Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 17
Butyrate and Propionate are Negatively Correlated with Obesity and Glucose Levels in Patients with Type 2 Diabetes and Obesity
Authors Zhang S, Zhang Y, Li J, Wang X, Zhang M , Du M, Jiang W, Li C
Received 8 August 2023
Accepted for publication 28 February 2024
Published 2 April 2024 Volume 2024:17 Pages 1533—1541
DOI https://doi.org/10.2147/DMSO.S434499
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Konstantinos Tziomalos
Shi Zhang,1 Yanju Zhang,2 Jing Li,1 Xincheng Wang,1 Minying Zhang,3 Meiyang Du,1 Weiran Jiang,4 Chunjun Li1
1Department of Endocrinology, Health Management Center, Tianjin Union Medical Center, Nankai University Affiliated Hospital, Tianjin, People’s Republic of China; 2Graduate School, Tianjin University of Traditional Chinese Medicine, Tianjin, People’s Republic of China; 3School of Medicine, Nankai University, Tianjin, People’s Republic of China; 4Eastman Institute for Oral Health, University of Rochester Medical Center, Rochester, NY, USA
Correspondence: Chunjun Li, Department of Endocrinology, Health Management Center, Tianjin Union Medical Center, Nankai University Affiliated Hospital, Tianjin, People’s Republic of China, Email [email protected]
Background: Growing evidence has demonstrated the important roles of gut microbiota and short chain fatty acids, especially acetate, propionate and butyrate, in the development of obesity and metabolic diseases. To date, the effects of acetate, propionate and butyrate on human adiposity and glucose metabolism remain controversial. This study aimed to explore the associations of systemically acetate, propionate and butyrate with obesity and glucose homeostasis in patients with type 2 diabetes (T2D) and obesity.
Methods: A total of 12 patients with T2D and obesity and 8 age- and sex-matched healthy individuals with BMI < 24 kg/m2 were enrolled in this study. Height, weight, body composition, blood pressure, biochemical indices, a 75-g oral glucose tolerance test, and plasma acetate, propionate and butyrate were measured at baseline. Then, participants in T2D group were given a weight control therapy, in addition to conventional medication, and all the measurements were repeated 12 months from baseline. The direct segmental multi-frequency bioelectrical impedance analysis was used to assess body composition. Acetate, propionate and butyrate levels were determined by liquid chromatography coupled to tandem mass spectrometry.
Results: Butyrate concentration significantly increased from baseline after obvious weight loss (P< 0.05). Correlation analysis showed that propionate was negatively correlated with percent of body fat (PBF) and 2-h plasma glucose (2-h PG) (P< 0.05), and butyrate was negatively associated with body mass index, visceral fat area, PBF and 2-h PG (P< 0.05). No association was found between acetate and obesity.
Conclusion: Butyrate and propionate are negatively correlated with obesity and glucose levels in patients with T2D and obesity.
Keywords: obesity, type 2 diabetes, gut microbiota, short chain fatty acids
Introduction
The prevalence of obesity and its related metabolic disorders has been ascending dramatically in the past few decades worldwide.1 Obesity is the result of a long-term imbalance between energy intake and energy expenditure, characterized by excessive deposition of adipose tissue leading to increased release of free fatty acids into the circulation, chronic low-grade inflammation of the body and eventually the development of metabolic diseases such as type 2 diabetes (T2D).2 Obesity and T2D pandemic have become a major public health problem globally. The pathogenesis and treatment of obesity have been the focus of researches in the past few years. Recently, the relationship between obesity and its related metabolic disturbances and gut microbiota and short chain fatty acids (SCFAs) derived from the gut has been gradually recognized.3,4
SCFAs are the end products of intestinal microbial fermentation of undigested foods, and the distal ileum and proximal colon are generally the major production sites of SCFAs. After formation in the gut, SCFAs are absorbed through the colon and transferred into the systemic circulation. There are seven types of SCFAs in peripheral circulation, among which acetate, propionate and butyrate account for the most majority.5 Acetate, propionate and butyrate have been shown to play important roles in host energy metabolism and glucose homeostasis.6 The results of a meta-analysis suggested that acetate administration (apple cider vinegar consumption) had a favorable effect on glycemic parameters and lipid profiles.7 A study conducted by Yoshida et al showed that propionate might have the potential preventive and therapeutic effects on diabetes by suppressing gluconeogenesis.8 Wu et al found that the abundance of butyrate-producing bacteria significantly decreased in patients with obesity and T2D, and this result was independent of metformin treatment.9
Growing evidence has demonstrated that SCFAs exert the beneficial effects on appetite control, weight loss, insulin resistance improvement and glucose homeostasis maintainment via increasing the secretion of gut hormones, particularly glucagon-like peptide‑1 (GLP-1) and peptide YY (PYY).10 Nevertheless, most of these data come from animal and in vitro studies, and the effects of SCFAs on human adiposity and glucose metabolism remain controversial.11 Therefore, this study aimed to explore the associations between systemically SCFAs, mainly acetate, propionate and butyrate, and obesity and glucose homeostasis in patients with T2D and obesity.
Materials and Methods
Study Subjects
Patients aged 18 to 60 years with obesity and T2D who visited our outpatient clinic from January 2022 to May 2022 were selected as the study subjects. Obesity was defined as body mass index (BMI) ≥28 kg/m2 based on the Asia-Pacific diagnostic criteria for obesity.12 The diagnosis of T2D was made according to the Chinese Guidelines for the Prevention and Treatment of T2D (2020 Edition).13 After excluding patients suffering from severe cerebro-cardiovascular diseases or uncontrolled mental disorders, complicated with severe liver or kidney dysfunction, being pregnant or lactating, or unwilling to provide written informed consent, a total of 12 patients were enrolled in this study. Meanwhile, 8 age- and sex-matched healthy individuals with BMI <24 kg/m2 (the threshold for overweight diagnosis in the Asia-Pacific region) were selected as the controls. Height, weight, body composition, blood pressure, biochemical indices, a 75-g oral glucose tolerance test (OGTT), and plasma SCFAs, including acetate, propionate, butyrate, isobutyrate, valerate, isovalerate, and caproate, were measured at baseline. Then, participants in T2D group were given a weight management regimen including a high-protein diet and moderate exercise training, in addition to conventional medication. All the measurements were repeated in patients of T2D group 12 months from baseline.
The study was approved by the Ethics Committee of Tianjin Union Medical Center (approval number: 2021C06) and registered at www.chictr.org.cn (registration number: ChiCTR2100044305). All the participants provided written informed consent.
Demographic Data and Clinical Information Collection
Information on gender, age, ethnicity and previous medical history including hyperglycemia, nonalcoholic fatty liver disease (NAFLD), hypertension, hyperlipidemia and hyperuricemia were recorded in detail. After a 10-minute rest, blood pressure was measured three times with an automatic electronic sphygmomanometer, and the average value was taken.
Anthropometry and Body Composition Evaluation
All participants were asked to come to our clinic in a fasting state for height, weight and body composition assessments. BMI was determined via the value of height and weight. The direct segmental multi-frequency bioelectrical impedance analysis (BIA) (InBody 770, Bio-space Inc., Korea) was used to measure body composition, including visceral fat area (VFA) and percent of body fat (PBF). The detailed procedure for measurement of body composition had been described in our previous study.14
Biochemical Indices Determinations and Homeostatic Model Assessment of Insulin Resistance (HOMA-IR) Calculation
After an overnight fast, blood samples were collected. Biochemical indices including fasting plasma glucose (FPG), uric acid (UA), alanine aminotransferase (ALT), aspartate aminotransferase (AST), total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C) were detected by an automatic biochemical analyzer (TBA-120FR, Toshiba, Tokyo). Fasting insulin (FINS) was determined by chemiluminescent immunoassay. HOMA-IR was calculated as FPG (mmol/L) × FINS (IU/L)/22.5.15 Hemoglobin A1c (HbA1c) was measured by high-performance chromatography. After completing fasting blood sample collection, all participants received a 75-g OGTT and 2-h plasma glucose (2-h PG) and 2-h insulin (2-h INS) were determined.
SCFAs Measurements
Plasma concentrations of SCFAs, including acetate, propionate, butyrate, isobutyrate, valerate, isovalerate and caproate, were detected using fasting serum samples by liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS) (Waters ACQUITY UPLC I-Class, SCIEX QTRAP 6500).
Statistical Analysis
SPSS statistical software (version 27.0) was used for statistical analyses. Normally distributed continuous data were expressed as mean ± standard deviation, and non-normally distributed continuous data were presented as median (interquartile range). Categorical variables were indicated as numbers (percentages). For comparing the differences of baseline indices between the control and T2D group, independent sample t–test was performed to analyze the normally distributed data, and Mann–Whitney U–test was used for non-normally distributed data analysis. Unpaired t–test and Wilcoxon test were adopted to analyze the normally and non-normally distributed data of the T2D group before and after intervention. Categorical variables were compared using chi-squared test. Pearson correlation analysis was used to explore the association between SCFAs and obesity and some biochemical indices. P value <0.05 was considered to be statistically significant.
Results
Baseline Characteristics of the Control and T2D Group
A cohort of 12 patients who met the inclusion criteria were recruited as the T2D group, and 8 healthy individuals without overweight or obesity were included as the control group. There was no significant difference in the proportion of male and female subjects between the two groups. Other baseline characteristics, including age and ethnicity, were similar between groups. However, the incidence of cardiometabolic diseases, such as NAFLD, hypertension, hyperlipidemia and hyperuricemia, was significantly higher in the T2D group, while none of these diseases, including hyperglycemia, was present in subjects of the control group. The baseline characteristics of the two groups are demonstrated in Table 1.
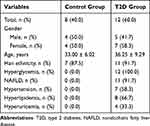 |
Table 1 Baseline Characteristics of the Control and T2D Group |
Clinical Features and SCFAs Levels of the Control and T2D Group Before and After Intervention
As for comparison between the control group and the T2D group before intervention, BMI, PBF and VFA were significantly higher in the T2D group than in the control group (P<0.05). Patients in the T2D group had obviously higher systolic blood pressure (SBP), diastolic blood pressure (DBP), FPG, 2-h PG, FINS, 2-h INS, HOMA-IR, HbA1c, UA, ALT, AST, TC, TG and LDL-C than subjects in the control group, except for HDL-C, which has cardiovascular protective effects (P<0.05). There were no significant differences of SCFAs levels between the two groups. Compared with before intervention, BMI, PBF, VFA, DBP, FPG, 2-h PG, FINS, 2-h INS, HOMA-IR, HbA1c, UA, ALT, AST, TC, TG and LDL-C significantly decreased, while HDL-C significantly increased, after intervention in the T2D group (P<0.05). Regarding the changes of SCFAs concentrations after significant weight loss, no obvious changes were observed except for an increase in butyrate and isobutyrate levels (P<0.05). The detailed results are shown in Table 2 and Figure 1.
 |
Table 2 Clinical Features and SCFAs Levels of the Control and T2D Group Before and After Intervention |
Correlations Between SCFAs and Obesity and Biochemical Indices
The associations of SCFAs and obesity, glucose and insulin secretion were analyzed when all the subjects were considered as a single cohort. Results showed that propionate was negatively correlated with PBF and 2-h PG (P<0.05), and butyrate was negatively associated with BMI, VFA, PBF and 2-h PG (P<0.05). No significant difference was found in other correlations. The results are exhibited in Table 3 and Figure 2.
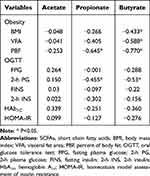 |
Table 3 Correlations Between SCFAs and Obesity and Biochemical Indices |
Discussion
In this study, we explored the associations between systemically SCFAs, mainly acetate, propionate and butyrate, and obesity, glucose and insulin secretion in patients with T2D and obesity. The results showed that butyrate significantly increased after dramatic weight loss, and butyrate and propionate were inversely correlated to obesity and glucose metabolism.
There is accumulating evidence indicating that SCFAs have anti-obesity and metabolic health protective effects. In studies on high-fat diet (HFD)-fed mice, Den et al found that adding oral sodium acetate for 12 weeks reduced weight gain by about 30% compared to mice fed HFD alone.16 Moreover, weight gain of HFD-fed mice was suppressed to a greater extent (around 72%) by prolonging the duration of sodium acetate supplementation.17 Another study conducted by Yamashita et al explored the effects of oral acetate administration on obesity and glucose metabolism in obesity-related type 2 diabetic rats, and the results showed that acetate treatment markedly reduced lipid and fat accumulation in adipose tissue and liver, significantly suppressed weight gain, and obviously ameliorated hyperglycemia.18 The underlying mechanisms by which SCFAs beneficially affect weight control and metabolic diseases may be primarily due to their ability to regulate appetite and satiety via the central hypothalamic and gut-derived hormone pathways. SCFAs are produced in the intestine, the most abundant of which are acetate, propionate and butyrate, and then transported into the systemic circulation. Acetate had been shown to be able to cross the blood–brain barrier, accumulate in the hypothalamus and act on the satiety center of the central nervous system in HFD-fed mice.19 Researches have demonstrated that the suppressive role of acetate on appetite in the central nervous system was mainly through the glutamate–glutamine circuitry, which led to increased production of gamma aminobutyric acid and neuropeptides with appetite inhibiting role.20 Additionally, in peripheral tissues, SCFAs can bind to the G-protein coupled receptors (GPR) 41 and GPR43, broadly expressed in the ileum and insulin sensitive tissues, including liver, adipose tissue, skeletal muscle and islet β cells, activate downstream signaling pathways and increase the secretion of gut-derived satiety hormones such as GLP-1 and PYY.21 Besides, a rodent study has found that acetate and propionate can promote the transcription of leptin gene and increase leptin release from the adipose tissue.22 Soliman et al found that acetate, butyrate and propionate enhanced the expression of leptin mRNA in in vitro cultured bovine adipocytes.23 Furthermore, a few studies have suggested that acetate may confer the bodyweight control effect via reducing lipid level, increasing fasting fat oxidation and energy expenditure in humans.24
The beneficial roles of SCFAs in obesity and metabolic diseases and the putative mechanisms have been clearly confirmed and elucidated in animal and in vitro cell studies. However, data on the roles of SCFAs in human obesity and metabolic diseases are scare, and the results of the existing human researches have demonstrated some discrepancies. A study conducted by Layden et al assessed the association of serum SCFAs with obesity and insulin secretion in women with obesity and polycystic ovary syndrome and found that serum acetate was inversely correlated with visceral adipose tissue mass and insulin levels in this population, suggesting that acetate might have anti-obesity and insulin resistance roles.25 The results of our current study were similar to those of Layden et al, which also found that SCFAs, in particular butyrate, were negatively associated with obesity, abdominal obesity and glucose metabolism. What’s more, butyrate levels significantly increased after dramatic weight loss, which further suggested that butyrate might have a protective effect against obesity. In this study, we also found the negative correlation between propionate and body fat mass and plasma glucose, while no correlation between propionate and BMI. The possible reasons might be that BMI does not reflect body fat and muscle mass, and so it is not an accurate indicator for assessing obesity, and the increase in body fat mass is the major cause of the development of metabolic diseases.26 On the contrary, another study conducted by Martina et al showed inconsistent findings with those of Layden et al and our study. They found a positive association of plasma acetate, propionate, and butyrate with the degree of obesity, total body fat, visceral fat and hepatic de novo lipogenesis in children and adolescents. Moreover, plasma concentrations of acetate, propionate, and butyrate were positively related with changes in adiposity over a mean follow-up time of 2.2 ± 1.7 years. The authors also analyzed the possible reason for this positive association between SCFAs and obesity, which might be due to the energy-harvesting role of SCFAs.27 Previous studies have suggested that SCFAs provide an additional energy source, which ultimately results in extra deposition of adipose tissue and abnormal lipid metabolism and glucose homeostasis. It has been estimated that the extra energy generated from SCFAs is equivalent to around 10% of the total daily energy supply.28
To our knowledge, this is the first study demonstrating the negative associations between butyrate and propionate and obesity and glucose metabolism and showing the increase of butyrate concentrations after significant weight loss in patients with diabetes and obesity. Nevertheless, this study has some limitations. Firstly, some factors, which may influence SCFAs levels such as alcohol intake, were not collected. Secondly, we do not measure fecal SCFAs levels, which may be more closely related to gut microbiota. Another limitation is that the results do not explain the casual relationship between SCFAs and obesity. Future studies, including animal experiments, are needed to verify the causality between SCFAs and obesity. Finally, the sample size of this study is relatively small, and the major study cohort is diabetes cohort.
In conclusion, this study revealed that butyrate was negatively associated with obesity, abdominal obesity and glucose metabolism, and butyrate levels increased after significant weight loss. Meanwhile, it showed the negative correlation between propionate and body fat mass and plasma glucose. Further researches with larger sample size, more diverse disease cohorts are required to elucidate the role of SCFAs in obesity and metabolic diseases and clarify the casual relationship between SCFAs and obesity.
Data Sharing Statement
All datasets generated for this study are available from the corresponding author upon reasonable request.
Ethics Statement
This study was reviewed and approved by the Medical Ethics Committee of Tianjin Union Medical Center (No. 2021C06). This study complied with the Declaration of Helsinki. The participants provided their written informed consent to participate in this study.
Acknowledgments
We gratefully appreciate all the participants in this study.
Funding
This study was supported by Grants from Tianjin Union Medical Center (2021YJ002) and Tianjin Health Research Project (TJWJ2022MS019).
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Pan XF, Wang L, Pan A. Epidemiology and determinants of obesity in China. Lancet Diabetes Endocrinol. 2021;9(6):373–392. doi:10.1016/S2213-8587(21)00045-0
2. Kawai T, Autieri MV, Scalia R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am J Physiol Cell Physiol. 2021;320(3):C375–91. doi:10.1152/ajpcell.00379.2020
3. Portincasa P, Bonfrate L, Vacca M, et al. Gut microbiota and short chain fatty acids: implications in Glucose homeostasis. Int J Mol Sci. 2022;23(3):1105. doi:10.3390/ijms23031105
4. Geng J, Qingqiang N, Sun W, Liangge L, Feng X. The links between gut microbiota and obesity and obesity related diseases. Biomed Pharmacother. 2022;147:112678. doi:10.1016/j.biopha.2022.112678
5. van der Hee B, Wells JM. Microbial regulation of host physiology by short-chain fatty acids. Trends Microbiol. 2021;29(8):700–712. doi:10.1016/j.tim.2021.02.001
6. Nogal A, Valdes AM, Menni C. The role of short-chain fatty acids in the interplay between gut microbiota and diet in cardio-metabolic health. Gut Microbes. 2021;13(1):1–24. doi:10.1080/19490976.2021.1897212
7. Hadi A, Pourmasoumi M, Najafgholizadeh A, Cain CTC, Esmaillzadeh A. The effect of apple cider vinegar on lipid profiles and glycemic parameters: a systematic review and meta-analysis of randomized clinical trials. BMC Complement Med Ther. 2021;21(1):179. doi:10.1186/s12906-021-03351-w
8. Yoshida H, Ishii M, Akagawa M. Propionate suppresses hepatic gluconeogenesis via GPR43/AMPK signaling pathway. Arch Biochem Biophys. 2019;672:108057. doi:10.1016/j.abb.2019.07.022
9. Hao W, Tremaroli V, Schmidt C, et al. The gut microbiota in prediabetes and diabetes: a population-based cross-sectional study. Cell Metab. 2020;32(3):379–390.e3. doi:10.1016/j.cmet.2020.06.011
10. Bayer Christiansen C, Buur Nordskov Gabe M, Svendsen B, Ove Dragsted L, Marie Rosenkilde M, Juul Holst J. The impact of short-chain fatty acids on GLP-1 and PYY secretion from the isolated perfused rat colon. Am J Physiol Gastrointest Liver Physiol. 2018;315(1):G53–5. doi:10.1152/ajpgi.00346.2017
11. González Hernández MA, Canfora EE, Johan WEJ, Blaak EE. The short-chain fatty acid acetate in body weight control and insulin sensitivity. Nutrients. 2019;11(8):1943. doi:10.3390/nu11081943
12. James WPT, Chunming C, Inoue S. Shuji inoue. appropriate asian body mass indices? Obes Rev. 2002;3(3):139. doi:10.1046/j.1467-789X.2002.00063.x
13. Chinese Diabetes Society. Guideline for the prevention and treatment of type 2 diabetes mellitus in China (2020 edition). Chin J Diabetes Mellitus. 2021;13:315–409.
14. Zhang S, Huang Y-P, Jing L, et al. The visceral-fat-area-to-hip-circumference ratio as a predictor for insulin resistance in a Chinese population with type 2 diabetes. Obes Facts. 2022;15(4):621–628. doi:10.1159/000525545
15. Son DH, Lee HS, Lee YJ, Lee JH, Han JH. Comparison of triglyceride-glucose index and HOMA-IR for predicting prevalence and incidence of metabolic syndrome. Nutr Metab Cardiovasc Dis. 2022;32(3):596–604. doi:10.1016/j.numecd.2021.11.017
16. den Besten G, Bleeker A, Gerding A, et al. Short-chain fatty acids protect against high-fat diet-induced obesity via a pparγ-dependent switch from lipogenesis to fat oxidation. Diabetes. 2015;64(7):2398–2408. doi:10.2337/db14-1213
17. Yanfei L, Fan C, Ping L, Yanfei L, Chang X, Kemin Q. Short chain fatty acids prevent high-fat-diet-induced obesity in mice by regulating g protein-coupled receptors and gut microbiota. Sci Rep. 2016;6(1):37589. doi:10.1038/srep37589
18. Yamashita H, Fujisawa K, Ito E, et al. Improvement of obesity and glucose tolerance by acetate in type 2 diabetic Otsuka Long-Evans Tokushima Fatty (OLETF) rats. Biosci Biotechnol Biochem. 2007;71(5):1236–1243. doi:10.1271/bbb.60668
19. Jiang L, Irene Gulanski B, De Feyter HM, et al. Increased brain uptake and oxidation of acetate in heavy drinkers. J Clin Invest. 2013;123(4):1605–1614. doi:10.1172/JCI65153
20. Frost G, Sleeth ML, Sahuri-Arisoylu M, et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat Commun. 2014;5(1):3611. doi:10.1038/ncomms4611
21. May KS, den Hartigh LJ. Gut microbial-derived short chain fatty acids: impact on adipose tissue physiology. Nutrients. 2023;15(2):272. doi:10.3390/nu15020272
22. May KS, den Hartigh LJ. Modulation of adipocyte metabolism by microbial short-chain fatty acids. Nutrients. 2021;13(10):3666. doi:10.3390/nu13103666
23. Soliman M, Kimura K, Ahmed M, et al. Inverse regulation of leptin mRNA expression by short- and long-chain fatty acids in cultured bovine adipocytes. Domest Anim Endocrinol. 2007;33(4):400–409. doi:10.1016/j.domaniend.2006.08.005
24. Birkeland E, Gharagozlian S, Valeur J, Aas A-M. Anne-Marie Aas. short-chain fatty acids as a link between diet and cardiometabolic risk: a narrative review. Lipids Health Dis. 2023;22(1):40. doi:10.1186/s12944-023-01803-5
25. Layden BT, Yalamanchi SK, Ms Wolever T, Dunaif A, Lowe WL Jr. Negative association of acetate with visceral adipose tissue and insulin levels. Diabetes Metab Syndr Obes. 2012;5:49–55. doi:10.2147/DMSO.S29244
26. Neeland IJ, Ross R, Després J-P, et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: a position statement. Lancet Diabetes Endocrinol. 2019;7(9):715–725. doi:10.1016/S2213-8587(19)30084-1
27. Goffredo M, Mass K, Parks EJ, et al. Role of gut microbiota and short chain fatty acids in modulating energy harvest and fat partitioning in youth. J Clin Endocrinol Metab. 2016;101(11):4367–4376. doi:10.1210/jc.2016-1797
28. Yazhong G, Ahmed S, Yao W, You L, Zheng J, Hileuskaya K. Regulation effects of indigestible dietary polysaccharides on intestinal microflora: an overview. J Food Biochem. 2021;45(1):e13564. doi:10.1111/jfbc.13564
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


