Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 18
Bu-Fei Yi-Shen Granules Reduce Acute Exacerbations in Patients with GOLD 3–4 COPD: A Randomized Controlled Trial
Authors Yu XQ, Di JQ, Zhang W, Wei GS, Ma ZP, Wu L, Yu XF, Zhu HZ, Zhou M, Feng CL, Feng JH, Fan P, Li JS , Yang JY
Received 10 May 2023
Accepted for publication 17 October 2023
Published 6 November 2023 Volume 2023:18 Pages 2439—2456
DOI https://doi.org/10.2147/COPD.S413754
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Xue-Qing Yu,1,2,* Jia-Qi Di,2,* Wei Zhang,3 Geng-Shu Wei,4 Zhan-Ping Ma,5 Lei Wu,6 Xue-Feng Yu,7 Hui-Zhi Zhu,8 Miao Zhou,9 Cui-Ling Feng,10 Ji-Hong Feng,11 Ping Fan,12 Jian-Sheng Li,1,2 Jian-Ya Yang1
1Department of Respiratory Disease, the First Affiliated Hospital of Henan University of Chinese Medicine, Zhengzhou, Henan Province, 450000, People’s Republic of China; 2Co-Construction Collaborative Innovation Center for Chinese Medicine and Respiratory Diseases by Henan & Education Ministry of P.R. China, Henan University of Chinese Medicine, Zhengzhou, Henan, 450046, People’s Republic of China; 3Department of Respiratory Disease, Shanghai Shuguang Hospital, Shanghai University of Chinese Medicine, Shanghai, 200000, People’s Republic of China; 4Department of Respiratory Disease, the Affiliated Hospital of Shaanxi University of Chinese Medicine, Xianyang, Shaanxi Province, 712000, People’s Republic of China; 5Department of Respiratory Disease, Shaanxi Province Hospital of Traditional Chinese Medicine, Xi’an, Shaanxi Province, 710000, People’s Republic of China; 6Department of Respiratory Disease, Hebei Province Hospital of Traditional Chinese Medicine, Shijiazhuang, Hebei, 050000, People’s Republic of China; 7Department of Respiratory Disease, the Second Affiliated Hospital of Liaoning University of Traditional Chinese Medicine, Shenyang, Liaoning Province, 110000, People’s Republic of China; 8Department of Respiratory Disease, the First Affiliated Hospital of Anhui University of Chinese Medicine, Hefei, Anhui Province, 230000, People’s Republic of China; 9Department of Respiratory Disease, the Third Affiliated Hospital of Henan University of Chinese Medicine, Zhengzhou, Henan Province, 450000, People’s Republic of China; 10Department of Traditional Chinese Medicine, People’s Hospital Affiliated to Peking University, Beijing, 100000, People’s Republic of China; 11Department of Respiratory Disease, the Second Affiliated Hospital of Tianjin University of Traditional Chinese Medicine, Tianjin, 300000, People’s Republic of China; 12Department of Respiratory Disease, the First Affiliated Hospital of Guangzhou Medical University, Guangzhou, Guangdong Province, 510000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jian-Sheng Li, Co-construction Collaborative Innovation Center for Chinese Medicine and Respiratory Diseases by Henan & Education Ministry of P.R. China, Henan University of Chinese Medicine, Zhengzhou, Henan, 450046, People’s Republic of China, Tel +86-371-65676568, Email [email protected]
Purpose: Chronic obstructive pulmonary disease (COPD) is a disease characterized by frequent acute exacerbations (AEs), especially in severe and very severe cases. We aimed to evaluate the efficacy and safety of Bu-fei Yi-shen granules (BYGs) for COPD.
Patients and Methods: We conducted a multicenter, randomized, double-blinded, placebo-controlled trial of 348 COPD patients with GOLD 3– 4 COPD. The patients were randomly assigned into experimental or control groups in a 1:1 ratio. Patients in the experimental group were prescribed BYG, while those in the control group were administered a placebo, orally, twice daily, with 5 days on and 2 days off per week for 52 weeks. The outcomes included AEs, pulmonary function, clinical signs and symptoms, dyspnea scores (mMRC), quality of life scores, and a 6-minute walk test (6MWT).
Results: A total of 280 patients completed the trial, including 135 patients in the experimental group and 145 in the control group. Compared to the control group, significant differences were observed in frequencies of AEs (mean difference: − 0.35; 95% CI: − 0.61, − 0.10; P = 0.006) and AE-related hospitalizations (− 0.18; 95% CI: − 0.36, − 0.01; P = 0.04), 6MWD (40.93 m; 95% CI: 32.03, 49.83; P < 0.001), mMRC (− 0.57; 95% CI: − 0.76, − 0.37; P < 0.001), total symptoms (− 2.18; 95% CI: − 2.84, − 1.53; P < 0.001), SF-36 (11.60; 95% CI: 8.23, 14.97; P < 0.001), and mCOPD-PRO (− 0.45; 95% CI: − 0.57, − 0.33; P < 0.001) after treatment. However, there were no significant differences in mortality, pulmonary function, and mESQ-PRO scores (P > 0.05). No obvious adverse events were observed.
Conclusion: BYG, as compared to a placebo, could significantly reduce the frequencies of AEs and AE-related hospitalizations for GOLD 3– 4 COPD patients. Clinical symptoms, treatment satisfaction, quality of life, and exercise capacity improved. There was no significant improvement in mortality and pulmonary function.
Keywords: chronic obstructive pulmonary disease, traditional Chinese medicine, efficacy, safety, RCT
Introduction
Chronic obstructive pulmonary disease (COPD) is a critical disease, characterized by long-term persistent respiratory symptoms such as cough, expectoration, chest tightness, and asthma. In later stages, COPD is often complicated with frequent acute exacerbations (AEs) and chronic respiratory failure, which seriously affect quality of life and lead to a worse prognosis.
The incidence rate and prevalence of COPD are both high, with an increasing trend and heavy disease burden for COPD all over the world, and chronic respiratory diseases have been the third leading cause of death, mostly due to COPD.1 In China, the prevalence rate of COPD can reach 8.6%, and is higher than 13% among people over 40 years old.2,3 Although the disease-associated burden decreased in the past 30 years, COPD remains a critical public health problem in China.4
In recent years, a series of studies have been performed and gratifying results have been obtained for COPD. Its occurrence and development have been delayed to a certain extent; however, although the constantly updated treatment measures help to improve the clinical symptoms and delay disease progression, most COPD patients are still undergoing rapid progression, especially for severe and very severe patients, and AEs are still critical events, causing decreased pulmonary function, increased hospitalizations, and worse prognoses such as death.5–7 Reducing AEs remains a key target for severe and very severe COPD patients.
Inflammation is an important pathogenesis for COPD, and corticosteroids are an important treatment option. However, oral corticosteroids increase mortality in stable COPD patients.8 Therefore, inhalation corticosteroids have garnered increased attention and are widely used. β2-agonist (LABA) and/or muscarinic antagonist (LAMA) and other combinations are important treatment options for COPD. Triple therapy has also been considered to be an effective treatment for severe and very severe COPD to improve pulmonary function.9 However, triple therapy cannot effectively reduce AEs and mortality, and the prognosis may not improve.10,11 New treatments to improve efficacy are urgently required.
Through long-term clinical practice and experience, traditional Chinese medicine (TCM) has proven to have good clinical efficacy and advantages in the treatment of COPD to improve symptoms, reduce AEs, and improve quality of life. However, there is a lack of uniformity on the prescription thereof, and evidence-based studies are insufficient. Consequently, we performed a series of studies of TCM for COPD.
The core TCM pathogenesis is deficiency of vital energy and accumulated damage.12 Deficiency is the root cause, with manifestation of phlegm and blood stasis. For severe and very severe COPD, treatments should focus on strengthening the vital energy (invigorating the lungs and kidneys, supplemented by strengthening the spleen), and assist in reducing phlegm and promoting blood circulation. We, therefore, previously established TCM treatment schemes and prescriptions for different stages and grades of COPD,13 which were then optimized through clinical practice and basic studies. Here, we aimed to formulate a Bu-fei Yi-shen granule (BYG) for the treatment of COPD and conduct a randomized controlled trial to provide critical references for the treatment of severe and very severe COPD.
Patients and Methods
Study Design
We conducted a multicenter, large sample, randomized, double-blind, placebo-controlled clinical trial. A total of 348 stable COPD patients were randomized in a ratio of 1:1 to receive either the BYGs or a placebo. Each of the treatment arms included 174 participants, recruited from 11 subcenters on the Chinese mainland, including the First Affiliated Hospital of Henan University of Traditional Chinese Medicine, Shuguang Hospital of Shanghai University of Traditional Chinese Medicine, the Third Affiliated Hospital of Henan University of Traditional Chinese Medicine, the First Affiliated Hospital of Guangzhou Medical University, Shaanxi Provincial Hospital of Traditional Chinese Medicine, Peking University People’s Hospital, the Second Affiliated Hospital of Liaoning University of Traditional Chinese Medicine, the First Affiliated Hospital of Anhui University of Traditional Chinese Medicine, the First Affiliated Hospital of Shaanxi University of Traditional Chinese Medicine, the Second Affiliated Hospital of Tianjin University of Traditional Chinese Medicine, and Hebei Hospital of Traditional Chinese Medicine.
The protocol (version 2019.04.25.1.0.3.0) was approved by the Institution Ethics Committee of the First Affiliated Hospital of Henan University of Traditional Chinese Medicine (No. 2019HL-010) and registered on ClinicalTrials.gov with an ID of NCT03976713. The study was conducted in strict accordance with the reviewed protocol and adhered to the tenets of the Declaration of Helsinki.
Patients
Diagnostic Criteria
We enrolled 348 stable COPD patients, diagnosed according to the following criteria:
(1) Referring to the 2019 edition of the Global Initiative for COPD (GOLD)14 and Chinese Experts’ Consensus on Diagnosis and Treatment of AECOPD (2017 update),15 we diagnosed patients with COPD when there was a history of exposure to risk factors such as smoking and dust harmful gases, characterized by long-term cough and expectoration and excluding other diseases that can cause similar symptoms. The presence of clear airflow restriction shown by pulmonary function examination after inhalation of bronchodilators was a necessary condition for diagnosis (FEV1/FVC < 0.70). AECOPD was diagnosed when patients had worsening symptoms (dyspnea, cough, and expectoration) beyond the daily range of variation characterized by increased shortness of breath, often accompanied by wheezing, chest tightness, increased cough, increased sputum volume, changes in sputum color and/or viscosity, and fever.
(2) Syndrome differentiation met the criteria of Qi deficiency of the lung and spleen ZHENG, Qi deficiency of the lung and kidney ZHENG, or Qi and Yin deficiency of the lung and kidney ZHENG as per the Diagnostic Standards for TCM Syndromes of COPD (2011 Edition).16
Inclusion and Exclusion Criteria
We included participants with (1) a confirmed diagnosis of GOLD 3–4 COPD (according to the GOLD spirometric criteria, GOLD 3 patients: 30%≤ FEV1% predicted <50%; GOLD 4 patients: FEV1% predicted < 30%); (2) syndrome differentiation meeting the criteria of Qi deficiency of the lungs and spleen, Qi deficiency of the lungs and kidneys, or Qi and Yin deficiency of the lungs and kidneys; (3) ages between 40 and 80 years (≥ 40 and ≤ 80), male or female; and (4) those who received the treatment voluntarily and provided informed consent. We excluded (1) pregnant and lactating women; (2) patients with severe cardiovascular and cerebrovascular diseases (malignant arrhythmia, unstable angina, acute myocardial infarction, cardiac function ≥ level 3, stroke, cerebral hemorrhage); (3) patients with bronchiectasis, bronchial asthma, active tuberculosis, obliterative bronchiolitis, diffuse pan bronchiolitis, pulmonary embolism, pneumothorax, and pleural effusion; (4) patients with respiratory failure requiring endotracheal intubation and invasive ventilator assistance; (5) severe hepatorenal diseases (severe liver diseases refer to cirrhosis, portal hypertension, and varicose bleeding; severe kidney diseases include kidney dialysis and kidney transplantation); (6) patients with tumors or neuromuscular diseases that affect respiratory motor function; (7) patients bedridden for a long time; (8) congenital or acquired immunodeficiency; (9) delirium, dementia, and other mental disorders; (10) patients taking oral glucocorticoids within one month before participation; and (11) clinical investigators or patients participating in other interventions within one month before the start of the study.
Elimination Criteria
The principal investigators and data managers made a final decision on the rejection of cases during blind verification. The participants were eliminated under any of the following conditions:
(1) Violation of the inclusion or exclusion criteria.
(2) Failure to take the investigational drug after entering the study group.
(3) Failure to visit for post-treatment follow-up studies.
(4) Those who seriously deviated from the protocol, such that it affected the judgment of curative effects and safety.
Termination Criteria
We would terminate the study under the following conditions:
(1) If a severe safety event occurred during the evaluation.
(2) If the clinical trial scheme was found to have significant errors during the trial. Although the scheme was reasonable, if serious deviations occurred during the implementation, making it difficult to evaluate the efficacy of the investigational drug, the trial would be terminated.
(3) It was found that the investigational drug treatment was ineffective and had no clinical value during the trial, the test would be stopped.
(4) The test was canceled by the administrative department.
Early termination of the clinical trial would be promptly conveyed to all research parties.
Withdraw Criteria
Under the following situations, participants would be withdrawn from the study at any time.
(1) In the case of an allergic reaction or serious adverse event.
(2) If disease/health conditions deteriorated during the study, the participants would quit the test and receive other effective treatments.
(3) The participants have poor compliance, and the use of the investigational drugs does not reach 80% or exceeds 120% of the prescribed amount.
(4) During the study, the participant changed medicines when the study was active or added Chinese and Western medicines prohibited by this program.
(5) Accidental unmasking of the study while the study is active.
Under the following situations, the study participant could withdraw from the study at any time.
(1) For any reason, if the participant wishes to withdraw from participating in the trial, the study investigator can withdraw the participant.
(2) Participants who do not wish to accept any more investigational drugs, although they do not state the withdrawal explicitly.
When the participants withdrew or dropped out of the trial, investigators took active measures to try to complete the last test to analyze the efficacy and safety. Moreover, for these cases, the study conclusions and reasons for dropping out were recorded in the research medical records/case report forms. In case of withdrawal from the trial due to allergic reaction, adverse reaction, and ineffective treatment, investigators took appropriate treatment measures according to the current situation of the participants during that time.
Sample Size
Based on previous relevant research results, the number of AEs decreased by 0.228 ± 1.109 times after treatment with BYG and by 0.138 ± 1.385 times after placebo treatment. In this study, class I error α and class II error β of the study were assumed to be 0.05 and 0.1, respectively, and the ratio of the sample size for the two groups was 1:1. Considering the potential absence from future visits and withdrawal, and according to the calculation formula of sample size to compare average values of two groups of independent samples, the sample size of each group was calculated by PASS software, and was approximately 174 participants. Therefore, the targeted total sample was 348 in this study, with 174 participants in the experimental group and 174 participants in the control group.
Interventions
According to the international guidelines, GOLD 3–4 COPD patients should also be given long-term maintenance medication. Based on routine treatment of modern medicine, patients in the experimental group were given BYG, with those in the control group receiving a placebo. All the treatments lasted for 52 weeks, and we prescribed other necessary treatments if the patients had AEs. The planned treatment measures continued when the patient’s conditions returned to stable.
Routine Treatment of Modern Medicine
According to the Chinese Guidelines for Diagnosis and Treatment of COPD (2013 Revision), patients could select β 2-Receptor agonists (terbutaline sulfate inhalation powder spray, salbutamol aerosol inhalation solution, or indacaterol inhalation powder spray), anticholinergic drugs (ipratropium bromide aerosol, or tiotropium bromide powder for inhalation), or aminophylline as routine treatment. Participants with hypertension, coronary heart disease, and other diseases were also treated with conventional drugs according to the relevant disease guidelines during the treatment and follow-up period. All the drug names, usage, and dosage were recorded in detail.
TCM Treatment
BYGs could tonify lung and kidney function by strengthening the spleen, promoting blood circulation, and reducing phlegm. The granules and placebo were produced, packaged, and transported by Jiangyin Tianjiang Pharmaceutical Co., Ltd, who meet GMP standards with strict quality control. The Bu-fei Yi-shen placebo granules contain 5% of BYGs; their appearance, weight, color, and smell are the same or similar; and they cannot be distinguished by the participants and/or researchers. BYGs or the placebo were administered orally, twice daily, with 5 days on and 2 days off a week. The raw material components of the daily dose for the BYGs are shown in Table 1.
 |
Table 1 Components of Bu-Fei Yi-Shen Granules |
Outcomes
Basic Characteristic Variables
We collected the following baseline data for the study: (1) demographic data, including age, height, and weight; and (2) general clinical data, including comorbidities and medications.
Primary Efficacy outcomes
We recorded the duration and frequency of any AEs, made judgments according to the standards of the stabilization time, and recorded the stable condition time in detail.
Secondary Indices of Curative Efficacy
(1) Mortality, calculated up to 52 weeks.
(2) Pulmonary function: values of FEV1, FVC, and FEV1% were recorded to evaluate the improvement of pulmonary function from baseline to weeks 26 and 52.
(3) Clinical symptoms and signs: including cough, expectoration, gasp, chest tightness, shortness of breath, fatigue, and cyanosis. During the study, data was collected at baseline and weeks 13, 26, 39, and 52.
(4) Exercise capacity: a 6-minute walking distance (6MWD) test was adopted to evaluate exercise capacity, performed and recorded at baseline and weeks 13, 26, 39, and 52.
(5) Evaluation of life quality: the COPD assessment test (CAT), SF-36, modified patient-reported outcome scale for COPD (mCOPD-PRO), and modified efficacy satisfaction questionnaire for COPD (mESQ-COPD) were used to measure quality of life of patients. These scale scores were recorded at baseline and weeks 13, 26, 39, and 52.
(6) Dyspnea: Modified Medical Research Council (mMRC) scores were adopted to evaluate dyspnea, recorded at baseline and weeks 13, 26, 39, and 52.
Safety Assessment
The following safety items were recorded before and after each test:
(1) Vital signs, such as blood pressure, respiration, heart rate, and pulse.
(2) Routine examination of urine and stool.
(3) Routine examination of blood.
(4) Chest CT.
(5) Electrocardiogram.
(6) Liver and renal function, blood glucose, blood lipid, ALT, AST, urea nitrogen, creatinine, and uric acid.
(7) Any adverse events, including dizziness, headache, pruritus, skin rashes, alopecia, gastrointestinal symptoms, hepatorenal toxicity, and leukopenia. The occurrence time, severity, frequency, duration, and outcomes of the adverse events were recorded.
Adverse Events
An adverse event form was set up in the Research Medical Record and Case Report Form (CRF), requiring investigators to record the occurrence time, severity, duration, measures taken, and outcomes of any adverse events. In case of adverse events during the trial, we took all necessary measures to ensure the safety of the participants, and immediately report the events to the local and provincial drug administration within 24 hours. If any aggravation of symptoms occurred, the patient was withdrawn from the study and referred for further treatment.
Randomization
The method of hierarchical block central random allocation was adopted. According to the clinical trial plan, the random allocation plan was constructed and subsequently implemented and managed through a central random network system. Participants were randomly assigned to the experimental and control groups, with 174 participants in each group. The study investigators acquired the assigned codes of the participants through the network.
The clinical trial doctor obtained the participant assignment code through the internet, and recruitment was performed in the 11 hospitals. Besides direct recruitment, other methods, such as multimedia advertising, were adopted to ensure an adequate sample size.
Masking
As this is a double-blind study, blinding was maintained for the study participants, the clinicians, the research assistants, the drug managers, and the statisticians, until the completion of the study. Blinding was completed by the person in charge of the project unit, the drug preparation personnel, and the statistician. Uniformity was maintained while packaging the test and placebo drugs. This was followed by uniform distribution of the packaging boxes. During the trial period, the clinicians, research assistants, and drug managers maintained confidentiality and shared the drug information only with associated physicians.
A detailed written record was maintained for the blinding process, which was signed by responsible and authorized persons. The drug containers were sealed and stamped soon after the drug was dispensed. A person in charge of the project, drug preparation personnel, and the statisticians adjudicated the whole process.
The investigator, and the study site personnel remained blinded to the participant’s treatment group. Blinding was only broken if the participant experienced a medical emergency and knowledge of the blinded treatment assignment was deemed necessary for further management of the participant. The date and reasons for the code break were documented in the source documents and on the appropriate CRF.
Procedures
After a wash-out of 2 weeks, patients in the experimental and control groups received the BYGs or placebo, respectively, for 52 weeks. We followed up and assessed each participant every 13 weeks. Quality control was performed by checking and recycling all the outer packaging of the experimental drugs at the next follow-up visit.
Data Management and Statistical Analysis
During project implementation, the quality supervision committee was established, composed of clinical and quality inspectors, who performed an inspection on the project every 12 months. An independent data management council (DMC) was also established by the trial steering committee. The DMC included clinical epidemiologists, data monitors, and statisticians.
Per protocol set (PPS) analysis was performed for the efficacy assessment, including data from all cases that met the inclusion and exclusion criteria and completed all treatment requirements. All randomized patients who received at least one dose of treatment and had actual data recorded by safety indices were included in the safety data set.
The enumeration data are described by frequency or composition ratio, while the measurement data are described by mean ± standard deviation (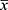 ± SD). Measurement data, such as pulmonary function, with normal distribution and homogeneous variance, were compared through t-tests with Wilcoxon rank-sum tests used for non-normal distribution or uneven variance. A repeated measurement analysis of variance (ANOVA) was used for data measured more than twice at different time points. Chi-square or Fisher’s exact tests were applied to compare differences in enumeration data, such as the frequencies of AEs and adverse events/reactions. Two-tailed tests were applied with P < 0.05 considered statistically significant. SPSS 25.0 software was used for data analysis, and GraphPad Prism 8 was applied for image generation. Data management and analysis was undertaken by Jiangsu famous Medical Technology Co., Ltd. A flow chart of the study is shown in Figure 1, and the schedule of enrollment, intervention, and assessments is presented in Figure 2.
± SD). Measurement data, such as pulmonary function, with normal distribution and homogeneous variance, were compared through t-tests with Wilcoxon rank-sum tests used for non-normal distribution or uneven variance. A repeated measurement analysis of variance (ANOVA) was used for data measured more than twice at different time points. Chi-square or Fisher’s exact tests were applied to compare differences in enumeration data, such as the frequencies of AEs and adverse events/reactions. Two-tailed tests were applied with P < 0.05 considered statistically significant. SPSS 25.0 software was used for data analysis, and GraphPad Prism 8 was applied for image generation. Data management and analysis was undertaken by Jiangsu famous Medical Technology Co., Ltd. A flow chart of the study is shown in Figure 1, and the schedule of enrollment, intervention, and assessments is presented in Figure 2.
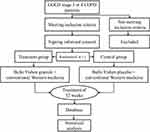 |
Figure 1 Study trial procedure. |
 |
Figure 2 Procedures for each patient in the study. |
Results
From July 2019 to September 2020, a total of 348 stable COPD patients with GOLD 3–4 were recruited and included, with 174 participants in each group. After treatment and follow-up, 44 patients were eliminated for not adhering to the clinical protocol (38 patients) or concealing a history of diabetes (6 patients). A total of 24 patients dropped out of the study (Table 2). Finally, a total of 280 patients (135 cases in the experimental group and 145 in the control group) were included in the data analysis.
 |
Table 2 Summary of Discontinuities/Dropouts/ Withdraw for the Two Groups |
Baseline Characteristics
There were no statistical differences in age, gender, nationality, BMI, heart rate, blood pressure, marriage, occupation, educational levels, smoking, drinking, and concomitant medications between the experimental and control groups. No intergroup significant differences were observed in annual numbers and durations of AEs, pulmonary function, or course of disease (Table 3).
 |
Table 3 Baseline Characteristics of Included COPD Patients§ |
Primary Outcomes
After treatment, significant differences were observed in frequency and duration of AEs and AE-related hospitalization between the experimental and control groups. As shown in Figure 3 and Table S1, frequencies of AEs in the experimental group were 0.35 times lower than those in the control group (95% CI: 0.10, 0.61; P = 0.006). For frequencies of AE-related hospitalizations, the numbers in both groups improved (P < 0.001), and were 0.18 times lower in the experimental group compared to the control group (95% CI: 0.01, 0.36; P < 0.05). Compared to the control group, the duration of AE-related hospitalizations was significantly lower in the experimental group (mean difference: −1.83 days; 95% CI: −2.97, −0.68; P = 0.002).
 |
Figure 3 Comparison of differences between groups regarding AEs. |
Secondary Outcomes
Mortality
After treatment, two patients died in each group. No statistical difference was observed for mortality.
Pulmonary Function
The repeated ANOVA data shows that there are no significant differences in FEV1, FVC, and FEV1% between the experimental and control groups after treatment (Figure 4).
Signs and Symptoms
During the follow-up period, cough, expectoration, wheezing, chest tightness, shortness of breath, fatigue, cyanosis scores, and total scores decreased in both groups. There were no significant intergroup differences at weeks 26, 39, and 52, respectively (P < 0.05). For wheezing, chest tightness scores, and total scores, significant differences were observed at week 13 (P < 0.05). The repeated ANOVA shows that time group effects were observed, indicating that there was a significant difference between the experimental and control groups in cough, with better outcomes in the experimental group (P < 0.001) (Figure 5 and Table S1).
6MWD
6MWD improved in both groups at different follow-up points during the treatment period, with intergroup statistical differences at week 26, 39, and 52, respectively (P = 0.025, P = 0.004, P < 0.001). We also observed time group effects by repeated ANOVA, indicating significant differences between the experimental and control groups in 6MWD, with better outcomes in the experimental group (P < 0.001). After treatment, 6MWD was higher in the experimental group than in the control group, by 40.93 m (95% CI: 32.03 m, 49.83 m, P < 0.001) (Figure 6 and Table S1).
 |
Figure 6 Comparison of differences between groups regarding 6MWD. |
Quality of Life
Quality of life assessed by CAT, SF-36, mCOPD-PRO, and mESQ-COPD improved in both groups, with better efficacy in the experimental group compared to the control group (P < 0.001). SF-36 scores, except for BP, improved in each aspect of the score (Figures 7–8 and Table S1).
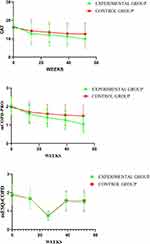 |
Figure 7 Comparison of differences between groups regarding CAT, mCOPD-PRO, and mESQ-COPD. |
 |
Figure 8 Comparison of differences between groups regarding SF-36. |
mMRC
mMRC decreased in both groups, and significant intergroup differences were found at weeks 26, 39, and 52 (P = 0.006, P < 0.001, P < 0.001). A repeated ANOVA shows that time group effects were also observed, indicating a significant difference between the experimental and control groups in mMRC, with better outcomes in the experimental group (P < 0.001). After treatment, mMRC was lower in the experimental group than in the control group (mean difference: −0.57; 95% CI: −0.76, −0.37; P < 0.001) (Figure 9 and Table S1).
 |
Figure 9 Comparison of differences between groups on mMRC. |
Adverse Events
Throughout the treatment and follow-up period, the biochemical tests, urine routine tests, and ECG from all the participants were approximately normal. In the experimental group, four adverse events were observed: two patients died, one patient was diagnosed with diabetes, and one patient was diagnosed with malignancy. In the control group, three adverse events were observed: two patients died, and one patient was diagnosed with malignancy. Additionally, one patient in each group had transient stomach pain. There was no statistical difference in adverse events.
In addition, we have also performed intention-to-treat (ITT) data analysis as a supplement. The results have been shown in the attachment materials (Table S2) not in the body manuscript. The analysis results of ITT and PP are not contradictory. Please find the specific results in Table S2.
Discussion
We found efficacy advantages of TCM for GOLD 3–4 COPD patients. Compared to the placebo, BYGs were effective in reducing AEs in the 52-week follow-up period. The frequencies and durations of AEs and AE-related hospitalizations decreased in our experimental group. Additionally, clinical symptoms, treatment satisfaction, quality of life, and exercise capacity improved. However, pulmonary function did not improve following treatment using BYGs for GOLD 3–4 COPD patients. To our knowledge, this is the first RCT involving TCM in GOLD 3–4 COPD patients without regard to TCM syndrome. Our results may highlight the core TCM pathogenesis and provide references for clinical applications, especially for severe and very severe COPD patients.
AEs induce worse symptoms and pulmonary function decline with additional treatments. Hospitalization and worse prognoses are also induced by severe AEs, especially for severe and very severe COPD patients.5 The incidence of AEs in COPD patients within one year is as high as 43.7% in China,17 and is 61.7% in Spain.18 Among COPD patients hospitalized due to AE, GOLD 3–4 patients account for 71.1%.19 Severe AE has also been confirmed to be an independent risk factor leading to patient death, with the risk thereof increasing as the frequency of AE increases.20 Therefore, reducing the frequencies of AEs has been the most important treatment target, especially for the GOLD 3–4 COPD patients.
Based on the clinical characteristics of GOLD 3–4 COPD patients with frequent AEs and serious clinical hazards, our research team have summarized the core TCM pathogenesis of deficiency of lung and kidney Qi, combined with spleen deficiency, phlegm turbidity, and blood stasis. We have also developed BYGs with increased efficacy of tonifying the lungs and kidneys, resolving phlegm, and promoting blood circulation. In this study, BYGs showed an advantage in reducing AEs in all the patients regardless of different TCM syndromes. The annual number of AEs in the experimental group decreased by 0.36 times compared to the control group, and the difference was statistically significant, which has confirmed the clinical efficacy of BYGs in reducing AEs. The results are worthy of further promotion.
Dyspnea is a common clinical symptom in COPD patients, gradually worsening with the decline of lung function assessed by the mMRC scale. Levels of dyspnea are also related to quality of life and prognosis.21 Despite standard treatment, 43% of COPD patients still have persistent dyspnea symptoms with mMRC scores ≥ 2.22 In this study, we found that BYGs could reduce the levels of dyspnea for GOLD 3–4 COPD patients compared to a placebo group; the improvement was more outstanding from week 26. The results may also indicate the late effect for TCM, which has been ignored in clinical studies to date.
The CAT scale is internationally recognized as a COPD assessment scale that can help patients understand the impact of COPD on their health and quality of life. The scale negatively correlates with the levels of pulmonary function and positively correlates with the frequency of AEs in the previous year.23 We showed that BYGs can reduce CAT scores, reducing disease severity for GOLD 3–4 COPD patients.
For COPD patients, quality of life will decrease with disease progression. In this study, SF −36, EQ-5D, and mCOPD-PRO scores were adopted to comprehensively assess quality of life. We showed improved quality of life in our experimental group, with some differences in areas related to TCM treatment.
Patients with COPD can also experience clinical symptoms such as cough, expectoration, chest tightness, and asthma. TCM treatment based on syndrome differentiation originates from the symptoms and signs. Therefore, the efficacy of TCM should first show the improvement of symptoms. However, this study was conducted based on disease classification, and summarizes the core pathogenesis and formulates core TCM prescriptions, which may be different and sublimated from TCM treatment based on syndrome differentiation. Our results may still indicate the efficacy of BYGs in improving clinical symptoms and signs, which will further verify the core pathogenesis, and provide critical references for the staging and grading treatment of COPD with TCM.
As pulmonary function declines, exercise capacity in COPD patients will also significantly decrease, especially for GOLD 3–4 patients. Referring to relevant guidelines and studies, we adopted the 6MWD to assess exercise capacity. For COPD patients, skeletal muscle atrophy is very common and may be an important factor leading to decreased exercise capacity.24 Treatment in modern medicine focuses on the lungs, regardless of the peripheral tissues. Although non-drug therapies, such as pulmonary rehabilitation, have shown some improvement in exercise capacity, the quality of evidence is still low.24 BYGs can treat COPD through multiple targets and pathways, and its action is not limited to the lungs. We showed improved 6MWD results, confirming the multi-target mechanism from a clinical perspective.
Pulmonary function did not improve in our follow-up period. This may be related to the effective link of the prescription as well as COPD disease characteristics at this stage. The long-term effects of TCM may also have been discounted. Therefore, the advantages of TCM should be reassessed, as well as the related evaluation of TCM clinical research. A suitable evaluation method and index for TCM to reflect the efficacy advantages should be urgently investigated.
We also considered the role of psychological factors in disease treatment and explored the impact of TCM treatment on patient satisfaction. Since the bio–psycho–social medical model has been recognized, more and more researchers have begun to focus on patient psychological factors and patient satisfaction, which can enrich the cognition of therapeutic effects from different perspectives. As an exploratory study in patient satisfaction, we adopted the ESQ-COPD as an assessment method, as developed by our research team previously.25 ESQ-COPD scores decreased in both groups in the all fields, with a significantly higher reduction rate in the experimental group, indicating that BYGs can significantly improve the satisfaction of patients with therapeutic effects. In addition, the side effects were also recorded and assessed. During the treatment period, there were no significant abnormalities in biochemical and electrocardiogram examinations in both groups. No serious adverse events related to the treatment drug were found.
Inhalers are the mainstay of treatment for stable COPD. Commonly used drugs include inhaled hormones and bronchodilators, which can effectively delay the decline of lung function to a certain extent, and are convenient to use. In the past, dual drugs have been the main treatment with general efficacy. Therefore, in recent years, new triple therapies have been developed, which could be applied in treating COPD and may improve lung function.26 Moreover, triple therapies may have good efficacy in patients without reversible airway lesions and airway eosinophilic inflammation.27 However, studies on clinical symptoms and acute exacerbation are lacking. Unfortunately, when our study protocol was formulated, triple therapy had not been widely promoted and applied.
Studies regarding TCM treatment for COPD are abundant; however, most are exploratory, with small samples and low quality of evidence.28 A study on the efficacy of TCM for a certain syndrome has been conducted.29 The overall efficacy of TCM has been assessed in some studies; however, there are few studies on certain pulmonary function levels.30 These studies failed to comprehensively and carefully evaluate the clinical efficacy of TCM in treating COPD.
We have studied the prevention and treatment of COPD with TCM for a long time. Different studies have been performed to explore ways to determine traditional syndrome differentiation and one-person treatment models for different disease stages and periods,13,31,32 providing critical references for further studies and more convenient clinical applications. This study is also an in-depth investigation based on the former foundations, with regard for the summary of the disease and pathogenesis characteristics of severe and extremely severe COPD patients with TCM. New studies need to clarify and improve the efficacy of TCM in patients with severe pulmonary function and extremely severe COPD, to further enrich the advantages and strategies thereof.
Limitations
This study had some limitations. First, the total target sample size was calculated based on the whole study, regardless of sub-group, which induces uneven results among different syndrome subgroups. Our sub-group analysis was abandoned. Second, because of COVID-19, some patients could only be followed up by telephone, which may not fully reflect the required information, and may cause some bias. Finally, due to a lack of time, we only conducted the study for 52 weeks, and failed to follow up on patient disease status after our treatment. We, therefore, failed to evaluate the late effect of the BYGs.
Conclusion
BYGs could reduce the frequencies of AEs and AE-related hospitalization for GOLD 3–4 COPD patients significantly with acceptable side effects. Patient clinical symptoms, treatment satisfaction, quality of life, and exercise capacity were improved by BYGs, with no significant improvement in pulmonary function and mortality.
Abbreviations
6MWD, 6-min walk distance; ANOVA, Analysis of Variance; CAT, COPD assessment test; COPD, chronic obstructive pulmonary disease; GOLD, global initiative for chronic obstructive lung disease; mCOPD-PRO, modified patient-reported outcome scale for COPD; mESQ-COPD, modified efficacy satisfaction questionnaire for COPD; mMRC, Modified Medical Research Council; PPS, Per Protocol Set; TCM, traditional Chinese Medicine.
Data Sharing Statement
The raw data for this study could be available from corresponding author under request only for researches, and cannot be disseminated.
Ethics Approval and Informed Consent
The trial protocol (version 2019.04.25.1.0.3.0) was approved by the Institution Ethics Committee of the First Affiliated Hospital of Henan University of Traditional Chinese Medicine (No. 2019HL-010). The study obtained informed consent and handwritten informed consent forms from each participant before trial. The study was conducted in strict accordance with the reviewed protocol and adhered to the tenets of the Declaration of Helsinki.
Consent for Publication
All authors have read the manuscript and agree to publish.
Funding
This study has been supported by the National Key Research & Development Program of China (grant number: 2018YFC1704800, 2018YFC1704802). All the participant experts are appreciated for their diligence in this study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Kendrick PJ, Paulson KR, Gupta V, et al. Prevalence and attributable health burden of chronic respiratory diseases, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Resp Med. 2020;8(6):585–596. doi:10.1016/S2213-2600(20)30105-3
2. Wang C, Xu J, Yang L, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China pulmonary health [CPH] study): a national cross-sectional study. Lancet. 2018;391(10131):1706–1717. doi:10.1016/S0140-6736(18)30841-9
3. Fang L, Gao P, Bao H, et al. Chronic obstructive pulmonary disease in China: a nationwide prevalence study. Lancet Resp Med. 2018;6(6):421–430. doi:10.1016/S2213-2600(18)30103-6
4. Yin P, Wu J, Wang L, et al. The burden of COPD in China and Its provinces: findings from the global burden of disease study 2019. Front Public Health. 2022;10:859499. doi:10.3389/fpubh.2022.859499
5. Flattet Y, Garin N, Serratrice J, Perrier A, Stirnemann J, Carballo S. Determining prognosis in acute exacerbation of COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:467–475. doi:10.2147/COPD.S122382
6. Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57(10):847–852. doi:10.1136/thorax.57.10.847
7. Han MK, Quibrera PM, Carretta EE, et al. Frequency of exacerbations in patients with chronic obstructive pulmonary disease: an analysis of the SPIROMICS cohort. Lancet Respir Med. 2017;5(8):619–626. doi:10.1016/S2213-2600(17)30207-2
8. Horita N, Miyazawa N, Morita S, et al. Evidence suggesting that oral corticosteroids increase mortality in stable chronic obstructive pulmonary disease. Respir Res. 2014;15(1):37. doi:10.1186/1465-9921-15-37
9. van den Berge M, De Backer J, Van Holsbeke C, et al. Functional respiratory imaging assessment of budesonide/glycopyrrolate/formoterol fumarate and glycopyrrolate/formoterol fumarate metered dose inhalers in patients with COPD: the value of inhaled corticosteroids. Respir Res. 2021;22(1):191. doi:10.1186/s12931-021-01772-2
10. Suissa S, Dell’Aniello S, Ernst P. Triple inhaler versus dual bronchodilator therapy in COPD: real-world effectiveness on mortality. J Chron Obstruct Pulmon Dis. 2022;19(1):1–9. doi:10.1080/15412555.2021.1977789
11. Suissa S, Dell’Aniello S, Ernst P. Single-inhaler triple versus dual bronchodilator therapy in COPD: real-world comparative effectiveness and safety. Int J Chron Obstruct Pulmon Dis. 2022;17:1975–1986. doi:10.2147/COPD.S378486
12. Jian-Sheng L. Positive deficiency and accumulated damage is the core TCM pathogenesis of chronic obstructive pulmonary disease. Chin J Traditional Chin Med. 2011;26(08):710–713.
13. Li JS, Li SY, Xie Y, et al. The effective evaluation on symptoms and quality of life of chronic obstructive pulmonary disease patients treated by comprehensive therapy based on traditional Chinese medicine patterns. Complement Ther Med. 2013;21(6):595–602. doi:10.1016/j.ctim.2013.09.006
14. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease; 2019. Available from: https://goldcopd.org/wp-content/uploads/2018/11/GOLD-2019-v1.7-FINAL-14Nov2018-WMS.pdf.
15. Expert Group on the Diagnosis and Treatment of Acute Exacerbation of Chronic Obstructive Pulmonary Disease. Chinese expert consensus on the diagnosis and treatment of acute exacerbation of chronic obstructive pulmonary disease (AECOPD) (2017 update). Int J Resp. 2017;37(14):1041–1057.
16. Li JS. Diagnostic criteria for traditional Chinese medicine syndromes of chronic obstructive pulmonary disease (2011 Edition). J Traditional Chin Med. 2012;53(2):177–178.
17. Huang J, Bian Y, Zhao Y, Jin Z, Liu L, Li G. The impact of depression and anxiety on chronic obstructive pulmonary disease acute exacerbations: a prospective cohort study. J Affect Disord. 2021;281:147–152. doi:10.1016/j.jad.2020.12.030
18. Montserrat-Capdevila J, Godoy P, Marsal JR, Barbé F, Galván L. Risk factors for exacerbation in chronic obstructive pulmonary disease: a prospective study. Int J Tuberc Lung Dis. 2016;20(3):389–395. doi:10.5588/ijtld.15.0441
19. Hartl S, Lopez-Campos JL, Pozo-Rodriguez F, et al. Risk of death and readmission of hospital-admitted COPD exacerbations: European COPD Audit. Eur Respir J. 2016;47(1):113–121. doi:10.1183/13993003.01391-2014
20. Soler-Cataluña JJ, Martínez-García MA, Román Sánchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60(11):925–931. doi:10.1136/thx.2005.040527
21. Casanova C, Marin JM, Martinez-Gonzalez C, et al. Differential effect of modified Medical Research Council dyspnea, COPD assessment test, and clinical COPD questionnaire for symptoms evaluation within the new GOLD staging and mortality in COPD. Chest. 2015;148(1):159–168. doi:10.1378/chest.14-2449
22. Sundh J, Ekström M. Persistent disabling breathlessness in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2016;11:2805–2812. doi:10.2147/COPD.S119992
23. Lee SD, Huang MS, Kang J, et al. The COPD assessment test (CAT) assists prediction of COPD exacerbations in high-risk patients. Resp Med. 2014;108(4):600–608. doi:10.1016/j.rmed.2013.12.014
24. Hopkinson NS, Tennant RC, Dayer MJ, et al. A prospective study of decline in fat free mass and skeletal muscle strength in chronic obstructive pulmonary disease. Respir Res. 2007;8(1):25. doi:10.1186/1465-9921-8-25
25. Yu XQ, Wang MH, Li JS, et al. Effect of comprehensive therapy based on Chinese medicine patterns on self-efficacy and effectiveness satisfaction in chronic obstructive pulmonary disease patients. Chin J Integr Med. 2019;25(10):736–742. doi:10.1007/s11655-017-2417-9
26. Ferguson GT, Rabe KF, Martinez FJ, et al. Triple therapy with budesonide/glycopyrrolate/formoterol fumarate with co-suspension delivery technology versus dual therapies in chronic obstructive pulmonary disease (KRONOS): a double-blind, parallel-group, multicentre, Phase 3 randomised controlled trial. Lancet Respir Med. 2018;6(10):747–758. doi:10.1016/S2213-2600(18)30327-8
27. Muro S, Sugiura H, Darken P, Dorinsky P. Efficacy of budesonide/glycopyrronium/formoterol metered dose inhaler in patients with COPD: post-hoc analysis from the KRONOS study excluding patients with airway reversibility and high eosinophil counts. Respir Res. 2021;22(1):187. doi:10.1186/s12931-021-01773-1
28. Zeng Y, Li Y, Wei H, et al. The effects and safety of Chinese oral herbal paste on stable chronic obstructive pulmonary disease: a systematic review and meta-analysis of randomized controlled trials. J Evid Based Complementary Altern Med. 2020;2020:5867086.
29. Sun Y, Chen X, Zhang L, et al. Efficiency and safety of baofei granules in chronic obstructive pulmonary disease (lung and spleen qi deficiency syndrome): a multicenter, randomized, double-blind, placebo-controlled Phase II clinical trial. Drug Des Devel Ther. 2022;16:4251–4267. doi:10.2147/DDDT.S382285
30. Mao Y, Hu G, Meng Q, et al. Efficacy of Shenling Baizhu San on stable chronic obstructive pulmonary disease patients: a systematic review and meta-analysis. J Ethnopharmacol. 2021;272:113927. doi:10.1016/j.jep.2021.113927
31. Li SY, Li JS, Wang MH, et al. Effects of comprehensive therapy based on traditional Chinese medicine patterns in stable chronic obstructive pulmonary disease: a four-center, open-label, randomized, controlled study. BMC Complement Altern Med. 2012;12:197. doi:10.1186/1472-6882-12-197
32. Haifeng W, Jiansheng L, Suyun L, et al. Effect of sequential treatment with syndrome differentiation on acute exacerbation of chronic obstructive pulmonary disease and “AECOPD Risk-Window”: study protocol for a randomized placebo-controlled trial. Trials. 2012;13(1):40. doi:10.1186/1745-6215-13-40
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


