Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 16
Bronchodilator Response in Patients with COPD, Asthma-COPD-Overlap (ACO) and Asthma, Evaluated by Plethysmographic and Spirometric z-Score Target Parameters
Authors Kraemer R , Smith HJ, Gardin F, Barandun J, Minder S, Kern L, Brutsche MH
Received 12 May 2021
Accepted for publication 2 August 2021
Published 1 September 2021 Volume 2021:16 Pages 2487—2500
DOI https://doi.org/10.2147/COPD.S319220
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Richard Kraemer,1,2 Hans-Jürgen Smith,3 Fabian Gardin,4 Jürg Barandun,4 Stefan Minder,3 Lukas Kern,5 Martin H Brutsche5
1Center of Pulmonary Medicine, Hirslanden Private Hospital Group, Salem-Hospital, Bern, Switzerland; 2Department of Biomedical Research, University of Bern, Bern, Switzerland; 3Medical Development, Research in Respiratory Diagnostics, Berlin, Germany; 4Center of Pulmonary Medicine, Hirslanden Private Hospital Group, Clinic Hirslanden, Zürich, Switzerland; 5Clinic of Pneumology, Cantonal Hospital St. Gallen, St. Gallen, Switzerland
Correspondence: Richard Kraemer
Center of Pulmonary Medicine, Hirslanden Private Hospital Group, Schänzlistrasse 39, Berne, CH-3013, Switzerland
Tel +41 79 300 26 53
Email [email protected]
Background: Airflow reversibility criteria in COPD are still debated – especially in situations of co-existing COPD and asthma. Bronchodilator response (BDR) is usually assessed by spirometric parameters. Changes assessed by plethysmographic parameters such as the effective, specific airway conductance (sGeff), and changes in end-expiratory resting level at functional residual capacity (FRCpleth) are rarely appreciated. We aimed to assess BDR by spirometric and concomitantly measured plethysmographic parameters. Moreover, BDR on the specific aerodynamic work of breathing (sWOB) was evaluated.
Methods: From databases of 3 pulmonary centers, BDR to 200 g salbutamol was retrospectively evaluated by spirometric (∆FEV1 and ∆FEF25– 75), and plethysmographic (∆sGeff, ∆FRCpleth, and ∆sWOB) parameters in a total of 843 patients diagnosed as COPD (478 = 57%), asthma-COPD-overlap (ACO) (139 = 17%), or asthma (226 = 27%), encountering 1686 BDR-measurement-sets (COPD n = 958; ACO n = 276; asthma n = 452).
Results: Evaluating z-score improvement taking into consideration the whole pre-test z-score range, highest BDR was achieved by combining ∆sGeff and ∆FRC detecting BDR in 62.2% (asthma: 71.4%; ACO: 56.7%; COPD: 59.8%), by ∆sGeff in 53.4% (asthma: 69.1%; ACO: 51.6%; COPD: 47.4%), whereas ∆FEV1 only distinguished in 10.6% (asthma: 21.8%; ACO: 18.6%; COPD: 4.2%). Remarkably, ∆sWOB detected BDR in 49.4% (asthma: 76.2%; ACO: 47.8%; COPD: 46.9%).
Conclusion: BDR largely depends on the pre-test functional severity and, therefore, should be evaluated in relation to the pre-test conditions expressed as ∆z-scores, considering changes in airway dynamics, changes in static lung volumes and changes in small airway function. Plethysmographic parameters demonstrated BDR at a significant higher rate than spirometric parameters.
Keywords: COPD, bronchodilator response, COPD and coexisting asthma, ACO, pulmonary hyperinflation, small airways dysfunction, aerodynamic work of breathing
Introduction
Chronic obstructive pulmonary disease (COPD) is a common, complex and heterogeneous disease, characterized by airflow limitation and an increased inflammatory response of the lung.1–3 The complexity refers to components with interactions, while heterogeneity is suggested because not all components are present in all patients at the same time,4,5 especially not over a lifetime.6 Bronchodilator response (BDR)– a major feature of asthma– is usually defined as an improvement of the FEV1, FVC, or the FEV1/FVC-ratio, and is included as a major criterion for the diagnosis of the asthma COPD overlap (ACO).7–12 There is, however, still increasing evidence that explanatory power of lung function trajectories, especially FEV1 and FVC, are poorly compelling with the complex clinical and functional facets of COPD. A staging system that could offer a composite picture of the whole pattern of functional disease severity is highly desirable.13 As previously proposed by both the American Thoracic Society (ATS) and the European Respiratory Society (ERS), the degree of flow limitation should be expressed by z-scores, using the lower limit of normal (LLN) for FEV1, FVC, FEV1/FVC ratio, and FEF25–75, as cut-offs,14–17 identical with −1.645 z-scores, or the 5th percentiles in the distribution from which the reference values are derived. In our study also measurements of sGeff, sReff and FRCpleth were z-transformed, for the latter both the upper limit of normal (ULN), identical with +1.645 z-scores or the 95th percentiles were defined as cut-off. By that procedure the positioning of an observed measurement value within the distribution of the reference population could be obtained, and hence gender-, age-, height-, and ethnicity-specific corrected. Moreover, between-subject and age- and growth-related variability has to be taken into account.15,18,19 There is growing understanding that due to the enormous clinical, functional, structural, and biological heterogeneity in these patients and the changes in functional dynamics over time, a more personalized approach in diagnosing COPD and its potential subtypes is needed.4 There are additional features of COPD such as small airways dysfunction (SAD),20–23 and/or dynamic pulmonary hyperinflation (PHI),24–29 consistently interacting with parameters of airflow limitation, airway obstruction, and pulmonary hyperinflation. Since BDR is still considered as an important, potential phenotypic marker for several subtypes of COPD, BDR could well serve as a candidate for COPD subtyping. Admittedly, we have been interested in the evaluation of BDR not only by a single LLN level, but in a larger scope over the whole range of pre-test z-scores.
The objectives of the present study, therefore, were to evaluate BDR in a multidimensional approach, looking at an entire set of lung function parameters (spirometric and plethysmographic), expressed as z-scores in relation to the pre-test functional severity, in order to identify relevant parameters assessing BDR in relation to pre-test functional severity. Moreover, we wanted to examine BDR within functional subgroups of COPD patients with PHI on one hand and SAD on the other hand.
Patients and Methods
Study Design and Ethics
In the present paper, we refer to retrospectively evaluated data obtained from three Swiss centers (Center of Pulmonary Diseases, Hirslanden Private Hospital Group, Salem-Hospital, Bern, Switzerland; Clinic of Pneumology, Cantonal Hospital St. Gallen, Switzerland; Center of Pulmonology, Clinic Hirslanden, Zürich, Switzerland). Patients’ records were anonymized before analysis to maintain their confidentiality. The patients have been referred to the centers for extended pulmonary function testing and optimizing therapy. Data were collected from patients with a clinical diagnosis of chronic obstructive pulmonary diseases, either as (i) COPD, or patients with (ii) COPD with coexisting asthma (ACO), or (iii) bronchial asthma. The anamnestic and clinical features were assessed by experienced pulmonary physicians, based on history-taking, chest radiographs, high-resolution CT-scans, and functional investigations, such as spirometry, whole-body plethysmography, measurements of the carbon monoxide diffusion capacity (DLCO) and measurement of the fraction of exhaled nitric oxide (FeNO). COPD was defined by a history of smoking (current or ex-tobacco smokers) and a previously documented airflow limitation (FEV1/FVC < 70%). ACO was diagnosed when the subject had features of COPD and asthma.7–12 Asthma was diagnosed based on symptoms such as wheezing, shortness of breath, chest tightness and cough that vary over time in their occurrence, frequency, and intensity. Patients previously diagnosed with cystic fibrosis, interstitial lung disease, pulmonary thromboembolic disease, active tuberculosis, chest wall disease, neuromuscular disorder, malignant tumor, or a history of thoracotomy with pulmonary resection, were excluded.
The study was planned according to the Federal Law of Human Research, conceptualized according to the Swiss Ethics Committees on research involving humans, and was conducted in accordance with the tenets of the Declaration of Helsinki. The study is part of the framework of the project entitled “Functional diversification of the Asthma-ACO-COPD multi-center study” (ID 2017–00259), approved by the Governmental Ethics Committee of the State of Bern, St. Gallen and Zürich (Project KEK-BE PB_2017-00104). Written informed consent was waived because of the retrospective study design, which follows the institutional and national policies concerning research approvals. Master-files have been stored and secured in the REDCap-system of the Clinical Trial Unit, Medical Faculty, University of Berne, Switzerland.
Patients
From the database of the three centers, 843 measurement-sets fulfilled the inclusion criteria of a correctly conducted post-bronchodilator response-test to 200 µg salbutamol, defined as to be positive, if the condition ∆FEV1 and/or ∆FVC ≥ 12%, and ≥ 200 mL is fulfilled.15,30 These data sets were obtained from a total of 843 patients (226 patients with asthma, 26.8%; 139 patients with ACO, 16.5%; and 478 patients with COPD, 56.7%), with a mean-age of 65.7 years (min. 34.1 years, max. 89.5 years) and no significant difference between the 3 diagnostic classes.
Pulmonary Function Procedures
In the present study plethysmography including spirometry was performed using standard techniques according to ATS-ERS criteria,15,31 previously established and extended subsequently,2,32,33 using a constant-volume body plethysmograph (Master Screen Body, Erich Jaeger GmbH, Würzburg, Germany). During tidal breathing within the closed plethysmograph recordings of specific airway loops (sRaw-loops) are generated, consisting of the shift volume (Vpleth) and the tidal flow (V’). After automated electronic loop compensation achieving “Body Temperature and Pressure Saturated” (BTPS) corrections, various parameters of airway dynamics can be computed.
The integral method of the Jaeger MasterLab software (JLab® and SentrySuite®) evaluates changes of airway dynamics as effective specific airway resistance (sReff), its reciprocal values the effective specific airway conductance (sGeff), and the specific aerodynamic work of breathing (sWOB) concomitantly with changes in the end-expiratory lung volume (EELV) at FRC. Therefore, it was important that parameters of airway dynamics are assessed in the first phase of plethysmographic measurements, and hence not influenced by deep inspiration or forced breathing maneuvers or other efforts, which may influence the broncho-motor tone.34–37 Details regarding the sequence of plethysmographic measurements are given in Supplemental Material Section 1. A special export software was developed by PanGas Ltd, Dagmersellen, Switzerland, enabling access to all routinely stored parameters in every JLab-, Sentry-Suite-databases, respectively.
Assessment of Airway Dynamics by the Integral Method
Although numerous parameters of airway dynamics can be calculated from the plethysmographic sRaw-loop,38 we used the approach proposed by Matthys and Orth39 defining the so called “effective specific resistance” (sReff) as the ratio of the area of the shift-volume versus tidal volume (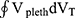 ), to the area of the tidal flow/volume loop (
), to the area of the tidal flow/volume loop (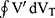 ) (see Figure S1). The mathematical background of the integral technique to obtain parameters of airway dynamics (sReff, sGeff, sWOB) has been previously established.33,39–41 Details of the methodological and mathematical approach of the so called “integral method” are given in the Supplemental Material Section 2. The advantage of this integral method compared with parameters of the two-point analysis defining sRaw, sGaw, respectively, is that data points throughout the entire respiratory cycle are evaluated. Moreover, the integral
) (see Figure S1). The mathematical background of the integral technique to obtain parameters of airway dynamics (sReff, sGeff, sWOB) has been previously established.33,39–41 Details of the methodological and mathematical approach of the so called “integral method” are given in the Supplemental Material Section 2. The advantage of this integral method compared with parameters of the two-point analysis defining sRaw, sGaw, respectively, is that data points throughout the entire respiratory cycle are evaluated. Moreover, the integral 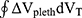 embodies the specific, aerodynamic work of breathing (sWOB) at rest.39 Pulmonary function test data were assessed in absolute values, percentage of predicted normal values, and as z-scores according to standard prediction equations.18,19 For the parameters of airway dynamics (sWOB, sGeff, and sReff), normative reference equations were used, recently worked out,42,43 and in details given in Supplemental Material Section 3.
embodies the specific, aerodynamic work of breathing (sWOB) at rest.39 Pulmonary function test data were assessed in absolute values, percentage of predicted normal values, and as z-scores according to standard prediction equations.18,19 For the parameters of airway dynamics (sWOB, sGeff, and sReff), normative reference equations were used, recently worked out,42,43 and in details given in Supplemental Material Section 3.
Data Analysis and Statistical Methods
Statistical analyses were performed with the IBM SPSS version 25.0 (SPSS Inc., Chicago, IL). The limit of significance was a p-value of 0.05. Data were transformed to z-scores as a standardized measure of the positioning of an observed measurement in the distribution of the reference population taking both between-subject as well as age- and growth-related variability into account.18,19 By this procedure the lower limits of normal (LLN), identical with −1.645 z-scores, or the 5th percentile in the distribution from which the reference values are derived for FEV1, FEF25–75, sGeff, as well as the upper limit of normal (ULN), identical with + 1.645 z-scores or the 95th percentiles for FRCpleth, and sWOB were obtained. The theoretical background on the Gaussian distribution, the z-score levels, and their parameter-specific pre-test z-score distribution over the whole range of z-scores are given in Table S1 and Figure S2 of Supplemental Material Section 3.
Functional Severity
Apart from the criteria given in the last issue of GOLD to use “LLN”, “ULN” respectively as a threshold to distinguish between “normal” from “abnormal”,3 we thought it suitable to define functional severity not only based on a one-dimensional criterion, but substantiated on the individual pre-test z-scores over the whole range of baseline z-scores. The baseline values of each lung function parameter have been transferred into z-scores. This allowed the option to study the graduated individual degree of severity within different functional subtypes, such as the degree of bronchial obstruction, flow limitation, small airway dysfunction, or pulmonary hyperinflation.
Bronchodilator Response (BDR)
Apart from the condition whether the LLN (FEV1, FEF25–75 sGeff), ULN (FRCpleth, sWOB) respectively, was reached after 400 ug salbutamol, we developed a multi-level approach, looking for ∆z-scores of BDR within the whole range pre-test z-score distribution and hence several levels of the Gaussian z-scores, as shown in Figure S2 of Supplemental Material Section 3. A positive BDR was specified, if a ∆FEV1-z-score equivalent to ∆FEV1 and/or ∆FVC ≥ 12%, and ≥ 200 mL was achieved as predictor for the ∆z-scores of each of the other 5 parameters after bronchodilation. Based on that regression model, equivalent improvement in lung function for each parameter could be determined.
Pearson’s χ2-test for categorical variables was performed for discriminating prevalence within patient collectives differentiating lung function within “normal” and “abnormal” ranges of airflow limitation, bronchial obstruction, small airway dysfunction and pulmonary hyperinflation, and finally for better accuracy of defining several functional subtypes in asthma versus ACO and COPD. Fisher’s linear discriminant analysis was used as a classification tool to maximize the criterion function of the target parameters in the discrimination between the diagnostic classes, to define the ranking order characterizing or separating the diagnostic classes as a classifier.
Results
Prevalence of Airflow-Limitation, Bronchial Obstruction, and Dynamic Pulmonary Hyperinflation
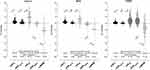
To allow a comparison with former studies, lung function measurements were first stratified according to the baseline values, i.e. whether they lay within the range of normal or not, the latter indicating the prevalence of each parameter (Table 1). According to the GOLD-Classification, prevalence of airflow limitation was in the global 85.2%, highest in COPD (90.6%), ACO (90.5%) respectively. Using the ATS/ERS criteria prevalence of airflow limitation was in the global 87.2%, highest in COPD (94.8%). Regarding the 5 target parameters, and taking LLN, ULN respectively as pre-test criteria of functional severity, the prevalence of airflow limitation in measurements of patients with asthma/ACO/COPD was 65.0%/86.0%/85.0% for FEV1; 50.0%/70.8%/70.0% for FEF25–75; 86.3%/91.9%/87.7% for sGeff; 82.7/83.9/94.6 for sWOB; and 35.1%/51.1%/63.3% for FRCpleth (Table 1; row b). Accordingly, the highest prevalence for asthma was found by sGeff and sWOB, for ACO by sGeff and FEV1, and for COPD by sGeff and sWOB. Noteworthy, a high percentage of abnormal pre-test measurements, and hence a marker for the prevalence of PHI, was found for FRCpleth (53.8%), indicating that PHI was initially present in 35.1% of asthmatics, 51.1% of patients with ACO and 63.3% of patients with COPD. The percent-distribution over the 4 z-score levels representing the distribution of functional severity at pre-test (row c) shows that sGeff and sWOB presented with the highest allocation of functional severity in the z-score range > 2.576 SD, especially in patients with COPD (93.3%, 91.0%, respectively; rows c). No differences in the z-score distributions were found regarding gender. The quantitative distribution of pre-test z-scores is synoptically presented in Figure 1. The largest z-score ranges are shown for sWOB (18.9 SD) followed by sGeff (13.9 SD).
 |
Figure 1 The quantitative distribution of z-scores pre-test of the parameters displayed over the whole z-score pre-test range of 3 spirometric and 3 plethysmographic lung function parameters. |
Bronchodilator Response (BDR)
Results of BDR taking pre-test values of ≤ LLN for FEV1, FEF25–75, sGeff, and of ≥ ULN for FRCpleth, sWOB respectively as baseline starting points and hence excluding measurements within the range of normal on one hand, and individual ∆z-scores on the other hand, obtained within the 3 diagnostic classes are given in Table 2. Pre-test measurements lying within ± 1.645 SD considered as “initially normal” are given in row a, showing that pre-test measurements were “initially normal” in 20.2% of FEV1, 35.2% of FEF25–75, but only in 11.6% of sGeff. Regarding sWOB only 10.3% “were initially normal”, whereas FRCpleth was normal in 46.2%. Noteworthy, sGeff in combination with FRCpleth presented only 9.3% as “initially normal”.
Bronchodilator Response Taking LLN, ULN Respectively as Thresholds
In Table 2 (row b) BDR is presented using the criterion whether or not reaching LLN, ULN respectively, as proposed by the ERS Task force.15,16,31,44 BRD including all diagnostic classes could be found for all parameters in range between 10–20%.
Bronchodilator Response Assessed by Improvement Within the Whole Range of z-Scores
To find an algorithm enabling a statistically and mathematically correct comparison of BDR of all 5 lung function parameters within the 3 diagnostic groups, the ATS-ERS-criterion (∆FEV1 ≥ 12%; ≤ 200 mL) was taken as primary criterion for sufficient BDR, corresponding to a ∆FEV1 of z-score = ∆ 0.778. Equivalent thresholds were computed for each of the other parameters differentiating significantly between “responders” and “non-responders” and the 3 diagnostic classes for each parameter (Table 2, row e; p <0.001). It can be demonstrated that by the assessment evaluating BDR over the whole z-score range much higher BDR percent responses were achieved than assessed by the criteria LLN, ULN, respectively. The highest BDR was achieved by the ∆sWOB in 72.6% of measurements (asthma: 76.2%; ACO: 71.1%; COPD: 72.6%), followed by the combination of ∆sGeff with ∆FRCpleth in 62.2% (asthma: 71.4%; ACO: 56.7%; COPD: 59.8%). BDR by ∆FEV1 was only seen in 10.6% of measurements (asthma: 21.8%; ACO: 18.6%; COPD: 4.2%). The differences between the two assessments of BDR are presented in row d, showing that highest differences were achieved by ∆sWOB (62.3%) followed by the combination of ∆sGeff with ∆FRCpleth (48.1%).
In Figure 2 the BDR, expressed as ∆z-scores, obtained by each target parameter within the 3 diagnostic classes are given. All parameters presented with significant responses (p <0.001). However, the distribution of BDR between the parameters was quite different. The most pronounced BDR was achieved by ∆sWOB (asthma: 76.6%; ACO: 72.4%; COPD: 71.1%), followed by ∆sGeff (asthma: 68.7%; ACO: 68.8%; COPD: 47.3%). It was supposed that BDR could be influenced by the degree of pre-test functional severity. However, that was only the case for ∆sWOB. Noteworthy, in patients with COPD, a considerable number of patients presented BDR apart from patients with bronchial obstruction (58.1%) also by a decrease of pulmonary hyperinflation in 23.1%, or both in 18.8%. In this particular plethysmographic sub-group, BDR has been observed by ∆FEV1 only in 5.1%.
Discussion
Findings of the Present Study
There are only a few studies reporting on a combination of spirometric and plethysmographic measurements to detect functional severity and BDR in patients with COPD.45–49 Jarenbäck et al. investigated the BDR based on advanced lung function parameters and brought to light that apart from flow response, also volume response, or a combination of both, have to be identified, if BDR is to be correctly assessed in COPD.49 Topalovic et al. and Borrill et al. demonstrated that the specific airway conductance (sGaw) could be a significant factor to differentiate asthma from COPD,45,47 and regarding BDR, Saito et al. showed that sGaw is a more sensitive measurement for detecting lung function changes in COPD patients than the more commonly used FEV1.48 However, to our knowledge, none of these author groups evaluated measurements assessing airway dynamic parameters by the plethysmographic integral method featuring the parameters sReff, sGeff, and sWOB. If the spirometric approach is combined with a plethysmographic assessment, a profound insight into lung physiology is provided, insofar as not only the degree of flow limitation, but also the degree of bronchial obstruction and small airways dysfunction, as well as the magnitude of pulmonary hyperinflation can be estimated in COPD.
The main findings of the present study are that a combination of spirometric and plethysmographic parameters (i) enlarge the scope of evaluation of different functional deficits, within (ii) different functional severity groups. Apparently, this is due to the appreciation of the whole z-score range at pre-test of different target parameters. Accordingly, (iii) BDR is distinctive for each single parameter. In consequence, our study shows that (iv) BDR largely depends on the type and degree of pre-test dysfunction49 and, therefore, the magnitude of the BDR was greater than expected, and as previously reported.22,49–51 Unmistakably, it must be recognized that the overwhelming belief that COPD patients mostly present with an irreversible flow-limitation, is only based on the worldwide approach that reversibility mostly was assessed by simple spirometric measurements. There is now increasing understanding that treatment objectives should improve the function not only in central airways, but especially also in the small peripheral airways, both by improving flow and by decreasing the degree of alveolar hypoventilation, as well as decreasing air trapping and hyperinflation in COPD. Regarding BDR, it is thus important to go beyond the information provided by FEV1, which mainly reflects flow limitation in the central airways.
Small Airways Dysfunction (SAD)
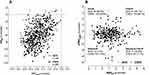
In recent years, there has been a resurgence of interest in the study of SAD from a pathophysiological viewpoint, especially as a precursor of emphysema.20,52 In support of this concept maximal mid-expiratory flow (MMEF) was found as a surrogate of small airways function.53 Cross-sectional studies suggest that airway narrowing eventually results in a loss of airways.54 It was anticipated that small airway disease can build up over time in peripheral zones of the lungs, without being detectable by FEV1.55 Regarding plethysmographic parameters, it has been anticipated that sReff, and hence its inverse, more robust measure sGeff, were closely associated with symptoms of dyspnea.56 Therefore, MMEF and hence FEF25–75 as well as sGeff have been esteemed as reliable measures of peripheral airway function. We were interested to elaborate the interrelationship between FEF25–75 and sGeff (both lower than LLN at pre-test) in a selective cohort of 616 measurements of patients with ACO (n = 136) and COPD (n = 480). Figure 3 demonstrates that there is a significant interrelationship between FEF25–75 and sGeff (Figure 3A, ACO: F = 10.2 p = 0.002; COPD: F = 120.8 p <0.0001). We like to conclude, that in contrast to sGaw, obtained by the angle-technique, sGeff computed by the integral method and hence considering the whole resistance slope, qualifies well as a parameter for central and peripheral airway function.
Pulmonary Hyperinflation (PHI)
Pulmonary hyperinflation, defined as an increased volume of air remaining in the lungs at the end of spontaneous expiration, is present when resting FRCpleth or EELV is increased above normal.26,27 The development of PHI in the disease course of COPD, therefore, is clinically important, mainly because it contributes to dyspnea and reduced physical activity.26,27 Research in recent years has clearly demonstrated that hyperinflation, at rest and/or during exercise, is more closely associated with important clinical outcomes such as dyspnea and exercise intolerance than with expiratory flow indices.26 Moreover, hyperinflation has become an important endpoint in several clinical trials evaluating the efficacy of pharmacological and non-pharmacological therapeutic approaches to COPD. Recently, a few studies have used the inspiratory capacity (IC) in relation to TLC as an indicator of static hyperinflation, since it correlated well with subjective dyspnea, exercise intolerance, and mortality in COPD patients.57,58 It was, however, recognized that using only IC as an indicator of a potential hyperinflation has limitations. Patients with mild airway obstruction and increased FRCpleth can have an IC within the normal range. Moreover, it has to be preconceived that after deep inspirations and/or bronchodilator administration changes in the EELV at FRC may occur,30 altering the distending forces of the bronchial tree, which may influence the flow in small airways, a dynamic functional aspect which has to be kept in mind.
BDR Within Functional Sub-Types in COPD
Response in Relation to Different Initial Functional Subtypes
In a subset of 842 measurements, 18.4% presented with a PHI (asthma: 28.0%; ACO: 11.0%; COPD: 16.6%), 32.0% suffered from a SAD (asthma: 49.7%; ACO: 36.7%; COPD: 23.8%), and a combination of both, was found in 49.6% (asthma: 22.3%; ACO: 52.3%; COPD: 59.6%) of abnormal pre-test measurements. Figure 3B shows that after bronchodilation the BDR can be allocated in an ∆sGeff-∆FRCpleth-plot into non-responder (left upper quadrant; approximately 30%), volume-responder (left lower quadrant; in COPD 13.9%), flow responder (right upper quadrant; approximately 50%) and volume- and flow-responder (right lower quadrant; 11–14%). Noteworthy, by assessing BDR by combining airway dynamics (∆sGeff) with changes in static lung volumes (∆FRCpleth) at least 25% of patients with COPD, which would remain undetected by simple spirometry, could be identified as responders. Such findings may serve as cornerstones in future treatment concepts, since it has reliably been shown that bronchodilators reduce the degree of PHI, and hence also the work of breathing in the absence of a significant spirometric response.59 Therefore, it is our belief that tailoring diagnostics and treatment in COPD would require that a distinction into diagnostic classes and functional subtypes, as well as influencing confounders, must carefully be assessed, especially when asthma and COPD coexist as overlap (ACO).
Potential Sources of Mis-Qualification
For most parameters of airflow limitation and bronchial obstruction, the pre-test z-scores were < −2.576 equal to the lower left of the Gaussian distribution (0.5 percentile) mostly expressed by sGeff (66.8%) compared with FEV1 (54.6%). In other words, more than half of the patients started before bronchodilation with a severe flow-limitation prior to bronchodilation. Looking at the sWOB, it can be demonstrated that most patients (87.6%) presented with a sWOB z-score > 6.9. This new discovery leads to the assumption that patients with the need of 5–6 times more energy for breathing pre-test at rest, are likely to be limited in their breathing capacities. Plethysmographic measurements are performed during tidal breathing, requesting only low cooperation and no coordination. It follows that severe pre-test functional derangement amplifies the specific aerodynamic work of breathing, potentially impeding the patient’s coordination and cooperation for optimal forced breathing maneuvers and hence mis-qualifying baseline functional conditions. The assumption stands to reason, whether some COPD-patients with clear BDR should not be classified as ACO and hence treated accordingly. This remains assumed because it must be evaluated prospectively.
Limitations and Strengths of the Study
The present study is a retrospective evaluation of lung function data obtained by various parameters and there are no longitudinal observations, a feature that can only be achieved by a prospectively designed study. Our actual challenge was to find surrogate markers superior to FEV1, which is still considered as the gold standard for both the diagnosis and assessment of BDR. As it turned out, the evaluation of a potential BDR obtained by ∆sGeff, and a combination of ∆sGeff with ∆FRCpleth as well as of by ∆sWOB disclosed a fundamentally different response archetype, which requires a new definition of lung function reversibility in COPD. The search for such alternatives is not new, as there has been a request for surrogate markers superior to FEV1 for several years. To differentiate the functional subtypes of COPD both spirometry and plethysmography are needed. Regarding BDR, our data show that sGeff is the most convincing target parameter to define reversibility of obstruction and flow limitation in central and peripheral airways.
The main limitation of our study is that it only addresses functional trajectories representing the complex lung physiology in COPD, that are not directly linked to clinical settings. However, the aerodynamic specific work of breathing at rest (sWOB) features presumably the closest parameter associated with clinical signs such as wheezing, shortness of breath, chest tightness and cough, and could well be taken as a marker for longitudinal follow-up and treatment efficacy. Other limitations are the relatively small number of subjects per center and within the sub-groups of COPD. However, there were no differences if the centers were compared with one another (data not shown). Therefore, the population-based retrospective nature of our study and its highly standardized multicenter framework has a reliable force of expression.
Conclusion
The enormous functional, structural, and biological heterogeneity in patients with COPD implicates an assessment of target parameters evaluating interactionally flow limitation, bronchial obstruction, small airways dysfunction and pulmonary hyperinflation, graduating precisely functional severity defined by a multi-level approach with pre-test z-scores, on which standardized changes regarding BDR can be apprised. Such an approach can be helpful in the tracking of dynamics and changes over time in patients with COPD. Moreover, the distinction of different sub-phenotypes on one hand, and corresponding treatment strategies on the other hand could be provided.4 In view of the serious disease burden in COPD we conclude that a more sophisticated assessment of functional deficits and their reversibility is justified.
Abbreviations
ACO, asthma COPD overlap; ATS, American Thoracic Society; BDR, bronchodilator response; COPD, chronic obstructive pulmonary disease; DLCO, carbon monoxide diffusion capacity; ∆Vpleth, plethysmographic shift volume: EELV, end-expiratory lung volume; ERS, European Respiratory Society; FEF25–75, forced expiratory flow between 25 and 75% vital capacity; FeNO, fraction of exhaled nitric oxide; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; FRCpleth, plethysmographic functional residual capacity; FVC, forced vital capacity; Gaw, airway conductance; IC, inspiratory capacity; LLN, lower limit of normal; LMM, linear mixed model; Ln, natural logarithm; MMEF, maximal mid-expiratory flow; Pamb, ambient pressure; PH2O, water pressure; PHI, pulmonary hyperinflation; RV, residual volume; SAD, small airways dysfunction; SD, standard deviation; Raw, airway resistance; sGaw, specific airway conductance (angle method); sGeff, effective specific airway conductance (integral method); sReff, effective specific airway resistance (integral method); sWOB, effective resistive work of breathing (integral method); TLC, total lung capacity; ULN, upper limit of normal; VC, vital capacity; VIF, variance inflation factor; V’, flow; VT, tidal volume.
Ethics Approval
The study was planned according to the Federal Law of Human Research, conceptualized according to the Swiss Ethics Committees on research involving humans, and was conducted in accordance with the tenets of the Declaration of Helsinki. The study is part of the framework of the project entitled “Functional diversification of the Asthma-ACO-COPD multi-center study” (ID 2017-00259), approved by the Governmental Ethics Committee of the State of Bern, St. Gallen and Zürich (Project KEK-BE PB_2017-00104). Written informed consent was waived because of the retrospective study design, which follows the institutional and national policies concerning research approvals. Master-files have been stored and secured in the REDCap-system of the Clinical Trial Unit, Medical Faculty, University of Berne, Switzerland.
Acknowledgment
We would also like to thank Sabina Gallati from the Division of Human Genetics, University of Berne for the critical reviews of the manuscripts and Mrs. Zoe Clerc, Laboratory Technician, Division of Human Genetics, University of Berne for reviewing the English style of the manuscript.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
No funding was received.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Agusti A, Vestbo J. Current controversies and future perspectives in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2011;184(5):507–513. doi:10.1164/rccm.201103-0405PP
2. Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD Executive Summary. Am J Respir Crit Care Med. 2017;195(5):557–582. doi:10.1164/rccm.201701-0218PP
3. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for diagnosis, management, and prevention, of chronic obstructive pulmonary disease; 2021. Available from: https://goldcopd.org.
4. Faner R, Agusti A. Multilevel, dynamic chronic obstructive pulmonary disease heterogeneity. A challenge for personalized medicine. Ann Am Thorac Soc. 2016;13(Suppl 2):S466–S470. doi:10.1513/AnnalsATS.201605-372AW
5. Han MK, Quibrera PM, Carretta EE, et al. Frequency of exacerbations in patients with chronic obstructive pulmonary disease: an analysis of the SPIROMICS cohort. Lancet Respir Med. 2017;5(8):619–626. doi:10.1016/S2213-2600(17)30207-2
6. Agusti A, MacNee W. The COPD control panel: towards personalised medicine in COPD. Thorax. 2013;68(7):687–690. doi:10.1136/thoraxjnl-2012-202772
7. Sin DD, Miravitlles M, Mannino DM, et al. What is asthma-COPD overlap syndrome? Towards a consensus definition from a round table discussion. Eur Respir J. 2016;48(3):664–673. doi:10.1183/13993003.00436-2016
8. Cosio BG, Soriano JB, Lopez-Campos JL, et al. Defining the asthma-COPD overlap syndrome in a COPD cohort. Chest. 2016;149(1):45–52. doi:10.1378/chest.15-1055
9. Postma DS, Rabe KF. The asthma-COPD overlap syndrome. N Engl J Med. 2015;373(13):1241–1249. doi:10.1056/NEJMra1411863
10. Suzuki M, Makita H, Konno S, et al. Asthma-like features and clinical course of chronic obstructive pulmonary disease. An analysis from the hokkaido COPD cohort study. Am J Respir Crit Care Med. 2016;194(11):1358–1365. doi:10.1164/rccm.201602-0353OC
11. de Marco R, Marcon A, Rossi A, et al. Asthma, COPD and overlap syndrome: a longitudinal study in young European adults. Eur Respir J. 2015;46(3):671–679. doi:10.1183/09031936.00008615
12. de Marco R, Pesce G, Marcon A, et al. The coexistence of asthma and chronic obstructive pulmonary disease (COPD): prevalence and risk factors in young, middle-aged and elderly people from the general population. PLoS One. 2013;8(5):e62985. doi:10.1371/journal.pone.0062985
13. Celli BR, MacNee W, Force AET. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23(6):932–946. doi:10.1183/09031936.04.00014304
14. Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) workshop summary. Am J Respir Crit Care Med. 2001;163(5):1256–1276. doi:10.1164/ajrccm.163.5.2101039
15. Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26(5):948–968. doi:10.1183/09031936.05.00035205
16. Swanney MP, Ruppel G, Enright PL, et al. Using the lower limit of normal for the FEV1/FVC ratio reduces the misclassification of airway obstruction. Thorax. 2008;63(12):1046–1051. doi:10.1136/thx.2008.098483
17. Quanjer PH, Enright PL, Miller MR, et al. The need to change the method for defining mild airway obstruction. Eur Respir J. 2011;37(3):720–722. doi:10.1183/09031936.00135110
18. Stocks J, Quanjer PH. Reference values for residual volume, functional residual capacity and total lung capacity. ATS workshop on lung volume measurements. Official statement of the European respiratory society. Eur Respir J. 1995;8(3):492–506. doi:10.1183/09031936.95.08030492
19. Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159(1):179–187. doi:10.1164/ajrccm.159.1.9712108
20. Hogg JC, Chu F, Utokaparch S, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(26):2645–2653. doi:10.1056/NEJMoa032158
21. Burgel PR. The role of small airways in obstructive airway diseases. Eur Respir Rev. 2011;20(119):23–33. doi:10.1183/09059180.00010410
22. Pisi R, Aiello M, Zanini A, et al. Small airway dysfunction and flow and volume bronchodilator responsiveness in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2015;10:1191–1197.
23. Crisafulli E, Pisi R, Aiello M, et al. Prevalence of small-airway dysfunction among COPD patients with different GOLD stages and its role in the impact of disease. Respiration. 2017;93(1):32–41. doi:10.1159/000452479
24. O’Donnell DE. Hyperinflation, dyspnea, and exercise intolerance in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2006;3(2):180–184. doi:10.1513/pats.200508-093DO
25. O’Donnell DE, Laveneziana P. The clinical importance of dynamic lung hyperinflation in COPD. Copd. 2006;3(4):219–232. doi:10.1080/15412550600977478
26. Gagnon P, Guenette JA, Langer D, et al. Pathogenesis of hyperinflation in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2014;9:187–201.
27. Rossi A, Aisanov Z, Avdeev S, et al. Mechanisms, assessment and therapeutic implications of lung hyperinflation in COPD. Respir Med. 2015;109(7):785–802. doi:10.1016/j.rmed.2015.03.010
28. Park J, Lee CH, Lee YJ, et al. Longitudinal changes in lung hyperinflation in COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:501–508. doi:10.2147/COPD.S122909
29. Alter P, Orszag J, Kellerer C, et al. Prediction of air trapping or pulmonary hyperinflation by forced spirometry in COPD patients: results from COSYCONET. ERJ Open Res. 2020;6(3):92–2020. doi:10.1183/23120541.00092-2020
30. Quanjer PH, Tammeling GJ, Cotes JE, Pedersen OF, Peslin R, Yernault J-C. Lung volumes and forced ventilatory flows. Eur Respir J. 1993;Suppl. 6(Suppl 16):5–40. doi:10.1183/09041950.005s1693
31. Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi:10.1183/09031936.05.00034805
32. Goldman M, Smith HJ, Ulmer WT. Lung function testing: whole-body plethysmography. Eur Respir Mon. 2005;31:15–43.
33. Kraemer R, Smith H-J, Sigrist T, Giger G, Keller R, Frey M. Diagnostic accuracy of methacholine challenge tests assessing airway hyperreactivity in asthmatic patients - a multifunctional approach. Respir Res. 2016;17(1):154. doi:10.1186/s12931-016-0470-0
34. Kapsali T, Permutt S, Laube B, Scichilone N, Togias A. Potent bronchoprotective effect of deep inspiration and its absence in asthma. J Appl Physiol. 2000;89(2):711–720. doi:10.1152/jappl.2000.89.2.711
35. Salome CM, Thorpe CW, Diba C, Brown NJ, Berend N, King GG. Airway re-narrowing following deep inspiration in asthmatic and nonasthmatic subjects. Eur Respir J. 2003;22(1):62–68. doi:10.1183/09031936.03.00117502
36. Slats AM, Janssen K, van Schadewijk A, et al. Bronchial inflammation and airway responses to deep inspiration in asthma and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;176(2):121–128. doi:10.1164/rccm.200612-1814OC
37. Nensa F, Marek W, Marek E, Smith HJ, Kohlhaufl M. Assessment of airway hyperreactivity: comparison of forced spirometry and body plethysmography for methacholine challenge tests. Eur J Med Res. 2009;14(Suppl 4):170–176. doi:10.1186/2047-783X-14-S4-170
38. Ulmer WT, Marek W, Rasche K. [Airway resistance curves in obstructive respiratory tract diseases. 8 types of airway resistance curves in spontaneous respiration]. Fortschr Med. 1988;106(33):663–667.
39. Matthys H, Orth U. Comparative measurements of airway resistance. Respiration. 1975;32(2):121–134. doi:10.1159/000193642
40. Kraemer R, Baldwin DN, Ammann RA, Frey U, Gallati S. Progression of pulmonary hyperinflation and trapped gas associated with genetic and environmental factors in children with cystic fibrosis. Respir Res. 2006;7(1):138. doi:10.1186/1465-9921-7-138
41. Kraemer R, Delosea N, Ballinari P, Gallati S, Crameri R. Effect of allergic bronchopulmonary aspergillosis on lung function in children with cystic fibrosis. Am J Respir Crit Care Med. 2006;174(11):1211–1220. doi:10.1164/rccm.200603-423OC
42. Kraemer R, Smith HJ, Matthys H. Normative reference equations of airway dynamics assessed by whole-body plethsmography during spontaneous breathing transitionally evaluated in infants, children and adults. Res Sq. 2020. doi:10.21203/rs.3.rs-39320/v1
43. Kraemer R, Smith HJ, Matthys H. Normative reference equations of airway dynamics assessed by whole-body plethysmography during spontaneous breathing evaluated in infants, children, and adults. Physiol Rep. In press 2021. doi:10.14814/phy2.15027
44. Bakke PS, Ronmark E, Eagan T, et al. Recommendations for epidemiological studies on COPD. Eur Respir J. 2011;38(6):1261–1277. doi:10.1183/09031936.00193809
45. Borrill ZL, Houghton CM, Woodcock AA, Vestbo J, Singh D. Measuring bronchodilation in COPD clinical trials. Br J Clin Pharmacol. 2005;59(4):379–384. doi:10.1111/j.1365-2125.2004.02261.x
46. Santus P, Radovanovic D, Henchi S, et al. Assessment of acute bronchodilator effects from specific airway resistance changes in stable COPD patients. Respir Physiol Neurobiol. 2014;197:36–45. doi:10.1016/j.resp.2014.03.012
47. Topalovic M, Derom E, Osadnik CR, et al. Airways resistance and specific conductance for the diagnosis of obstructive airways diseases. Respir Res. 2015;16(1):88. doi:10.1186/s12931-015-0252-0
48. Saito T, Takeda A, Hashimoto K, Kobayashi A, Hayamizu T, Hagan GW. Triple therapy with salmeterol/fluticasone propionate 50/250 plus tiotropium bromide improve lung function versus individual treatments in moderate-to-severe Japanese COPD patients: a randomized controlled trial - Evaluation of Airway sGaw after treatment with tripLE. Int J Chron Obstruct Pulmon Dis. 2015;10:2393–2404.
49. Jarenback L, Eriksson G, Peterson S, Ankerst J, Bjermer L, Tufvesson E. Bronchodilator response of advanced lung function parameters depending on COPD severity. Int J Chron Obstruct Pulmon Dis. 2016;11:2939–2950. doi:10.2147/COPD.S111573
50. Tashkin DP, Celli B, Decramer M, et al. Bronchodilator responsiveness in patients with COPD. Eur Respir J. 2008;31(4):742–750. doi:10.1183/09031936.00129607
51. Albert P, Agusti A, Edwards L, et al. Bronchodilator responsiveness as a phenotypic characteristic of established chronic obstructive pulmonary disease. Thorax. 2012;67(8):701–708. doi:10.1136/thoraxjnl-2011-201458
52. McDonough JE, Yuan R, Suzuki M, et al. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. N Engl J Med. 2011;365(17):1567–1575. doi:10.1056/NEJMoa1106955
53. Stockley JA, Ismail AM, Hughes SM, Edgar R, Stockley RA, Sapey E. Maximal mid-expiratory flow detects early lung disease in α1 -antitrypsin deficiency. Eur Respir J. 2017;49(3):1602055. doi:10.1183/13993003.02055-2016
54. Bodduluri S, Kizhakke Puliyakote A, Nakhmani A, Charbonnier J-P. Computed tomography–based airway surface area–to-volume ratio for phenotyping airway remodeling in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2021;203(2):185–191. doi:10.1164/rccm.202004-0951OC
55. Bhatt SP, Soler X, Wang X, et al. Association between functional small airway disease and FEV1 decline in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2016;194(2):178–184. doi:10.1164/rccm.201511-2219OC
56. Mahut B, Caumont-Prim A, Plantier L, et al. Relationships between respiratory and airway resistances and activity-related dyspnea in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2012;7:165–171.
57. French A, Balfe D, Mirocha JM, Falk JA, Mosenifar Z. The inspiratory capacity/total lung capacity ratio as a predictor of survival in an emphysematous phenotype of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2015;10:1305–1312.
58. Salomon J, Stolz D, Domenighetti G, et al. Indacaterol and glycopyrronium versus indacaterol on body plethysmography measurements in COPD-a randomised controlled study. Respir Res. 2017;18(1):13. doi:10.1186/s12931-016-0498-1
59. Newton MF, O’Donnell DE, Forkert L. Response of lung volumes to inhaled salbutamol in a large population of patients with severe hyperinflation. Chest. 2002;121(4):1042–1050. doi:10.1378/chest.121.4.1042
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.