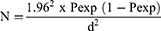Back to Journals » Veterinary Medicine: Research and Reports » Volume 13
Bovine Mastitis: Prevalence, Risk Factors, and Bacterial Pathogens Isolated in Lactating Cows in Gamo Zone, Southern Ethiopia
Authors Belay N , Mohammed N, Seyoum W
Received 3 November 2021
Accepted for publication 27 December 2021
Published 7 January 2022 Volume 2022:13 Pages 9—19
DOI https://doi.org/10.2147/VMRR.S344024
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Young Lyoo
Nahom Belay,1 Nejib Mohammed,2 Wasihun Seyoum2
1Southern Agricultural Research Institute, Bonga Agricultural Research Center, Bonga, Ethiopia; 2Arba Minch University, College of Agricultural Science, Department of Animal Science, Arba Minch, Ethiopia
Correspondence: Nahom Belay Email [email protected]
Purpose: Mastitis is a disease known to cause a great deal of loss of production and has a major economic impact. In the study area, there is little current information on bovine mastitis. Therefore, this study aimed to determine the overall prevalence of bovine mastitis and its associated risk factors and isolate the major pathogenic bacteria.
Methods: A cross-sectional study was conducted from February 2020 to September 2020 in selected dairy farms of Gamo Zone, southern Ethiopia. A total of 422 lactating cows were diagnosed for mastitis using the California mastitis test, clinical examination, and bacteriological methods.
Results: The overall prevalence of bovine mastitis determined in the area was 17.1% (72 of 422), of which 1.9% (eight of 422) was clinical and 15.2% (64 of 422) subclinical. Of 1,662 quarters examined, 7.94% (132) were positive. Bacteriological methods were also used to isolate the major pathogenic bacterial species associated with bovine mastitis. From 72 composite milk samples, growth of six different groups of bacteria was recorded in 64 (88.9%) samples. The most predominant bacterial pathogens isolated were Staphylococcus aureus (42.6%), ahead of Streptococcus spp. (26.2%), non-aureus staphylococci (14.8%), and Escherichia coli (11.5%). Salmonella spp. (3.3%) and Klebsiella pneumoniae (1.6%) were the least isolated bacterial pathogens. Among risk factors, breed, parity, udder depth, and tick infestation of the udder showed statistically significant differences (P< 0.05) regarding the occurrence of mastitis.
Conclusion: The current study revealed that mastitis is one of the health problems affecting dairy cows in Gamo. Enhancing the awareness of dairy farmers, regular screening, and improving hygienic conditions are critically important to control and prevent bovine mastitis in the study area.
Keywords: Arba Minch, major pathogenic bacteria, mastitis, prevalence, risk factors
Introduction
Ethiopia has the largest livestock population in Africa, which plays a significant role in the economy and livelihoods of farmers and pastoralists. Cows represent 54.68% of the total cattle population of the country, of which 20.7% are milking cows. Of the female cattle population of the country, 97.9%, 1.82%, and 0.28% are local, cross-, and exotic breeds, respectively. The subsector comprises about 16.5% of national gross domestic product (GDP) and 35.6% of agricultural gross domestic product. Livestock products and by-products, such as meat, milk, cheese, butter, honey, and eggs, provide the main animal protein required for better nutritional status of the people.10
Milk and its by-products produced from lactating cows provide a crucial nutritional and dietary source of energy and protein for much of the rural, urban, and periurban populations. According to a 2009 FAO report, the overall annual milk-production potential of Ethiopia is 797,900–1,197,500 mt of raw-milk equivalents. Of this production capacity, 85%–89% comes from cattle, followed by goats, camels, and sheep. However, this is a great deal less than the national demand for milk and milk products within the country.13 Both the standard quality and quantity of milk production in Ethiopia are too low and even below the average for most Eastern and sub-Saharan African countries, due to a number of complex and interrelated factors, such as the presence of widespread diseases, inadequate feed and nutrition, poor genetic potential of local breeds, and inefficiency of livestock-development services. The mammary gland disease known as mastitis is the most widespread and costly disease in dairy farms worldwide and a particular issue for farmers in developing countries like Ethiopia.2,23
Bovine mastitis is an inflammation of mammary glands caused by a wide range of pathogens epidemiologically classified as contagious and environmental. It is a complex and multifactorial disease resulting from the interaction of three major factors: the animal, pathogens, and environmental and management factors.9,29 Contagious mastitis refers to udders of infected lactating cows serving as the major reservoir of the pathogens. Such bacteria as Staphylococcus aureus, Mycoplasma spp., Corynebacterium bovis, and Streptococcus agalactiae are the best examples of contagious pathogens. Contrarily, environmental mastitis can be associated with those intramammary infections caused by microorganisms whose primary source is the environment in which the lactating cows live. Bacteria like Escherichia coli, Streptococcus dysgalactiae, Streptococcus uberis, and Klebsiella spp. are the best examples of environmental pathogens. A majority of environmental mastitis caused by these pathogens are clinical and of short duration.29
Bovine mastitis can occur in clinical or subclinical forms based on the presence or absence of observable manifestations of clinical signs. Clinical mastitis is characterized by sudden onset, the presence of one or more of symptoms like udder swelling and abnormal milk, and systemic signs, such as lethargy, anorexia, and elevated body temperature.12 Subclinical mastitis is the most common form and characterized by increased somatic cell count in the milk and absence of visible clinical signs.4 Mastitis is a worldwide problem highly affecting animal health, quality, quantity, and the economics of milk production. It has been known to cause large losses in productivity, and it can cause huge financial losses due to its impact on quantity and quality of milk yield, veterinary expenses, condemnation of milk due to antibiotic residues, culling of mastitis cows at an early age, and occasional deaths. Furthermore, mastitis has a serious zoonotic impact associated with a shedding of pathogenic bacteria and their toxins in the milk of lactating cows.34
Bovine mastitis has been a serious issue for farmers in most developing countries, such as Ethiopia. The disease has been reported in different parts of the country, with overall prevalence of 39.5%–62.6%.2,18,30,35,38 Epidemiological research on its prevalence and associated factors, as well as the pathogens involved, is essential in designing prevention and control strategies against the disease in a given area. According to data from zonal and district-level livestock-resource and -management offices of the study area, the disease is poorly investigated and data on its distribution, magnitude, associated risk factors, and resultant economic loss scant. Therefore, this study aimed to estimate the prevalence of mastitis in lactating cows, identify associated risk factors, and isolate major pathogenic bacteria on dairy farms of Gamo Zone, southern Ethiopia.
Methods
Study Area
The study was conducted on small-scale dairy farms and households with lactating cows in selected areas of Gamo, southern Ethiopia from February 2020 to September 2020 (Figure 1). The town of Arba Minch is the center of administration for Gamo, a zone bordering Wolayta to the north, Lake Abaya and Chamo to the east, Segene and part of south Omo to the south, and Dawuro and Gofa zone to the west. Arba Minch is 446 km south of Addis Ababa at an elevation of 1,285 MASL. It receives 600–1,000 mm rainfall per annum and the annual temperature range is 26°C–34°C. The cattle population of Arba Minch at the time of writing was 19,554, of which 8,551 were cows.14
 |
Figure 1 Location of study area. |
Arba Minch is additionally the administrative center of Arba Minch Zuriya District. The district receives 800–1,200 mm of rainfall annually, has an average 10°–38°C temperature, and is at an altitude of 1,200–3,300 MASL. It is bordered on the north by Dita and Chencha, on the south by Derashe, on the northeast by Mirab Abaya, on the west by Bonke, on the southeast by Amaro, and on the east by Oromia. The main system of agriculture is mixed farming, whereby livestock and crops are managed. Farmers in and around Arba Minch commonly such produce crops as coffee, maize, mangoes, avocados, bananas, papaya, and apples. The cattle population of the district is 101,628.14 Chencha is 37 kilometers north of Arba Minch, 298 km from Hawassa and 530 km southwest of Addis Ababa, is at 2,732 MASL. Mean annual temperature and rainfall of the town are 22.5°C and 810–1,600 mm, respectively. Its cattle population is 109,690.14
Study Design
A cross-sectional study design was used to estimate the prevalence of bovine mastitis and associated risk factors and identify the major pathogenic bacteria in the study areas.
Study Population and Husbandry Practices
The study population was lactating cows on smallholder dairy farms managed under semi-intensive or intensive systems. On intensive farms, cattle were kept indoors all the time and given roughage and concentrates. The semi-intensive farms were characterized by outdoor grazing during the day. The study animals were categorized based on age, breed, and physiological status. Breed was classified as local and cross-breeds. Age was categorized as old (>9 years), adult (6–9 years), and young adult (3–5 years). Stocking density was grouped as low (≤ 5), medium (6–10) and high (>10). Physiological status was categorized based on Parker26 as poor, moderate, and good.
Sample-Size Determination and Sampling Technique
Sample size was computed based on the formula developed by Thrusfield et al37 for random sampling considering expected prevalence of 50%, 95% CI, and 5% absolute precision:
Where, Pexp = expected prevalence
d = desired absolute precision
N = the total sample size
The computed minimum sample size for the study was 384, though 422 lactating cows were included to increase precision and make data representative. Study areas were chosen purposively based on their level of milk production and livestock population using data from livestock and fishery offices. Areas covered were Shara, Lante, Kola Shele, Ganta Kanchama, Weze, Gurba, Bere, Chamo, Doysa, and Chencha. Farms and households were purposively selected based on the presence of lactating cows, high milk production, and husbandry practices. Simple random sampling was used for each lactating cow. Accordingly, 120 cows from Arba Minch and 144 and 158 were proportionally allocated and tested for bovine mastitis from Chencha and Arba Minch Zuriya, respectively, depending on the population of cows in the area (Table 1).
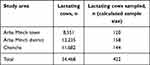 |
Table 1 Proportional allocation of samples |
Risk-Factor Assessment
Various farm- and animal-level risk factorswere considered in this study. Animal-level factors assessed were breed, age, parity, history of mastitis, body condition, udder depth, udder and leg hygiene, and teat-end shape. Farm-level factors assessed were stocking density, udder hygiene, floor type, and tick infestation of the udder. In sum, 138 small-scale farms were involved in the study. Each farm and household was visited just once during the study period.
Physical Examination of Udders
Udders were first inspected visibly and then carefully palpated to spot possible signs of inflammation, fibrosis, tick infestation, visible injury, swelling of the supramammary lymph nodes, and tissue atrophy. For cows with clinical mastitis, rectal temperature was taken to check for systemic involvement. Appearance and viscosity of milk secreted from each mammary quarter was observed for the existence of watery secretions, flakes, clots, and blood.28
Preparation of Udders and Teats
Before the collection of milk samples, the udder and teats were thoroughly cleaned and dried. Using a brush and dry towel, surfaces of the teats and udder were cleaned of dust, particles of bedding, and other contamination. Teats were cleaned with tap water and dried, then swabbed with cotton and soaked in 70% alcohol. During scrubbing with alcohol, teats on the far side of the udder were washed with alcohol first, then those on the near side to prevent recontamination of teats.24
Sample Collection, Handling, and Storage
Standard milk-sampling techniques was employed for the collection of milk samples. The near teats were sampled first then the far to reduce contamination of the teat ends during the collection of samples. After discarding the first three milking streams, about 10 mL milk was was put in a sterile sample cup. For handling and transport to the laboratory, samples were placed in an icebox. In the laboratory, samples were stored at 4°C for a maximum of 24 hours until inoculation in a standard bacteriological medium.24
California Mastitis Test (CMT)
In cows where clinical mastitis was not detected, milk samples were also collected to observe for subclinical mastitis. Milk samples were aseptically collected from each quarter and tested with a CMT kit. A squirt of milk sample from each quarter of the udder was added to each cup on the CMT paddle and the same amount of 3% CMT reagent placed in every cup and blended well. Reactions were ranked as 0 and trace for negative and 1, 2, and 3 for positive.28
Bacteriological Isolation and Characterization
Bacteriological examination of milk samples was done in accordance with the procedures used by Quinn et al.28 Samples were cultured straight away or stored at 4°C for a maximum of 24 hours before inoculation in a standard bacteriological medium.24 A loopful of milk sample was taken from each composite milk sample and inoculated solely on to MacConkey agar and and blood-agar base fixed with ovine blood. Plates inoculated with a sample were then aerobically incubated at 37°C for 24–48 hours. Identification of bacteria on primary culture was done based on hemolytic characteristics, colony morphology, Gram-stain reaction, including shape and arrangements of the bacteria, O-F, and catalase tests. Staphylococci were identified using growth characteristics, tube coagulase tests, and catalase tests. Identification of Streptococcus isolates was made on the basis of growth characteristics and catalase tests. Gram-negative bacteria grown on MacConkey agar were differentiated according to their growth characteristics, catalase test, oxidase reaction, triple sugar–iron agar test, and the IMViC (indole, methyl red, Voges–Proskauer, citrate) test.28
Data Analysis
Data (both qualitative and quantitative) were cleaned and put into Microsoft Excel every day after collection prevent loss. All data were analyzed using Stata 14. Associations between the dependent variable, mastitis status (1 = positive and 0 = negative), and independent categorical variables were analyzed using logistic regression analyses, with significance at P<0.05. The degree of association between various risk factors and the prevalence of bovine mastitis was assessed using ORs. All risk factors with P<0.25 on initial univariate logistic regression were analyzed for multicollinearity employing a correlation matrix, and those risk factors whose γ-value was between −0.6 and 0.6 were examined with multivariate logistic regression. The final model was checked for goodness of fit with the Hosmer–Lemeshow method. Descriptive statistics (count and proportion) are used to present results in tables.
Results
Prevalence of Mastitis
A total of 422 lactating cows (1,688 quarters) were tested for bovine mastitis using clinical examinations of the udder and the CMT, and the overall prevalence of mastitis at the cow level was found to be 17.1% (72 of 422). The prevalence of clinical mastitis was 1.9% (eight of 422) and subclinical mastitis 15.2% (64 of 422, Table 2). All quarters were examined for the existence of gross abnormalities, and it was observed that 26 (1.56%) teats were blind. Upon screening of the functional teats (1,662), 132 (7.94%) quarters were positive for mastitis (Table 3). Of the two types of mastitis, subclinical mastitis was predominant at the cow and quarter levels. For quarter-level mastitis, right rear teats had the highest rate of infection (9.9%), the next being left rear (9.7%), right front (6.5%), and left front (5.75%, Table 3). Among the three study areas, 422 lactating cows were involved in the study — 120 from Arba Minch, 158 from Arba Minch Zuriya, and 144 from Chencha — and 18.3%, 9.5%, and 24.3% were found to be positive for mastitis, respectively (Table 4).
 |
Table 2 Prevalence of mastitis |
 |
Table 3 Prevalence of mastitis at quarter level |
 |
Table 4 Prevalence of clinical and subclinical mastitis |
Risk Factors Associated with Bovine Mastitis
Ten possible cow-level risk actors were assessed for statistically significant associations between categories and the prevalence of bovine mastitis using univariate logistic regression analysis. Among these risk factors, seven were significantly associated with prevalence of bovine mastitis (Table 5). Univariate logistic regression analysis of management-level explanatory variables found a statistically significant association between bovine mastitis and udder infestation by ticks (Table 6). Essentially, bovine mastitis more likely occurred in cows with tick infestations than in those without.
 |
Table 5 Univariate logistic regression analysis of cow-level risk factors |
 |
Table 6 Univariate logistic regression analysis of management-level risk factors |
Among the risk factors examined in initial univariate logistic regression, milk yield per day was withdrawn from further analysis in consideration of multicollinearity with breed (γ=0.71), but breed as a risk factor was retained, due to its important implications regarding mastitis based on biological plausibility. Risk factors yielding P>0.25 on initial univariate analysis were also withdrawn from further analysis. Therefore, the variables considered for further analysis with multivariate logistic regression were breed, age, parity, history of mastitis, body condition, udder and leg hygiene, udder depth, teat-end shape, and tick infestation. The final logistic regression model showed that age, history of mastitis, body condition, udder and leg hygiene, and teat-end shape were not significant, and breed, parity, udder depth, and tick infestation were significant risk factors of mastitis in the cows (Table 7). The Hosmer–Lemeshow goodness-of-fit test indicated that the final model fit the data (χ2=3.94, P=0.86).
 |
Table 7 Multivariate logistic regression analysis of assorted risk factors |
Bacterial Isolates
Milk samples from cows positive for mastitis were bacteriologically examined to identify the major pathogenic bacteria involved in the disease. Microorganisms were determined based on their cultural characteristics, biochemical test reactions, and staining characteristics. Milk samples positive for mastitis at the cow level (72) were cultured for microbiological examination. Subsequently, growth of six groups of bacteria was examined in 64 (88.9%) samples. The predominant mastitis-causing bacterial pathogens isolated were S. aureus (42.6%) followed by Streptococcus spp. (26.2%), non-aureus staphylococci (NAS; 14.8%), and E. coli (11.5%). Klebsiella pneumoniae (1.6%) and Salmonella spp. (3.3%) were the least isolated bacterial pathogens (Table 8).
 |
Table 8 Bacterial species isolated |
Discussion
The current study revealed an overall prevalence of 17.1% in 422 lactating cows in and around Arba Minch, comparable with the 16.1% prevalence reported in Tullo,19 19% in Addis Ababa,25 23% in West Harerghe,17 but higher than the 6%35 in Debrezeit, 5.1%3 in and around Wolaita Sodo, and 9.9%30 in Ambo, central Ethiopia. On the other hand, the current result was much lower than the recent findings of Amin et al,5 Tesfaye and Abera,36 and Abebe et al,1 who reported 49.2% in and around Haramaya, 60.65% in Jimma, and 54.2% in southern Ethiopia, respectively. Variations between this and other reports of prevalence might be due to the complex nature of mastitis and its occurrence through the interactions of several factors, such as management and husbandry practices, environmental conditions, animal-level factors, and causative agents.29
The prevalence of subclinical mastitis (15.2%) was higher than that of clinical mastitis (1.9%) in the current study, which is in the line with several earlier reports from various parts of Ethiopia.1,2,5,36,39,40 As reported by Seegers et al,31 the subclinical form is 15–40 times as prevalent as the clinical form, is of long duration and of high economic consequence, and usually precedes the clinical form. As a result of the defense mechanism of the udder, which tends to reduce the severity of the disease, the subclinical form of mastitis has also been suggested to be higher than that of clinical mastitis.12
Overall, the quarter-level prevalence of 7.94% recorded in this study was in agreement with a report of 8.03%17 in West Harerghe, but lower than the 29.4%,1 45.68%,5 54.75%,11 21.48%,20 29.04%,21 and 39.4%36 found in other studies. Compared to other quarters, the right rear showed the highest proportion of infection (9.9%), followed by the left rear (9.7%). This is in line with other reports.2,36,40 The highest infection level in the rear quarters might be as a result of the hindquarters’ greater production capacity and higher chance of environmental and fecal contamination, owing to their anatomical location.33
The prevalence of bovine mastitis was found to be significantly associated with breed. The odds of finding cows with bovine mastitis in cross-breeds were 4.55 times those of local ones. This was in line with previous reports, where there was a statistically significant association between breeds and presence of mastitis, cross-breeds being predominant.2,21,36,39,40 This indicates that pure local breeds are highly resistant to infection by mastitis compared to cross- and exotic breeds. This might be because of diversity of breeds in genetic potential for disease resistance and adaptability to the environment. Moreover, the udder in cross- and exotic-breed cows is bigger, which can surely be contaminated and prone to different microorganisms.
Cows with moderate parity had higher odds of having inflammation of the mammary glands than cows with low parity. The odds of bovine mastitis in cows with moderate parity were 3.56 times those in cows with low parity, but no significant difference was found between cows with low and high parity. This result was in accordance with the result reported by Mekibib et al,22 where cows with moderate parity were were more prone to be affected than cows with lower parity.
We also found cows with more pendulous udders were the most prone to mammary infections. The likelihood of getting mastitis was greater in cows with pendulous udders than cows with normal udder position: 3.29 times that of the latter. As reported by Girma16 and Sori et al,33 animals with pendulous udders had a higher incidence of mastitis than cows with nonpendulous udders. It has been noted that cows with pendulous udders turn out to be the most prone to mammary infections. Pendulous udders open the teats and udders to injury, and microbes readily adhere to the teats and gain entry to gland tissue.6
There was a statistical association between tick infestation and bovine mastitis: the existence of tick infestation elevated the likelihood of mastitis by 5.57 times compared to those with no tick infestation. This result was in line with the report of Tolosa et al38 in Wolayta Soddo and Biffa et al7 in southern Ethiopia. Injuries produced by ticks are believed to create direct inflammatory reactions, necrosis, and abscess formation within the mammary gland, which can cause udder damage or risk of serious secondary infections.7
Bacteriological analysis of 72 milk samples revealed growth of various groups of bacteria in 64 (88.9%) samples, comparable to Mekibib et al22 and Yenew and Addis,39 who reported 90% and 85.7%, respectively, but higher than the 38.5% of Amin et al,5 66.12% of Dereje et al,11 46.97% of Kumbe et al,20 and 31.5% of Zeryehun and Abera.40 Also, it was lower than Abebe et al2 who recorded 98.8% growth. These variations could be a result of differences in sample size, use of quarter-level samples, methods employed, and proficiency of laboratory professionals. Being unable to isolate bacteria from all the collected milk specimen might be related to instinctive elimination of infection, low concentration of pathogens in milk, periodic shedding of pathogens, intracellular localization of pathogens, and inhibitory substances in the milk.29 It could also be because of cases of slow healing from infection where organisms are eliminated or decreased, while infiltration of leukocytes continues till full healing.33
The most predominant mastitis causing bacterial pathogens isolated in this study was S. aureus (42.6%), followed by Streptococcus spp. (26.2%), which is in line with Abebe et al1 and Kumbe et al.20 The predominance of these two bacterial species could be through constant colonization of teats, as they are commensals to the skin. They can then get ready entry to the teat canal while milking or suckling and can be transmitted from quarter to quarter and cow to cow during milking. Their intracellular location and capability to localize in microabscesses within the udder and consequent resistance to antibiotic therapy may also be a crucial factor that contributes to the predominance of these pathogens.29
The high prevalence of S. aureus (42.6%) in this study is in accordance with the reports of Dereje et al,11 Kumbe et al,20 and Mekibib et al,22 who reported predominance of S. aureus in causing bovine mastitis. High prevalence of S. aureus is related to poor milking hygiene, as this pathogen primarily spreads during milking through milkers’ hands and towels.8 Likewise, the higher isolation rate of S. aureus may well be due to vast ecological distribution in the mammary gland and skin, its localization intracellularly and in microabscesses within the udder, and its resistance to antibiotics. In settings where hand-milking and unwarranted usage of drugs is exercised to treat mastitis, its dominance has been reported.11
Streptococcus spp. (26.2%) were the second–most predominant bacterial species isolated during this study. This is in line with Abebe et al1 and Kumbe et al,20 who reported 18.6% and 21.29%, respectively, though higher than the 7.18% of Mekibib et al22 and 9.1% of Tesfaye and Abera,36 where Streptococcus spp. were the third–predominant bacterial species. Radostits et al29 noted that Streptococcus spp. are among the most prevalent bacterial species isolated, along with Staphylococcus spp.
In this study NAS were the third–most predominantly isolated bacteria, with frequency of 14.8%, in accordance with Dereje et al,11 and Seid et al,32 who reported 14.43% and 16.9% in different parts of Ethiopia, but lower than the 30.1%, 34.2, and 26.6%, reported by Mekibib et al,22 Tesfaye and Abera,36 and Zeryehun and Abera,40 respectively. NAS are seen as unimportant pathogens and typically treated as normal inhabitants of the bovine udder.15 NAS are the most frequently isolated microorganisms in cows with mastitis and are now considered an emerging pathogen of bovine mastitis.27
The isolation rate of E. coli (11.5%) was similar to Tesfaye and Abera36 who reported 13.31% in Jimma and higher than Dereje et al11 (6.18%) and Mekibib et al22 (4.6%) in Holeta. K. pneumoniae (1.6%) and Salmonella spp. (3.3%) were the least isolated bacterial pathogens in the current study. Our results for Klebsiella were aligned with the the 2.05% found by Dereje et al11 in Holeta. Differences between isolation rates of coliform organisms and other environmental mastitis-inducing bacteria may be related to poor farm hygiene, poor slope of stable settings, poor sanitation of milking materials, absence of use of individual towels, and no use of dry-cow therapy. Above all, feces, a typical origin of E. coli, can cause contamination of the udder over bedding, calving stalls, udder-washing water, and milkers’ hands.29
Conclusion
The present study affirmed that mastitis is one of the health problems affecting lactating cows in the study area, with an overall prevalence of 17.1%. Subclinical mastitis was the major type of mastitis, with 15.2% prevalence. Analysis of potential risk factors found statistically significant associations between bovine mastitis and breed, parity, udder depth, and tick infestation of udder. The present study also showed S. aureus, Streptococcus spp., NAS, E. coli, K. pneumoniae, and Salmonella spp. were possible causes of mastitis. Inadequate sanitation of the dairy setting, poor milking hygiene, and lack of adequate attention to the health of the mammary glands were major factors contributing to the prevalence of mastitis. Therefore, for improved control of mastitis in the study area, awareness of farmers on hygienic milking practices should be enhanced through animal-health extension services, implementation of regular screening of subclinical mastitis, and provision of treatment to positive cases on dairy farms. Since the current study on the causative agents was focused only on isolation of bacterial pathogens, further study on bovine mastitis pathogens and antimicrobial-sensitivity testing should be carried out.
Abbreviations
CMT, California mastitis test; CSA, Central Statistical Agency; FAO, Food and Agriculture Organization; GZLFO, Gamo Zone Livestock and Fishery Office; NAS, non-aureus staphylococci; NMC, National Mastitis Council.
Data Sharing
The data sets used and/or analyzed in the current study will be provided from the corresponding author on reasonable request.
Ethics
The Arba Minch University College of Agricultural Science Animal Research Ethics Review Committee ruled that no formal ethics approval was needed to conduct this study, because there were no invasive procedures or experiments to be conducted and no risk of harm for the research subjects involved. Before the study, informed consent was obtained from the owners and/or managers of the dairy farms included. Best-practice veterinary care was utilized in this research and, all procedures followed the proper guidelines and regulations.
Acknowledgments
The authors express their gratitude to the owners and/or managers of the dairy farms included in the study for their participation during the study period. Members of the office of Livestock and Fishery Resource Development in the study districts are very much acknowledged as well for their collaboration in the fieldwork. The authors are grateful to the editor and reviewer for their valuable comments in improving the quality of this manuscript.
Author Contributions
All authors took part in drafting, revising, or critically reviewing the article, gave final approval to the version to be published, have agreed on the journal to which the article has been submitted, and agree to be accountable for all aspects of the work.
Funding
The authors received no funding from any institute or organization.
Disclosure
The authors have declared there is no conflict of interests.
References
1. Abebe R, Abera M, Denbarga Y, et al. Prevalence, risk factors and bacterial causes of bovine mastitis in southern Ethiopia. Ethio Vet J. 2020;24(1):52–68. doi:10.4314/evj.v24i1.4
2. Abebe R, Hatiya H, Abera M, Megersa B, Asmare K. Bovine mastitis: prevalence, risk factors and isolation of Staphylococcus aureus in dairy herds at Hawassa milk shed, South Ethiopia. BMC Vet Res. 2016;12:270. doi:10.1186/s12917-016-0905-3
3. Abraham F, Zeleke MM. Prevalence of Bovine Clinical mastitis and farmer’s awareness in and around Wolaita Sodo, Southern Ethiopia. J Adv Dairy Res. 2017;5:184. doi:10.4172/2329-888X.1000184
4. Akers RM. Lactation and the Mammary Gland. Ames, Iowa, USA: Iowa State Press; 2002:278.
5. Amin B, Deneke Y, Abdela N. Bovine mastitis: prevalence, risk factors and isolation of Streptococcus species from small holders dairy farms in and around Haramaya Town, Eastern Ethiopia. Global J of Med Res. 2017;17(1):27–38.
6. Awale MM, Dudhatra GB, Avinash K, Chauhan BN, Kamani DR. Bovine mastitis: a threat to economy. Open Access Sci Rep. 2012;1:295.
7. Biffa D, Debela E, Beyene F. Prevalence and risk factors of mastitis in lactating dairy cows in Southern Ethiopia. Inter J Appl Res Vet Med. 2005;3(3):189–192.
8. Bradley AJ. Bovine mastitis: an evolving cattle disease. Vet J. 2002;164:116–128. doi:10.1053/tvjl.2002.0724
9. Cervinkova D, Vlkova H, Borodacova I, et al. Prevalence of mastitis pathogens in milk from clinically healthy cows. Vet Med. 2013;58(11):567–575. doi:10.17221/7138-VETMED
10. Central Statistical Agency. Agricultural sample survey. Report on livestock and livestock Characteristics, Federal Democratic Republic of Ethiopia, Addis Ababa; 2018:12–20.
11. Dereje K, Kebede A, Abebe N, Tamiru Y. Isolation, identification and antimicrobial susceptibility test of mastitis causing bacteria at Holeta Agricultural Research Center dairy farms. Int J Animal Sci Technol. 2018;2(1):6–13. doi:10.11648/j.ijast.20180201.12
12. Erskine RJ. Intramuscular administration of ceftiofursodiu versus intra mammary infusion of penicillin/novobiocin for treatment of Streptococcus agalactiae mastitis in dairy cows. J Am Vet Med Assoc. 2001;208:258–260.
13. Food and Agriculture Organization. Crop diversification and marketing development project, Interim report. Addis Ababa, Ethiopia; 2009:5–12.
14. Gamo Zone Livestock and Fishery Office. Report on livestock and livestock characteristics. Arbaminch, Gamo, Ethiopia: Gamo Zone Livestock and Fishery Office; 2020:1–5.
15. Gentilini E, Denamiel G, Betancor A, Rebuelto A, Rodriguez M. Antimicrobial susceptibility of Coagulase Negative Staphylococcus isolated from bovine mastitis. J Dairy Sci. 2002;85(8):1913–1917. doi:10.3168/jds.S0022-0302(02)74267-7
16. Girma D. Study on prevalence of bovine mastitis on cross breed dairy cows around Holeta area. Global Vet. 2010;5:318–321.
17. Girma S, Mammo A, Bogele K, Sori T, Tadesse F, Jibat T. Study on prevalence of bovine mastitis and its major causative agents in West Harerghe zone, Doba district, Ethiopia. J Vet Med and Anim Health. 2012;4(8):116–123.
18. Ismael A. Epidemiology of Bovine mastitis in Ethiopia. J Vet Med Health. 2018;2(1):104.
19. Kasech A, Alebachew T, Alemu A. Study on prevalence of Bovine mastitis in Tullo District of West Hararghe, Ethiopia: a cross sectional study. Adv Bio Res. 2016;10:147–153.
20. Kumbe A, Bekele B, Hussien B, Onate A, Teshome D. Study on Bovine mastitis under different management in pastoral and agro-pastoral areas of Borana Zone, Southern Ethiopia. J Vet Sci Res. 2020;5(1):000192.
21. Lakew BT, Fayera T, Ali YM. Risk factors for bovine mastitis with the isolation and identification of Streptococcus agalactiae from farms in and around Haramaya district, eastern Ethiopia. Trop Anim Health Prod. 2019;51:1507–1513. doi:10.1007/s11250-019-01838-w
22. Mekibib B, Furgasa M, Abunna F, Megersa B, Regassa A. Bovine mastitis: prevalence, risk factors and major pathogens in dairy farms of Holeta Town, Central Ethiopia. Vet World. 2010;3(9):397–403. doi:10.5455/vetworld.2010.397-403
23. Mohamed A, Simeon E, Yemesrach A. Dairy development in Ethiopia. EPTD Discussion Paper No. 123; Washington, D.C., U.S.A: International Food Policy Research Institute; 2004.
24. National Mastitis Council. Microbiological Procedures for the Diagnosis of Udder Infection.
25. Nesru H, Yohualashet T, Tilahun G. Prevalence of mastitis in different local and exotic breeds of milking cows. Proceedings of Institute of Agricultural Research (IAR), Addis Ababa, Ethiopia. 1997:256–262.
26. Parker R. Body condition scoring of dairy cattle: Ontario Ministry of Agriculture and Food. Ontario, Canada; 1989:1–10.
27. Pyöräla S. Mastitis in post-partum dairy cows. Reprod Dom Anim. 2009;43:252–259.
28. Quinn PJ, Carter ME, Markey BK, Carter GR. Veterinary Microbiology and Microbial Diseases, Bacterial Causes of Bovine Mastitis.
29. Radostits OM, Gay CC, Hinchcliff KW, Constable PD. Veterinary Medicine: A Textbook of the Disease of Cattle, Horses, Sheep, Pigs and Goats.
30. Sarba JE, Tola KG. Cross-sectional study on bovine mastitis and its associated risk factors in Ambo district of West Shewa Zone, Oromia, Ethiopia. Vet World. 2017;10:398–402. doi:10.14202/vetworld.2017.398-402
31. Seegers H, Fouricho C, Beaudeau F. Production effects related to mastitis and mastitis economics in dairy cattle herds. Vet Rec. 2003;34:475–491. doi:10.1051/vetres:2003027
32. Seid U, Zenebe T, Almaw G, et al. Prevalence, risk factors and major bacterial causes of Bovine mastitis in West Arsi Zone of Oromia Region, Southern Ethiopia. Nat Sci. 2015;13(8):19–27.
33. Sori H, Zerihun A, Abdicho S. Dairy cattle mastitis in and around Sebeta, Ethiopia. Intern J Appl Res Vet Med. 2005;3:332–338.
34. Suriyasathaporn W, Schukken YH, Nielsen M, Brand A. Low somatic cell count: a risk factor for subsequent clinical mastitis in dairy herd. J Dairy Sci. 2000;83:1248–1255. doi:10.3168/jds.S0022-0302(00)74991-5
35. Tesfaye B. Bovine mastitis: prevalence, isolation of bacterial species involved and its antimicrobial susceptibility test around Debrezeit, Ethiopia: a cross-sectional study. J Vet Sci Technol. 2016;7:396.
36. Tesfaye B, Abera A. Prevalence of mastitis and associated risk factors in Jimma town dairy farms, Western Ethiopia. J Vet Sci Anim Husb. 2018;6(3):307.
37. Thrusfield M, Christley R, Brown H, et al. Veterinary Epidemiology.
38. Tolosa T, Geberetsadik Z, Regassa F. Bovine mastitis and its associated risk factor in lactating cow in Wolayta Sodo, Southern Ethiopia. Anim Health Prod. 2009;57(4):311–319.
39. Yenew M, Addis H. Study on the prevalence and associated risk factors of bovine mastitis in and around Dessie town, South Wollo, northeastern Ethiopia. Biomed Nurs. 2020;6(3):59–76.
40. Zeryehun T, Abera G. Prevalence and bacterial isolates of mastitis in dairy farms in selected districts of Eastern Harrarghe Zone, Eastern Ethiopia. Hindawi J Vet Med. 2017;7:1–3.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.