). Moreover, IOP at Month 12 for the 18 patients at the non-participating sites was similar to that for the patients treated at the CC sites (14.4 vs 14.3 mmHg), while medication usage was slightly lower (0.7 vs 1.1 medications).
Group 1: Baseline IOP >18 mmHg
Mean medicated IOP for each timepoint is depicted in Figure 1A and C. Mean BL IOP was 21.7 and 22.1 mmHg for the combined with cataract surgery and standalone subgroups, which decreased to 15.0 and 16.3 mmHg at 12 months (−6.7 and −5.8 mmHg, respectively) and 15.6 and 14.7 mmHg at 2 years (−6.1 and −7.4 mmHg, respectively). These differences were all statistically significant (P<0.001). The median percent reduction in IOP (95% CI) from BL was 24% (9.4%, 38.2%) at Month 12 and 30% (13.6%, 46.4%) at Year 2 (n=34 Month 12, n=30 Year 2) for all Group 1 patients.
Patient level outcomes for all Group 1 patients excepting 4 with an SSI are shown as a scatterplot (Figure 2) of BL versus Year 2 IOP. With a single exception (BL IOP=22 mmHg on 3 medications, Year 2 IOP=24 mmHg on 1 medication), all patients had a lower IOP at Year 2 than at BL and were at or below 18 mmHg at Year 2 whereas none were at BL.
Most patients in Group 1 achieved a 20% or greater reduction in IOP at Month 12 (22 of 34, 64.7%, CI 49.4%, 80.0%) and at Year 2 (24 of 34, 70.6%, CI 55.3%, 85.9%), with slightly lower percentages for those on the same or fewer medications as at BL (19 of 34, 55.9%, CI 43.2%, 68.6% and 20 of 34, 58.8%, CI 42.3%, 75.3%) (Table 2). Most patients had Month 12 and Year 2 IOP less than or equal to 18 mmHg (Month 12, 31 of 34, 91.2%, CI 81.6%, 100; Year 2, 29 of 34, 85.3%, CI 73.3%, 97.3%), with somewhat lower proportions considering only those on the same or fewer medications (Month 12, 28 of 34, 82.4%, CI 69.5%, 95.3%; Year 2, 79.4%, CI 65.7, 93.1%). Results for the combined with cataract surgery and standalone procedure subgroups are provided in Table 2.
 |
Table 2 Responder Analyses Groups 1 and 2 |
Group 2: Baseline IOP ≤18 mmHg
Group 2 patients remained controlled with modest, albeit not statistically significant, decreases in IOP from BL, through 2 years (Figure 1B and D). The median percent reduction in IOP (95% CI) from BL was 3% (−2.7%, 8.7%) at Month 12 and 9% (−0.3%, 18.3%) at Year 2 for all Group 2 patients (n=38 and 36). Of Group 2 patients, 44.7% (CI 28.8%, 60.6%) had a 20% or greater reduction in IOP at Year 2 (Table 2). Like Group 1, the majority of patients had IOP less than or equal to 18 mmHg on the same or fewer medications, which remained between 69 and 75% at Year 2 depending on the subgroup (Table 2). Figure 2 shows patient level outcomes at Year 2 relative to BL. In this group with BL IOP ≤18 mmHg, the majority of patients (31 of 36) remained controlled (ie IOP ≤18 mmHg) at Year 2, with twice as many patients experiencing a decrease in IOP (23 of 36) than an increase (11 of 36). Two patients had no change in IOP.
Ocular Hypotensive Medications
Medications required for IOP control at 2 years were similar to 12 months considering the CC as a whole (1.24 at 12 months, 1.33 at 2 years, P=0.645), with the Year 2 outcome representing an average decrease from pre-operative BL of 0.72 medications (P <0.001). Results for each of the subgroups (combined with cataract groups 1 and 2, standalone groups 1 and 2) were consistent with this overall trend; slightly greater (but not statistically significant) medication usage at 2 years than at 12 months while remaining statistically significantly lower than at BL (Table 2). The sole exception was standalone Group 2, where medication usage at 2 years was numerically lower than at 12 months (1.18 versus 1.33, P=0.78). The number of patients on 1, 2, 3, or ≥4 medications is shown in Figure 3. While half of patients (36 of 72) were on 1 or 2 medications at BL, and this proportion remained fairly consistent through month 12 and year 2 (47.2% and 43.9%, respectively), the proportion on no medications increased from 12.5% at BL to 34.7% (2.8X) and 33.3% (2.7X) at month 12 and 2 years. Conversely, there were 16.7% of patients on 4 or more medications at BL, which decreased to 5.6% at month 12 and just 3% at 2 years.
 |
Figure 3 Number of patients on 1, 2, 3, or ≥4 medications at baseline (BL), 12 months (M12), and Year 2 (2Y). |
Five of the 72 patients had a diagnosis of pseudoexfoliation glaucoma; two each in combined with cataract surgery Groups 1 and 2, one in the standalone Group 2 cohort. Subgroup analyses of only POAG patients (67 of 72) had IOP and medication results essentially identical to those for the overall study population (Table 3).
 |
Table 3 IOP and IOP-Lowering Medication Usage: All Patients and POAG Subgroup |
Safety
Safety outcomes from the ROMEO study including intra-operative and post-operative adverse events through 12 months have been reported in detail previously.6,7 Over the first 12 months, adverse events were typical for cataract or angle surgery and included mild inflammation (14 of 129, 11%), IOP spikes (6 of 129, 5%), and clinically significant hyphema (layered >1mm, persistent for 1 week or more, and/or requiring intervention; 5 of 129, 4%). There were no device-related adverse events over the post-12-month period.
Nine subjects of the original 129 required an SSI during the first post-operative year, a rate of 7%.6,7 Between Month 12 and Year 2, an additional 6 of 72 (8.3%) had an SSI. These were an SLT for a patient with an IOP of 14 but on 4 medications at Month 12; a Baerveldt device for a patient with IOP of 22 on 3 medications at Month 12; SLT where IOP was 21 on 1 medication at Month 12; SLT where IOP was 16 on no medications (2 patients) at Month 12; and an XEN gel stent where IOP was 19.5 on 1 medication at Month 12.
Discussion
Glaucoma is a life-long, chronic disease that often progressively worsens despite the best efforts of ophthalmologists. Available treatments include topical medications for IOP-lowering and selective laser trabeculoplasty as first line treatment options, minimally invasive glaucoma surgery and ciliary ablation procedures, which are generally employed where first-line treatments are not sufficiently effective, and traditional glaucoma surgeries, including trabeculectomy and tube-shunts, where less invasive or medical approaches have failed to provide the necessary level of IOP control.9 The longevity of a given treatment’s effectiveness is thus a key factor in the therapeutic decision-making process and longer-term data is critical to understanding this longevity. Many MIGS procedures and devices, particularly non-implants, are relatively new and received marketing authorizations through the 510(k) regulatory pathway without a requirement for either a large randomized controlled trial or long-term (>3-year) follow-up data. Nevertheless, with time, ophthalmic surgeons are gaining more clinical experience with these procedures and publishing their findings.
Gallardo et al published single-center 24-month data for a series of 53 patients (60 eyes, 46 with 24-month data) treated with ab interno canaloplasty using the iTrack microcatheter. IOP was essentially unchanged from Month 12 to Month 24, 13.6 and 13.5 mmHg (baseline=20.7 mmHg), respectively, while medication usage at 24 months was intermediate between baseline and 12 months (baseline=2.8, 12 months=1.1, 24 months=1.7).10 A subsequent report of outcomes at 36 months (44 eyes of 44 patients) confirmed the stability of IOP (baseline=20.5 mmHg; 12, 24, 36 months=13.3, 13.1, and 13.3 mmHg, respectively) and medication use (baseline=2.8; 12, 24, 36 months=1.1, 1.0, and 1.3, respectively).11 Ondrejka et al reported 36-month outcomes for ab interno canaloplasty using the OSS or its predecessor, VISCO360, in a series of 206 eyes of 130 patients. Pre-operative baseline IOP was 21.1 mmHg on an average of 2.0 medications, which was reduced to an average of 15.3 mmHg on slightly less than 1 medication through the 36-month follow-up period. Figures in this publication show no indication of diminution of the IOP-lowering effect over this period.12 Klabe and Kaymak followed a series of 38 eyes from 27 patients that had undergone canaloplasty followed by trabeculotomy with the OSS for 24 months. Baseline IOP (washed out; 1.9 average medications prior to washout) was 24.6 mmHg and was 14.7 and 14.9 mmHg on an average 0.4 and 0.5 medications at 12 and 24 months, respectively.13 Three-year outcomes presented at the European Glaucoma Society 2022 annual meeting in Athens, Greece showed continued durability of the treatment effect (15.0 mmHg, 0.5 medications). Taken together, these studies show consistent post-operative IOP outcomes in the 15 mmHg range, and significant medication reduction sustained through 1, 2, and 3 years post-operatively.
The results of the present multicenter, multi-surgeon study agree with the single-center studies discussed above. IOP at 24 months remained similar to the IOP observed during the first 12 months of follow up. The difference observed between IOP at Month 12 and Month 24 was small across all 4 subgroups, with the high and low (Groups 1 and 2) baseline IOP combined with cataract surgery groups having small average increases of 0.6 and 0.3 mmHg and the Group 1 and Group 2 standalone groups showing modest decreases over this interval (−1.6 mmHg and −0.9 mmHg). While average IOP did not change significantly in any of these groups, it is interesting that the directionality of change, albeit small and not significant, was an increase only for the combined with cataract cohorts. This could reflect a waning of IOP-lowering effect provided by the cataract procedure itself. In a meta-analysis examining the effect of cataract surgery on IOP through 36 months, Armstrong et al noted that, “These effects appear to last at least 36 months with gradual loss of the initial effect noted after 2 years.”14
Between 12 and 24 months there were 6 patients for whom the surgeon deemed it necessary to perform an additional IOP-lowering procedure and were therefore considered “treatment failures” in our analysis. These included 2 patients with an IOP of 16 mmHg on no medications at Month 12. From a procedural perspective, it is difficult to consider these patients as failures since IOP was reduced at Month 12 by 3 and 9 mmHg, respectively, without any medications; in other words, the procedure did provide an IOP-lowering benefit, but perhaps not to the target deemed necessary for these individuals. Baseline mean deviation for these 6 patients was not remarkably different from that of the overall study population, with one exception. Five of the 6 had an MD of −5.8 dB or better (similar to the study average), while one had an MD of −10 dB. Data available for this analysis did not include sequential, historical visual fields that could possibly provide insight into rate of progression and additional context for secondary interventions.
We acknowledge that our study has some limitations. First, it is a retrospective chart review and therefore is dependent on data collected in routine medical practice and not for the purpose of research. This means that there is inherently more variability in the way in which procedures are performed and data collected than would be the case in a prospective trial. It also means that there is inevitably more missing data, including factors that could influence outcomes which were not recorded. Nevertheless, the study includes a relatively large number of patients from multiple investigative sites and multiple surgeons. We believe that such outcomes do provide a realistic view of the effectiveness of the OSS in actual clinical practice and, importantly, durability of the treatment effect beyond 12 months. Such information will be of great value to surgeons considering the OSS for use in their practices. Second, the extended follow-up reported herein includes only 7 of the original 11 ROMEO study sites. While the participating sites account for 86% of the enrollment, the potential for unintended bias must be acknowledged. As the non-participating sites were, in general, low enrollers in ROMEO, there is arguably the possibility that higher enrollment sites were more experienced with the OSS, which might lead to a bias toward better outcomes reported here. We believe this unlikely based on two observations: comparison of outcomes for the first 12 months from the overall ROMEO study population with those for the consistent cohort included in this study found no differences; 12-month mean IOP for the 18 patients at the non-participating sites was essentially identical to the mean for the 111 patients from participating sites (14.4 vs 14.3 mmHg). Third, there was a 35% lost to follow-up rate at 24 months. While significant loss to follow-up is commonplace in retrospective studies15–17 and it is not possible to ascertain the reasons for loss to follow-up, drop-outs due to adverse events and non-response cannot be ruled out. However, attrition was not unexpected given the restrictions imposed on routine clinical visits at ophthalmic clinics due to the COVID-19 pandemic.
Safety and effectiveness data beyond 12 months supporting laser or surgical intervention in glaucoma is essential for surgeons in order to strategically plan individualized care for their patients with glaucoma. One size does not fit all, as stage of disease, degree of disability, age and life expectancy, ability to self-administer medications reliably, and cost of therapy must all be considered.18–20 The span of time that a given treatment can be reasonably expected to provide the necessary level of IOP control is a key element in this planning. The present study extends the follow-up for IOP, medication, and safety outcomes of the original 12-month ROMEO study6,7 to 24 months and demonstrates continued and stable control of IOP, medication usage similar to that seen at 12 months and significantly lower than the pre-surgical baseline, with a high level of safety. Ab interno canaloplasty and trabeculotomy with the OSS is therefore a reasonable treatment option in mild-to-moderate glaucoma used in conjunction with cataract surgery or as a standalone procedure with demonstrable safety and efficacy through at least 2 years.
Data Sharing Statement
The authors do not intend to share participant-level data. Other queries or requests should be directed to the corresponding author (JD).
Disclosure
This study was supported by Sight Sciences Inc., the manufacturer of the OMNI Surgical System. JED is an employee of Sight Sciences, Inc. SDV consults for Sight Sciences and reports grants from Sight Sciences, grants from Alcon, grants from Glaukos, grants, personal fees from Carl Zeiss Meditec, patent royalty from Iridex, grants, personal fees from AbbVie, patent royalty from Volk, grants from RxSight, grants, personal fees from iStar Medical, grants, personal fees from Intellon, grants from Bausch & Lomb, grants from Santen, during the conduct of the study. BKW consults and speaks for Sight Sciences. AC reports compensation as PI in ROMEO Study from Sight Sciences, during the conduct of the study; compensation as PI in ROMEO Study from Sight Sciences, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Caprioli J, Kim JH, Friedman DS, et al. Special commentary: supporting innovation for safe and effective minimally invasive glaucoma surgery: summary of a joint meeting of the American Glaucoma Society and the Food and Drug Administration, Washington, DC, February 26, 2014. Ophthalmology. 2015;122:1795–1801. doi:10.1016/j.ophtha.2015.02.029
2. Lass JH, Benetz BA, He J, et al. Corneal endothelial cell loss and morphometric changes 5 years after phacoemulsification with or without CyPass micro-stent. Am J Ophthalmol. 2019;208:211–218. doi:10.1016/j.ajo.2019.07.016
3. Ahmed IIK, De Francesco T, Rhee D, et al. Long-term outcomes from the HORIZON randomized trial for a Schlemm’s canal microstent in combination cataract and glaucoma surgery. Ophthalmology. 2022;129:742–751. doi:10.1016/j.ophtha.2022.02.021
4. Reiss G, Clifford B, Vold S, et al. Safety and effectiveness of CyPass supraciliary micro-stent in primary open-angle glaucoma: 5-year results from the COMPASS XT study. Am J Ophthalmol. 2019;208:219–225. doi:10.1016/j.ajo.2019.07.015
5. Vinod K, Gedde SJ. Safety profile of minimally invasive glaucoma surgery. Curr Opin Ophthalmol. 2021;32:160–168. doi:10.1097/ICU.0000000000000731
6. Hirsch L, Cotliar J, Vold S, et al. Canaloplasty and trabeculotomy ab interno with the OMNI system combined with cataract surgery in open-angle glaucoma: 12-month outcomes from the ROMEO study. J Cataract Refract Surg. 2021;47:907–915. doi:10.1097/j.jcrs.0000000000000552
7. Vold SD, Williamson BK, Hirsch L, et al. Canaloplasty and trabeculotomy with the OMNI system in pseudophakic patients with open-angle glaucoma: the ROMEO study. Ophthalmol Glaucoma. 2021;4:173–181. doi:10.1016/j.ogla.2020.10.001
8. The Advanced Glaucoma Intervention Study (AGIS). 7. The relationship between control of intraocular pressure and visual field deterioration.The AGIS Investigators. Am J Ophthalmol. 2000;130:429–440. doi:10.1016/S0002-9394(00)00538-9
9. Gedde SJ, Vinod K, Wright MM, et al. Primary open-angle glaucoma preferred practice pattern. Ophthalmology. 2021;128:P71–P150. doi:10.1016/j.ophtha.2020.10.022
10. Gallardo MJ. 24-month efficacy of viscodilation of Schlemm’s canal and the distal outflow system with iTrack ab-interno canaloplasty for the treatment of primary open-angle glaucoma. Clin Ophthalmol. 2021;15:1591–1599. doi:10.2147/OPTH.S272506
11. Gallardo MJ. 36-month effectiveness of ab-interno canaloplasty standalone versus combined with cataract surgery for the treatment of open-angle glaucoma. Ophthalmol Glaucoma. 2022;5:476–482. doi:10.1016/j.ogla.2022.02.007
12. Ondrejka S, Körber N, Dhamdhere K. Long term effect of canaloplasty on IOP and use of IOP-lowering medications in patients with open angle glaucoma: canaloplasty and IOP/medications in open angle glaucoma. J Cataract Refract Surg. 2022;48:1388–1393. doi:10.1097/j.jcrs.0000000000001000
13. Klabe K, Kaymak H. Standalone trabeculotomy and viscodilation of Schlemm’s canal and collector channels in open-angle glaucoma using the OMNI surgical system: 24-month outcomes. Clin Ophthalmol. 2021;15:3121–3129. doi:10.2147/OPTH.S325394
14. Armstrong JJ, Wasiuta T, Kiatos E, et al. The effects of phacoemulsification on intraocular pressure and topical medication use in patients with glaucoma: a systematic review and meta-analysis of 3-year data. J Glaucoma. 2017;26:511–522. doi:10.1097/IJG.0000000000000643
15. Ferguson TJ, Swan RJ, Bleeker A, et al. Trabecular microbypass stent implantation in pseudoexfoliative glaucoma: long-term results. J Cataract Refract Surg. 2020;46:1284–1289. doi:10.1097/j.jcrs.0000000000000243
16. Ferguson TJ, Mechels KB, Dockter Z, et al. iStent trabecular microbypass stent implantation with phacoemulsification in patients with open-angle glaucoma: 6-year outcomes. Clin Ophthalmol. 2020;14:1859–1866. doi:10.2147/OPTH.S247910
17. Polat J, Grantham L, Mitchell K, Realini T. Repeatability of selective laser trabeculoplasty. Br J Ophthalmol. 2016;100:1437–1441. doi:10.1136/bjophthalmol-2015-307486
18. Spaeth GL, Shields MB. The stages of glaucoma. Am J Ophthalmol. 2006;141:147–148. doi:10.1016/j.ajo.2005.08.026
19. Spaeth GL, Cvintal V, Figueiredo A. Is there a need for new surgical procedures for glaucoma? Yes! Open Ophthalmol J. 2015;9:101–103. doi:10.2174/1874364101509010101
20. European Glaucoma Society. Terminology and guidelines for glaucoma, 5th edition. Br J Ophthalmol. 2021;105(Suppl 1):1. doi:10.1136/bjophthalmol-2021-egsguidelines
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Canaloplasty and Trabeculotomy Combined with Phacoemulsification for Glaucoma: 12-Month Results of the GEMINI Study
Gallardo MJ, Pyfer MF, Vold SD, Sarkisian SR Jr, Campbell A, Singh IP, Flowers B, Dhamdhere K
Clinical Ophthalmology 2022, 16:1225-1234
Published Date: 21 April 2022
Interim Analysis of STREAMLINE® Surgical System Clinical Outcomes in Eyes with Glaucoma
Lazcano-Gomez G, Garg SJ, Yeu E, Kahook MY
Clinical Ophthalmology 2022, 16:1313-1320
Published Date: 27 April 2022
Short-Term Efficacy of Combined ab Interno Canaloplasty and Trabeculotomy in Pseudophakic Eyes with Open-Angle Glaucoma
Bleeker AR, Litchfield WR, Ibach MJ, Greenwood MD, Ristvedt D, Berdahl JP, Terveen DC
Clinical Ophthalmology 2022, 16:2295-2303
Published Date: 21 July 2022
A Multicenter 12-Month Retrospective Evaluation of Canaloplasty and Trabeculotomy in Patients with Open-Angle Glaucoma: The ROMEO 2 Study
Murphy III JT, Terveen DC, Aminlari AE, Dhamdhere K, Dickerson Jr JE
Clinical Ophthalmology 2022, 16:3043-3052
Published Date: 14 September 2022
Prospective Study of Canaloplasty and Trabeculotomy Performed by Trainees
Smith AK, Kwan CC, Fox A, Noh S, Gustafson K, Lin KY, Mosaed S
Clinical Ophthalmology 2024, 18:17-26
Published Date: 3 January 2024


