Back to Journals » Pathology and Laboratory Medicine International » Volume 7
BRAF mutational analysis in ovarian tumors: recent perspectives
Authors Wong K, Tsai C, Gershenson D
Received 28 April 2015
Accepted for publication 15 July 2015
Published 18 September 2015 Volume 2015:7 Pages 75—82
DOI https://doi.org/10.2147/PLMI.S64383
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Paul Zhang
Kwong-Kwok Wong,1 Ching-Chou Tsai,2 David M Gershenson1
1Department of Gynecologic Oncology and Reproductive Medicine, The University of Texas MD Anderson Cancer Center, Houston, TX, USA; 2Department of Obstetrics and Gynecology, Kaohsiung Chang Gung Memorial Hospital, Chang Gung University College of Medicine, Kaohsiung, Taiwan, Republic of China
Abstract: BRAF mutations are rare in ovarian cancer and mainly occur in indolent serous borderline tumors (SBTs), also known as serous tumors of low malignant potential or atypical proliferative serous tumors. The reported percentage of BRAF mutations in SBTs varies from 23% to 71%. Although a high percentage of stage II–IV SBTs with noninvasive implants have progressed to invasive low-grade serous carcinomas when patients were observed for 5 years or longer, BRAF mutations are rare in low-grade serous carcinomas as well as in invasive implants associated with SBTs. BRAF mutations in SBTs may prevent SBTs from progressing to invasive carcinomas. On the other hand, the reported percentage of BRAF mutations in mucinous carcinoma (20%) is much higher than that of mucinous borderline tumor (5%). Further investigation of the role of BRAF mutations in SBTs and mucinous tumor will shed light on the molecular mechanism underlying the role of BRAF mutations in tumor progression in different cellular context and the clinical utility of BRAF mutations in SBTs as a biomarker of favorable prognosis.
Keywords: BRAF V600E, ovarian cancer, COLD-PCR
Introduction
BRAF is a serine/threonine protein kinase of the RAF family that also includes ARAF and RAF1. BRAF has the highest basal level in the RAF family and is part of the mitogen-activated RAS/RAF/MEK/ERK protein kinase pathway, which acts as a signal transducer between the extracellular signals and the nucleus. Extracellular signals such as hormones, cytokines, and various growth factors interact with their receptors to activate the small G-proteins of the RAS family and subsequently activate BRAF. Active BRAF then activates MEK1/2 to phosphorylate ERK1/2, which leads to the expression of several downstream transcription factors that regulate cell growth, differentiation, and survival.
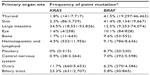
Mutations in the BRAF gene were first discovered by Davies et al1 in 2002 through a systematic and genome-wide assessment of cancer-associated pathways in human cancer. The Catalogue of Somatic Mutations in Cancer (COSMIC, version 71)2 database identified BRAF point mutations in over 40,000 cancer samples (Table 1). The frequency of BRAF mutations varies from over 40% in thyroid and skin tumors to 0%–12.5% in tumors in other organs. Coexisting mutations of BRAF and KRAS are very rare; however, such phenomena have been observed in a hyperplastic polyp of the colon3 and in ovarian mucinous carcinoma.4
The vast majority of missense mutations in BRAF involve a thymine to adenine substitution at nucleotide 1799 (c.1799T>A), which results in an amino acid change from valine (V) to glutamate (E) at codon 600. This V600E mutation represents approximately 95% of all identified BRAF point mutations (Table 2). Mutated BRAF V600E activates ERK1/2 without the need for extracellular signals. Other relatively frequent BRAF missense mutations include mutations at codons 466, 594, and 601.
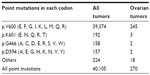  | Table 2 Frequency of common BRAF point mutations in all tumors and in ovarian tumors, according to the Catalogue of Somatic Mutations in Cancer (COSMIC) database |
BRAF point mutation has been reported in approximately 6% (270/4,386) of ovarian tumor samples tested for BRAF mutations in the COSMIC database (Tables 2 and 3). The majority of these ovarian tumor samples (245/270) have a mutation in codon 600, with p.V600E being the predominant missense mutation. BRAF gene amplification and overexpression were found in approximately 12% and 7%, respectively, of high-grade ovarian serous carcinomas using the cBio Cancer Genomics Portal5,6 to interrogate the data generated from The Cancer Genome Atlas study.7
  | Table 3 Frequency of BRAF point mutation detected in histologic subtypes of ovarian tumors, according to the Catalogue of Somatic Mutations in Cancer (COSMIC) database |
The available data indicate that BRAF mutations mainly occur in serous borderline tumors (SBTs),8 also known as serous tumors of low malignant potential or atypical proliferative serous tumors; SBTs also include micropapillary serous carcinoma (MPSC),9 a minor morphologically distinct subgroup that was first described by Burks et al10 in 1996. The reported frequency of BRAF mutations in SBTs ranges from 23% to 71%. We previously found that BRAF mutation is rare in advanced-stage ovarian low-grade serous carcinoma (LGSC).11 BRAF mutation is also rare in primary clear cell ovarian carcinoma (1%),12,13 mucinous borderline tumors (2%),13–15 and endometrioid carcinoma (3%),16 but is relatively more frequent in mucinous carcinoma4,17 and serous adenoma.18
Detection of mutated BRAF
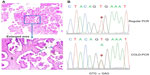
Most sequencing analyses of BRAF mutations in ovarian cancer have used direct Sanger sequencing. Standard polymerase chain reaction (PCR)-Sanger sequencing has a mutation detection sensitivity of approximately 10%.19 Since SBTs have a high component of stromal cells, the sensitivity for detecting the BRAF mutation may be compromised if DNA is extracted from bulk tissue for direct sequencing using regular PCR amplification of the targeted region (Figure 1A). To increase the mutation detection sensitivity, researchers can microdissect tumor cells from the paraffin section, but this strategy can be quite tedious. Several other methods, such as pyrosequencing,20 matrix-assisted laser desorption/ionization time of flight mass spectrometry,21 and comparative allele-specific TaqMan PCR,22 can be used to screen for BRAF mutations in samples with low tumor cell purity. We have adopted the co-amplification at lower denaturation temperature (COLD)-PCR approach, described previously,23 to enrich low-level mutant BRAF alleles in the DNA samples for detecting BRAF mutations by Sanger sequencing (Figure 1B).24 As shown in Figure 1B, COLD-PCR amplifies the mutated allele (A) to have the same signal intensity as the wild-type allele (T).
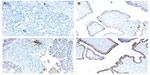
Immunohistochemical staining for the BRAF V600E mutation–specific monoclonal antibody VE125 can also be used to detect BRAF mutation. The sensitivity of VE1 immunostaining has been validated for detecting the BRAF V600E mutation in formalin-fixed and paraffin-embedded ovarian SBT tissues.26 Immunostaining was evaluable in most cases with sufficient tumor cells, but rare cases with scant cytoplasm and diffuse staining may be difficult to interpret.27 The false-positive rate of VE1 immunostaining can be as high as 30%, so initial VE1 immunostaining should be validated with sequencing.28 Figure 2 is an illustration of VE1 immunostaining on four ovarian tumor samples with confirmed BRAF mutation status by DNA sequencing. One of the SBT with wild-type BRAF gene (Figure 2B) (Figure 1A and 1B had wild-type BRAF; Figure 2C and 2D had mutated BRAF V600E) had faint false positive staining, which could be a result of an edge effect during staining and should be interpreted cautiously.
Role of BRAF mutation in the pathogenesis of ovarian cancer
There are four major histological subtypes of epithelial ovarian cancer (EOC), which is thought to arise from the surface epithelium of the ovaries but could also be from extra-ovarian origins.29 An ovarian tumorigenesis model based on morphology and molecular genetics has been proposed.30,31 EOCs are classified as Type I or Type II. Type I tumors include low-grade MPSC, mucinous, endometrioid, and clear cell carcinomas and are characterized by high frequency of KRAS, BRAF, PTEN, or beta-catenin mutations.30–34 Type II tumors include high-grade serous carcinoma, malignant mixed mesodermal tumors (carcinosarcomas) and undifferentiated carcinomas and are characterized by high genetic instability and high frequency of TP53 mutation.31,32 Separating EOC into Type I and Type II based on genetic mutations is controversial. High frequency of TP53 (57%, 8/14) and KRAS (57%, 8/14) mutations has also been found in Type I mucinous EOC.35 Similarly, Type I endometrioid cancer also has a high frequency of TP53 mutation (63%, 17/27).36 Both endometriosis-related cancers (endometrioid and clear cell cancer) have high frequency of ARID1A mutations.37,38 SBT has high frequency of BRAF and KRAS mutations while LGSC has high frequency of KRAS mutation. Although the progression of LGSC to high-grade serous carcinoma is very rare, several studies have reported the recurrence of high-grade serous carcinoma from SBT or LGSC.34,39,40 Thus, each ovarian carcinoma subtype should be treated as a different disease as suggested previously for future biomarker studies and clinical trials.41
No germline mutation of BRAF has been found, and thus the presence of BRAF mutation is probably not a genetic predisposition for the development of SBTs.16 Although a high percentage of stage II–IV SBTs with noninvasive implants have progressed to invasive LGSCs when patients were observed for 5 years or longer,42 BRAF mutations are rare in LGSCs as well as in invasive implants associated with SBTs.43,44 BRAF mutations are also rare in high-grade serous carcinomas. BRAF mutation is mainly found in ovarian serous adenoma, SBTs, invasive MPSC, and mucinous carcinomas.4,17,18,45,46
It is generally believed that adenoma progresses to SBT and then to LGSC. The pathologic difference between SBTs and LGSC is the destructive stromal invasion in LGSC. One study proposed that MPSC is a step in the progression from SBTs to LGSC.47 Destructive invasion of the ovarian stroma is rare in MPSC, but those with invasion are called invasive MPSC or are considered LGSC. MPSC represents approximately 6.5%–33% of all SBTs.10,16,48–51 However, there is a consensus that both typical SBTs and MPSC should be classified as LGSC when areas of stromal invasion are greater than 5 mm. Patients with SBT with or without MPSC features have no difference in recurrence or disease-related mortality, although noninvasive SBTs with a micropapillary pattern may have invasive peritoneal implants more often than those without the micropapillary pattern.50–54
Using sequencing analysis, Singer et al45 found that ovarian SBTs and low-grade invasive MPSC shared similar frequencies of both KRAS and BRAF mutations, which are involved in the progression of SBTs to LGSC.45 Another study found the same mutations in ovarian SBTs and the cystadenoma epithelium bordering the SBTs in six (86%) of seven samples.18 However, other studies suggest that ovarian SBTs with BRAF mutations may be less likely to spread or progress to LGSC. Most studies of ovarian LGSC reported a low frequency of BRAF mutations (Table 4).11,27,45,55–59 In one study of the implants that accompany ovarian SBTs, only six (13%) of 45 patients had BRAF mutations in SBTs with noninvasive peritoneal implants, and none of the patients had BRAF mutations in SBTs with invasive implants.43 Heublein et al44 detected BRAF mutations in only noninvasive implants associated with ovarian SBTs. Similarly, in our analysis of 36 patients with advanced-stage ovarian SBTs, we found that BRAF mutation was mainly associated with SBTs that did not progress to low-grade invasive tumors.23 BRAF mutation is likely involved in the initiation and progression of ovarian adenoma to SBTs, but may not be involved in the progression of SBTs to carcinoma. By comparing the gene expression profiles between ovarian SBTs with wild-type BRAF and those with mutated BRAF V600E, we previously found that SBTs with BRAF V600E express high levels of genes associated with cell growth-inhibitory functions, including CDC20B, PMEPA1, PAEP, FOXC1, and SFN.11 Using immunohistochemical analysis, Heublein et al44 also observed that ovarian SBTs with BRAF-mutated implants tended to express high levels of p16. High expression of p16 may attenuate the mutated BRAF-induced MAPK signals that affect cell-cycle progression and thus may prevent further tumor progression.
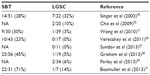  | Table 4 BRAF mutation frequency in serous borderline tumors (SBTs) and low-grade serous carcinomas (LGSCs) |
On the other hand, the total reported percentage of BRAF mutations in mucinous carcinoma (20%) is much higher than that of mucinous borderline tumor (5%) (Table 5).4,13,15,17,46,60–62 Unlike the serous adenoma with similar BRAF mutations as SBTs, none of the mucinous adenomas (n=40) had a detectable BRAF mutation.15 Since it is believed that mucinous adenoma can progress as mucinous borderline tumor and then mucinous carcinoma, a higher frequency of BRAF mutations in mucinous carcinoma may suggest a driver role of BRAF mutation in the pathogenesis of some mucinous carcinomas in contrast with that of SBTs.
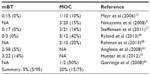  | Table 5 BRAF mutation frequency in mucinous borderline tumors (mBT) and mucinous carcinomas (MOC) |
Mutated BRAF as a potential prognostic marker for ovarian SBT or LGSC
Several studies have reported the association of BRAF mutation with a better clinical outcome for patients with ovarian cancer. Preusser et al28 reported that patients with invasive ovarian carcinomas that stained positive for the BRAF V600E monoclonal antibody had a strong trend toward better survival. Similarly, in a study by Grisham et al,58 BRAF V600E mutations were identified in 35% of 75 patients with ovarian SBTs or LGSC, and the BRAF V600E mutation in these tumors was associated with early-stage disease and improved prognosis. Grisham et al also concluded that patients with ovarian SBTs or LGSC who need systemic therapy are unlikely to have BRAF mutant tumors.55 Pathologically, ovarian SBTs with BRAF mutation are associated with cellular features indicative of senescence, such as the expression of senescence-associated beta-galactosidase activity and abundant eosinophilic cells.63 These data suggest that BRAF mutation is a biomarker of favorable prognosis and may prevent the progression of ovarian SBTs or early-stage LGSC to more aggressive disease, despite the fact that BRAF mutation is predictive of poor prognosis in other malignancies such as thyroid cancer and melanoma.
Future perspectives
SBT appears to be a unique disease with high frequency of BRAF mutations. Validation of BRAF mutations as a potential prognostic marker in a large cohort of patients with ovarian SBTs or LGSC is necessary to determine the clinical significance of BRAF status in the management of patients with this disease. Once the BRAF mutation has been confirmed as a protective factor against the progression of ovarian SBTs and early-stage LGSC into more aggressive disease,11,23,43,58 clinicians will be able to use the BRAF status of surgically resected ovarian SBTs to predict the risk of recurrence. Moreover, for progressive SBT/LGSC with BRAF V600E mutation, treatment with BRAF V600E specific inhibitor could be an alternative regimen.64,65 Furthermore, investigation of ovarian LGSC and mucinous carcinoma with BRAF mutation may unveil why BRAF mutations are associated with poor clinical outcomes in other cancers such as microsatellite-stable colon cancer,66 melanoma,67 and thyroid cancer.68 In summary, further study of BRAF mutation status will have a significant impact on the management of ovarian cancer.
Disclosure
The authors report no conflicts of interest in this work.
References
Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417(6892):949–954. | |
Forbes SA, Bindal N, Bamford S, et al. COSMIC: mining complete cancer genomes in the Catalogue of Somatic Mutations in Cancer. Nucleic Acids Res. 2011;39(Database issue):D945–D950. | |
Yachida S, Mudali S, Martin SA, Montgomery EA, Iacobuzio-Donahue CA. Beta-catenin nuclear labeling is a common feature of sessile serrated adenomas and correlates with early neoplastic progression after BRAF activation. Am J Surg Pathol. 2009;33(12):1823–1832. | |
Nakayama N, Nakayama K, Yeasmin S, et al. KRAS or BRAF mutation status is a useful predictor of sensitivity to MEK inhibition in ovarian cancer. British Journal of Cancer. 2008;99(12):2020–2028. | |
Cerami E, Gao J, Dogrusoz U, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2(5):401–404. | |
Gao J, Aksoy BA, Dogrusoz U, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6(269):pl1. | |
Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474(7353):609–615. | |
Sieben NL, Macropoulos P, Roemen GM, et al. In ovarian neoplasms, BRAF, but not KRAS, mutations are restricted to low-grade serous tumours. J Pathol. 2004;202(3):336–340. | |
Seidman JD, Kurman RJ. Ovarian serous borderline tumors: a critical review of the literature with emphasis on prognostic indicators. Hum Pathol. 2000;31(5):539–557. | |
Burks RT, Sherman ME, Kurman RJ. Micropapillary serous carcinoma of the ovary. A distinctive low-grade carcinoma related to serous borderline tumors. Am J Surg Pathol. 1996;20(11):1319–1330. | |
Wong KK, Tsang YT, Deavers MT, et al. BRAF mutation is rare in advanced-stage low-grade ovarian serous carcinomas. Am J Pathol. 2010;177(4):1611–1617. | |
Zannoni GF, Improta G, Chiarello G, et al. Mutational status of KRAS, NRAS, and BRAF in primary clear cell ovarian carcinoma. Virchows Arch. 2014;465(2):193–198. | |
Mayr D, Hirschmann A, Lohrs U, Diebold J. KRAS and BRAF mutations in ovarian tumors: a comprehensive study of invasive carcinomas, borderline tumors and extraovarian implants. Gynecol Oncol. 2006;103(3):883–887. | |
Gemignani ML, Schlaerth AC, Bogomolniy F, et al. Role of KRAS and BRAF gene mutations in mucinous ovarian carcinoma. Gynecol Oncol. 2003;90(2):378–381. | |
Hunter SM, Gorringe KL, Christie M, et al. Pre-invasive ovarian mucinous tumors are characterized by CDKN2A and RAS pathway aberrations. Clin Cancer Res. 2012;18(19):5267–5277. | |
Ueda M, Toji E, Noda S. Germ line and somatic mutations of BRAF V599E in ovarian carcinoma. Int J Gynecol Cancer. 2007;17(4):794–797. | |
Steffensen KD, Waldstrom M, Grove A, et al. Improved classification of epithelial ovarian cancer: results of 3 danish cohorts. Int J Gynecol Cancer. 2011;21(9):1592–1600. | |
Ho CL, Kurman RJ, Dehari R, Wang TL, Shih IeM. Mutations of BRAF and KRAS precede the development of ovarian serous borderline tumors. Cancer Res. 2004;64(19):6915–6918. | |
Thomas RK, Nickerson E, Simons JF, et al. Sensitive mutation detection in heterogeneous cancer specimens by massively parallel picoliter reactor sequencing. Nat Med. 2006;12(7):852–855. | |
Tan YH, Liu Y, Eu KW, et al. Detection of BRAF V600E mutation by pyrosequencing. Pathology. 2008;40(3):295–298. | |
Greaves WO, Verma S, Patel KP, et al. Frequency and spectrum of BRAF mutations in a retrospective, single-institution study of 1112 cases of melanoma. J Mol Diagn. 2013;15(2):220–226. | |
Richter A, Grieu F, Carrello A, et al. A multisite blinded study for the detection of BRAF mutations in formalin-fixed, paraffin-embedded malignant melanoma. Sci Rep. 2013;3:1659. | |
Tsang YT, Deavers MT, Sun CC, et al. KRAS (but not BRAF) mutations in ovarian serous borderline tumour are associated with recurrent low-grade serous carcinoma. J Pathol. 2013;231(4):449–456. | |
Milbury CA, Li J, Makrigiorgos GM. COLD-PCR-enhanced high-resolution melting enables rapid and selective identification of low-level unknown mutations. Clin Chem. 2009;55(12):2130–2143. | |
Capper D, Preusser M, Habel A, et al. Assessment of BRAF V600E mutation status by immunohistochemistry with a mutation-specific monoclonal antibody. Acta Neuropathol. 2011;122(1):11–19. | |
Hayashi Y, Sasaki H, Takeshita S, et al. Usefulness of immunohistochemistry for the detection of the BRAF V600E mutation in ovarian serous borderline tumors. Oncol Rep. 2014;32(5):1815–1819. | |
Bosmuller H, Fischer A, Pham DL, et al. Detection of the BRAF V600E mutation in serous ovarian tumors: a comparative analysis of immunohistochemistry with a mutation-specific monoclonal antibody and allele-specific PCR. Hum Pathol. 2013;44(3):329–335. | |
Preusser M, Capper D, Berghoff AS, et al. Expression of BRAF V600E mutant protein in epithelial ovarian tumors. Appl Immunohistochem Mol Morphol. 2013;21(2):159–164. | |
Kurman RJ, Shih IeM. Molecular pathogenesis and extraovarian origin of epithelial ovarian cancer – shifting the paradigm. Hum Pathol. 2011;42(7):918–931. | |
Shih IeM, Kurman RJ. Ovarian tumorigenesis: a proposed model based on morphological and molecular genetic analysis. Am J Pathol. 2004;164(5):1511–1518. | |
Kurman RJ, Shih IeM. The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol. 2010;34(3):433–443. | |
Cho KR, Shih IeM. Ovarian cancer. Annu Rev Pathol. 2009;4:287–313. | |
Kurman RJ, Shih IeM. Pathogenesis of ovarian cancer: lessons from morphology and molecular biology and their clinical implications. Int J Gynecol Pathol. 2008;27(2):151–160. | |
Vang R, Shih IeM, Kurman RJ. Ovarian low-grade and high-grade serous carcinoma: pathogenesis, clinicopathologic and molecular biologic features, and diagnostic problems. Adv Anat Pathol. 2009; 16(5):267–282. | |
Rechsteiner M, Zimmermann AK, Wild PJ, et al. TP53 mutations are common in all subtypes of epithelial ovarian cancer and occur concomitantly with KRAS mutations in the mucinous type. Exp Mol Pathol. 2013;95(2):235–241. | |
Okuda T, Otsuka J, Sekizawa A, et al. p53 mutations and overexpression affect prognosis of ovarian endometrioid cancer but not clear cell cancer. Gynecol Oncol. 2003;88(3):318–325. | |
Wiegand KC, Shah SP, Al-Agha OM, et al. ARID1A mutations in endometriosis-associated ovarian carcinomas. N Engl J Med. 2010; 363(16):1532–1543. | |
Jones S, Wang TL, Shih IeM, et al. Frequent mutations of chromatin remodeling gene ARID1A in ovarian clear cell carcinoma. Science. 2010;330(6001):228–231. | |
Dehari R, Kurman RJ, Logani S, Shih IeM. The development of high-grade serous carcinoma from atypical proliferative (borderline) serous tumors and low-grade micropapillary serous carcinoma: a morphologic and molecular genetic analysis. Am J Surg Pathol. 2007; 31(7):1007–1012. | |
Parker RL, Clement PB, Chercover DJ, Sornarajah T, Gilks CB. Early recurrence of ovarian serous borderline tumor as high-grade carcinoma: a report of two cases. Int J Gynecol Pathol. 2004;23(3):265–272. | |
Kobel M, Kalloger SE, Boyd N, et al. Ovarian carcinoma subtypes are different diseases: implications for biomarker studies. PLoS Med. 2008;5(12):e232. | |
Silva EG, Gershenson DM, Malpica A, Deavers M. The recurrence and the overall survival rates of ovarian serous borderline neoplasms with noninvasive implants is time dependent. Am J Surg Pathol. 2006;30(11):1367–1371. | |
Ardighieri L, Zeppernick F, Hannibal CG, et al. Mutational analysis of BRAF and KRAS in ovarian serous borderline (atypical proliferative) tumours and associated peritoneal implants. J Pathol. 2014;232(1):16–22. | |
Heublein S, Grasse K, Hessel H, et al. KRAS, BRAF genotyping reveals genetic heterogeneity of ovarian borderline tumors and associated implants. BMC Cancer. 2013;13:483. | |
Singer G, Oldt R 3rd, Cohen Y, et al. Mutations in BRAF and KRAS characterize the development of low-grade ovarian serous carcinoma. J Natl Cancer Inst. 2003;95(6):484–486. | |
Ryland GL, Hunter SM, Doyle MA, et al. RNF43 is a tumour suppressor gene mutated in mucinous tumours of the ovary. J Pathol. 2013;229(3):469–476. | |
Gershenson DM. Is micropapillary serous carcinoma for real? Cancer. 2002;95(4):677–680. | |
Uzan C, Nikpayam M, Ribassin-Majed L, et al. Influence of histological subtypes on the risk of an invasive recurrence in a large series of stage I borderline ovarian tumor including 191 conservative treatments. Ann Oncol. 2014;25(7):1312–1319. | |
Lazarou A, Fotopoulou C, Coumbos A, et al. Long-term follow-up of borderline ovarian tumors clinical outcome and prognostic factors. Anticancer Res. 2014;34(11):6725–6730. | |
Fauvet R, Demblocque E, Morice P, Querleu D, Darai E. Behavior of serous borderline ovarian tumors with and without micropapillary patterns: results of a French multicenter study. Ann Surg Oncol. 2012;19(3):941–947. | |
Uzan C, Kane A, Rey A, et al. Prognosis and prognostic factors of the micropapillary pattern in patients treated for stage II and III serous borderline tumors of the ovary. Oncologist. 2011;16(2):189–196. | |
Deavers MT, Gershenson DM, Tortolero-Luna G, et al. Micropapillary and cribriform patterns in ovarian serous tumors of low malignant potential: a study of 99 advanced stage cases. Am J Surg Pathol. 2002; 26(9):1129–1141. | |
Longacre TA, McKenney JK, Tazelaar HD, Kempson RL, Hendrickson MR. Ovarian serous tumors of low malignant potential (borderline tumors): outcome-based study of 276 patients with long-term(≥5-year) follow-up. Am J Surg Pathol. 2005;29(6):707–723. | |
Prat J, De Nictolis M. Serous borderline tumors of the ovary: a long-term follow-up study of 137 cases, including 18 with a micropapillary pattern and 20 with microinvasion. Am J Surg Pathol. 2002; 26(9):1111–1128. | |
Cho YH, Kim DY, Kim JH, et al. Mutational analysis of KRAS, BRAF, and TP53 genes of ovarian serous carcinomas in Korean women. Yonsei medical journal. 2009;50(2):266–272. | |
Vereczkey I, Serester O, Dobos J, et al. Molecular characterization of 103 ovarian serous and mucinous tumors. Pathology oncology research: POR. 2011;17(3):551–559. | |
Sundov D, Caric A, Mrklic I, et al. P53, MAPK, topoisomerase II alpha and Ki67 immunohistochemical expression and KRAS/BRAF mutation in ovarian serous carcinomas. Diagnostic pathology. 2013;8:21. | |
Grisham RN, Iyer G, Garg K, et al. BRAF mutation is associated with early stage disease and improved outcome in patients with low-grade serous ovarian cancer. Cancer. 2013;119(3):548–554. | |
Farley J, Brady WE, Vathipadiekal V, et al. Selumetinib in women with recurrent low-grade serous carcinoma of the ovary or peritoneum: an open-label, single-arm, phase 2 study. The Lancet. Oncology. 2013;14(2):134–140. | |
Rahman M, Nakayama K, Rahman MT, et al. PPP2R1A mutation is a rare event in ovarian carcinoma across histological subtypes. Anticancer research. 2013;33(1):113–118. | |
Anglesio MS, Arnold JM, George J, et al. Mutation of ERBB2 provides a novel alternative mechanism for the ubiquitous activation of RAS-MAPK in ovarian serous low malignant potential tumors. Molecular cancer research: MCR. 2008;6(11):1678–1690. | |
Gorringe KL, Choong DY, Williams LH, et al. Mutation and methylation analysis of the chromodomain-helicase-DNA binding 5 gene in ovarian cancer. Neoplasia. 2008;10(11):1253–1258. | |
Zeppernick F, Ardighieri L, Hannibal CG, et al. BRAF mutation is associated with a specific cell type with features suggestive of senescence in ovarian serous borderline (atypical proliferative) tumors. The American journal of surgical pathology. 2014;38(12):1603–1611. | |
Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363(9):809–819. | |
Robinson GW, Orr BA, Gajjar A. Complete clinical regression of a BRAF V600E-mutant pediatric glioblastoma multiforme after BRAF inhibitor therapy. BMC cancer. 2014;14:258. | |
Samowitz WS, Sweeney C, Herrick J, et al. Poor survival associated with the BRAF V600E mutation in microsatellite-stable colon cancers. Cancer research. 2005;65(14):6063–6069. | |
Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364(26):2507–2516. | |
Kebebew E, Weng J, Bauer J, et al. The prevalence and prognostic value of BRAF mutation in thyroid cancer. Ann Surg. 2007;246(3):466–470; discussion 470–461. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.