Back to Journals » Drug Design, Development and Therapy » Volume 12
Bispecific antibodies: design, therapy, perspectives
Authors Sedykh SE , Prinz VV, Buneva VN, Nevinsky GA
Received 9 September 2017
Accepted for publication 21 November 2017
Published 22 January 2018 Volume 2018:12 Pages 195—208
DOI https://doi.org/10.2147/DDDT.S151282
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jianbo Sun
Sergey E Sedykh, Victor V Prinz, Valentina N Buneva, Georgy A Nevinsky
Laboratory of Repair Enzymes, Siberian Branch of Russian Academy of Sciences Institute of Chemical Biology and Fundamental Medicine, Novosibirsk State University, Novosibirsk, Russia
Abstract: Antibodies (Abs) containing two different antigen-binding sites in one molecule are called bispecific. Bispecific Abs (BsAbs) were first described in the 1960s, the first monoclonal BsAbs were generated in the 1980s by hybridoma technology, and the first article describing the therapeutic use of BsAbs was published in 1992, but the number of papers devoted to BsAbs has increased significantly in the last 10 years. Particular interest in BsAbs is due to their therapeutic use. In the last decade, two BsAbs – catumaxomab in 2009 and blinatumomab in 2014, were approved for therapeutic use. Papers published in recent years have been devoted to various methods of BsAb generation by genetic engineering and chemical conjugation, and describe preclinical and clinical trials of these drugs in a variety of diseases. This review considers diverse BsAb-production methods, describes features of therapeutic BsAbs approved for medical use, and summarizes the prospects of practical application of promising new BsAbs.
Keywords: bispecific antibodies, therapeutic antibodies, monoclonal antibodies
Introduction
Immunoglobulins (antibodies [Abs]) are major protein components of the adaptive immune system, directed against foreign compounds and infectious agents. The IgG molecule consists of two light and two heavy chains connected by disulfide bonds; IgG is a monomer with a molecular weight of 146–160 kDa. Antigen-binding centers of Abs are formed by hypervariable regions of heavy and light chains. In the classical point of view, the Ab molecule contains two identical antigen-binding sites (two HL fragments) and is monospecific and bivalent. Immunoglobulins are expressed as receptors of the cell membrane of B lymphocytes, as well as in the form of soluble molecules secreted by plasma cells. Soluble Abs can bind virtually any natural and artificial molecules (antigens) with high affinity and specificity. The ability of Abs to recognize and bind a broad spectrum of antigens is ensured by their extraordinary diversity, reaching 108–1010 different variants of antigen-binding centers. Bispecific immunoglobulins contain two different antigen-binding sites. In the last 10 years, the number of articles devoted to bispecific Abs (BsAbs) has been steadily increasing. The particular interest in these molecules is due to their potential for therapeutic use. In 2014, the journal Nature Reviews Drug Discovery called BsAbs “next-generation antibodies.”
Monoclonal Abs (mAbs) are secreted by identical immune cells, clones of a single parent cell. mAbs are not just monospecific bivalent molecules, but in contrast to polyclonal Abs, bind the same epitope (antigen fragment recognized by Abs). In this regard, mAbs are widely used for the treatment of cancer: Avastin (bevacizumab, anti-VEGF), Herceptin (trastuzumab, anti-HER2-receptor antagonist), and rituximab (anti-CD20) have been on the pharmaceutical market for more than 15 years. However, these and other drugs based on mAbs are not able to cure some cases of cancer in monotherapy, particularly due to T lymphocytes not taking an active part in the destruction of tumors, while mAb molecules only prevent the binding of growth factors to the receptors. mAbs blocking the inhibitory signals that protect tumors from immune cells show excellent results in the treatment of particular types of tumors. Nevertheless, high expectations are focused on Abs binding two or more antigens (BsAbs), as well as conjugated to agents for chemo- and radiotherapy.1
BsAbs have been developed in which one antigen-binding site is directed against the CD3 receptor (activates cytotoxic T lymphocytes) and the other against specific antigens of tumor cells (CD19, CD20, CD33, CD123, HER2, epithelial cell adhesion molecule [EpCAM], BCMA, CEA, and others).2 The convergence of cytotoxic T lymphocytes and tumor cells due to BsAb binding activates cytotoxic T cells and promotes the destruction of tumor cells. In addition to a wide range BsAbs directed against tumors, several bispecific molecules for the treatment of other diseases have been developed. The BsAb for the treatment of osteoporosis blocks the factors of Wnt signal-transduction pathway (sclerostin and Dkk1); it enhances the formation of osteoblasts and growth of bone tissue.3 ACE910 binds blood-coagulation factors IX and X and is designed to reduce bleeding rate in hemophilia A. The convergence of coagulation factors enhances the coagulation cascade.4 A BsAb against the transferrin receptor (provides passage through the blood–brain barrier) and protease BACE1 (accumulates amyloid peptides) is a candidate for an anti-Alzheimer’s disease drug.5 BsAbs that are focused on autoimmune diseases usually bind cytokines: TNF, IL1, IL4, IL14, IL17, IL23, and others.2,6 It has been shown that simultaneous use of two mAbs against cytokines in autoimmune diseases has severe side effects without superior efficiency. In this regard, BsAbs against autoimmune diseases usually combine two anticytokine antigen-binding sites and provide higher therapeutic potential than a mixture of two mAbs.7,8 In particular, the most therapeutically important cytokines in psoriasis are IL17, IL23, IL6, and TNF.9 ABT122 against TNFα and IL17A has clinical effects in rheumatoid arthritis and psoriatic arthritis.10 In contrast, Phase I/II clinical trials of COVA322 (same specificity as ABT122) in psoriasis11 were preliminary terminated, due to safety concerns.8 Antigen-binding sites of ABT981 are directed against IL1α and IL1β, inflammatory cytokines found in the cartilage and synovial fluid of patients with osteoarthritis.12
BsAbs have several significant advantages over monospecific Abs. BsAbs direct specific effectors of the immune system to target tumor cells, enhancing their cytotoxicity. BsAbs can provide higher binding specificity, since in contrast to monospecific Abs, they interact with two different surface antigens. The use of BsAbs compared to combination therapy with two monospecific drugs makes it possible to optimize expenses by reducing the cost of development and clinical trials. Since one disease modulator may play an essential role in several independent pathways and coexpression of different receptors has been found in many tumors, targeting of two different growth-promoting receptors on a single tumor cell may increase the antiproliferative effect and help to avoid the development of resistance.13,14
The sales of more than 50 mAbs presented on the pharmaceutical market have reached more than US$60 billion per year. According to a 2014 estimation, the market of therapeutic BsAbs will grow up to $5.8 billion per year by 2024.15 Therapeutic BsAbs approved for medical use are directed for the treatment of “liquid” tumors: blood cancer (leukemia and lymphoma). The specialty of “liquid tumors” is that palpable tumors that can be mechanically probed do not form in the body, unlike solid tumors (breast, uterus, rectum). Leukemia and lymphoma never develop symptoms typical of other oncological pathologies, since malignant leukocytes proliferate in the bone marrow and come into the bloodstream as individual cells, making them available for BsAb therapy. There are other diseases for which BsAbs are being developed: autoimmune (arthritis, asthma, diabetes), infectious (pneumonia), hemophilia, and Alzheimer’s disease.6
Therapeutic bispecific antibodies
To date, two BsAbs have been approved for use in the US (blinatumomab; Amgen) and Europe (catumaxomab; Trion Pharma). More than 60 drugs are in preclinical and 30 in clinical trials, and two-thirds of them are focused on cancer treatment.16 Two therapeutic BsAbs that are on the market and some of the drugs in clinical and preclinical trials bring T lymphocytes (or natural killers) closer to cells expressing specific antigens on the surface, or simultaneously bind two antigens on the surface of a target cell.
Therapy with BsAbs constructed on whole IgG molecules shows active immunization against specific tumors, which in the future can lead to increased use of this format to provide long-lasting antitumor immunity in the organism. In the case of catumaxomab, the induction of a prolonged immunoresponse after treatment is due to the affinity of fragment crystallizable region (Fc) for dendritic cells presenting the antigen.17 In the case of BsAbs generated by chemical heteroconjugation (when Fc of two IgG molecules are covalently attached), immunization occurs due to the formation of an immunoactivating environment that attracts and activates dendritic cells.18
Blinatumomab
The drug blinatumomab (Amgen) is the first representative of bispecific T-cell engagers (BiTEs) authorized for use in the US. The efficacy of blinatumomab as a therapeutic drug against B-cell tumors was first shown in 2008 in 38 patients with refractory non-Hodgkin’s lymphoma,19 and the results of other preclinical and clinical studies have been published in a number of works.20–23 At the end of 2014, the US Food and Drug Administration approved the treatment with blinatumomab of acute lymphoblastic leukemia without the Philadelphia chromosome as a second-line drug.24 In the EU, the drug was registered in 2015. Therapy with blinatumomab leads to the depletion of B lymphocytes and precursors in peripheral blood, which is gradually restored after the end of treatment.19
The bispecific blinatumomab molecule has been developed using diabody technology: the first antigen-binding site is directed against a CD19 protein on the surface of B lymphocytes, the second against the CD3 receptor on the surface of cytotoxic T lymphocytes (Figure 1). The single-stranded structure of blinatumomab allows easy protein expression in monomeric form in significant amounts and provides broad therapeutic potential for use in lymphoma and leukemia.25 Unfortunately, this feature is the reason continuous intravenous administration of the drug is required. The blinatumomab molecule directs primary CD3+ T cells against CD19+ lymphoma cells, and provides cytotoxicity at very low concentrations (~10–100 pg/mL).26 The direction of cytotoxic T lymphocytes to tumor B cells, bypassing T-cell receptors and major histocompatibility complex, is a significant advantage of the drug.27 Blinatumomab increases the secretion of anti-inflammatory cytokines (IL2, IL4, IL6, IL10, IFNγ, TNFα).26 Data from animal models also confirm the high efficacy of blinatumomab against tumor cells in leukemia and lymphoma at very low concentrations.26,28
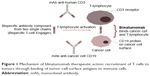  | Figure 1 Mechanism of blinatumomab therapeutic action: recruitment of T cells to tumors through binding of tumor-cell-surface antigens to immune cells. |
Blinatumomab therapy in adult patients with recurrent acute lymphoblastic leukemia leads to entirely positive results in 72%, achievement of minimal residual disease (tumor cells remaining in the organism after remission) in 88%, and average life expectancy after therapy of 9 months.21 Therapy for non-Hodgkin’s lymphoma with blinatumomab also shows good efficacy: in monotherapy clinical trials, blinatumomab significantly exceeded the effect of mAb therapy at much-lower final blood concentrations.22 Treatment of non-Hodgkin’s lymphoma patients with blinatumomab results in minimal residual disease, even after induction and consolidation,20 but clinical trials are still under way. In refractory and recurrent acute lymphoblastic leukemia in cases of CD19 absence on the lymphocyte surface and extramedullary hematopoiesis (formation of lymphocytes outside the bone marrow), therapy with blinatumomab is ineffective.29
After initiation of blinatumomab administration, the number of B lymphocytes decreases to under one cell/μL for 2 days and remains virtually undetectable until the end of therapy. On the contrary, the number of T lymphocytes lowers in all patients to a minimum level for 1 day and is then restored to normal within a few days. Moreover, within 2–3 weeks, the number of T cells doubles in most patients, dominated by an expansion of memory T cells expressing CD45RA. This can be explained by differences in signaling pathways used by memory T cells and naïve T cells.30 Rapid removal of blinatumomab from the bloodstream due to low molecular weight and the necessity of regular intravenous administration is partially resolved in newer therapeutics of tetravalent Abs – AFM11 and AFM13, developed by Affimed31,32 – which can be administered once or twice a week. T lymphocytes with chimeric CD19 receptors (CAR-T) provide complete remission in 90% of patients with refractory acute lymphoblastic leukemia, and in vivo such cells can proliferate up to 1,000 times.33 However, in the case of CAR-T therapy, the incidence of lymphocyte-release syndrome is much higher (up to 27%) than in the case of blinatumomab (up to 2%).26 Abs against other antigens (eg, CD79B) developed using BiTE technology directed to the treatment of myeloid leukemia and lymphoma are currently undergoing clinical trials.34
Catumaxomab
Catumaxomab (Removab, Trion) was the first bispecific trifunctional drug approved in 2009 by the European Medicines Agency for the treatment of malignant ascites.35–37 Catumaxomab redirects T cells to tumor cells, expressing EpCAM, ascites secondary to epithelial forms of cancer, especially gastric cancer. The results of clinical and preclinical studies of catumaxomab have been described in detail in many reviews.37–42 Catumaxomab is produced using the “quadroma” technology: HL fragments of mouse mAbs against CD3 (IgG2a) and rat mAbs (IgG2b) against EpCAM secreted by the corresponding hybridomas are combined in one bispecific molecule, which also binds the Fc-receptor.43 The Fc of catumaxomab preferentially binds to FcγRI, FcγRIIa, and FcγRIII activation receptors, but not to the FcγRIIb inhibitory receptor. This determines the recruitment and activation of macrophages, NK cells, and dendritic cells (FcγR+), leading to a complex immunoreaction.40 The use of HL fragments obtained from different host organisms reduces the possibility of BsAb formation with mismatched light chains, since light chains of rat Abs predominantly interact with rat heavy chains, and vice versa; light chains of mouse Abs are preferably associated with heavy mouse chains.43
The application of catumaxomab in clinical trials and therapy proves to be successful, since there is no barrier for T lymphocytes or catumaxomab molecules to penetrating ascites tumors. Ascites tumors are represented by separate cells that float separately from one another in liquid, which unites them with lymphoma and leukemia, two other liquid tumors, against which is directed blinatumomab. As in the case of blinatumomab, the antitumor effect of catumaxomab is due to the colocalization of a T-lymphocyte, of a tumor cell expressing EpCAM, and of the cell with the Fc receptor on the surface (macrophage, dendritic cell, natural killer) (Figure 2). Therefore, catumaxomab enhances activation of the patient’s immune system against the tumor. Binding of catumaxomab to Fcγ receptors of antigen-presenting cells turns inflammatory monocytes to express costimulatory molecules, which are necessary for T-cell activation.44,45 During preclinical studies, it was shown that catumaxomab could provide immunoresponse due to B- and T-memory cells activated by the Fc receptor.46 In a Phase IIIB clinical trial, intraperitoneal infusion of catumaxomab favored accumulation of and activated macrophages, NK cells, and CD4+ and CD8+ T-cells in ascites of peritoneal cavities.47,48
  | Figure 2 Mechanism of action: catumaxomab is a trifunctional antibody that accelerates the recognition and destruction of tumor cells by different immune cells. |
The interaction of patient immune cells with tumor cells leads to a complicated reaction, resulting in the elimination of tumor cells. Studies have shown several mechanisms of cytotoxicity: lymphocyte-mediated lysis, cytokine action (IL1β, IL2, IL6, IL12, CCL18 chemokine), phagocytosis, and Ab-dependent cellular toxicity. Compared to BsAbs, individual mouse and rat mAbs (anti-CD3 and anti-EpCAM) show significantly lower antitumor potential.37 Catumaxomab has high therapeutic potential with acceptable safety: intraperitoneal administration of low doses (10–100 mg) of the drug are carried out four to five times with an interval of 10–14 days. Catumaxomab is approved for the treatment of malignant ascites and is currently undergoing clinical trials in ovarian, stomach, and epithelial cancer. At the same time, a similar therapeutic effect is observed in some other tumors, eg, in ovarian carcinoma, catumaxomab reduces the formation of ascitic fluid.49 Interestingly, one of the catumaxomab-therapy side effects is the formation of Abs against mouse and rat Abs, and notably immunoresponse against mouse Abs correlates with positive response to treatment.50
Other BsAbs undergoing preclinical and clinical trials
Most of the antitumor BsAbs currently undergoing clinical and preclinical trials, such as blinatumomab and catumaxomab, contain one anti-CD3 antigen-binding site, attracting the T lymphocyte to the tumor cell. The second antigen-binding site can be directed against CD19, CD20, CD33, CD123, HER1, HER2, CEA, disialoganglioside GD2, PSMA, gpA33, and other proteins (Table 1). Also, many BsAbs with other combinations of antigen-binding sites (usually anticytokines) are in trials: HER2 + HER3, IL1α + IL1β, IL13 + IL17, IL17A/IL17F, and CD30 + CD16A. Data on BsAbs undergoing clinical trials have been published in many reviews and are combined in Table 1; the list of terminated clinical trials is combined in Table 2. The structures of most common BsAbs formats are presented in Figure 3.
  | Table 1 Clinical trials of bispecific antibodies |
  | Table 2 Terminated clinical trials of therapeutic bispecific antibodies |
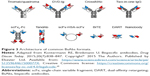  | Figure 3 Architecture of common BsAbs formats. |
Adverse effects of BsAb treatment
Like other methods of therapy for severe diseases, therapeutic BsAbs cause different side effects, the most common of which are nausea, vomiting, abdominal pain, fatigue, leukopenia, neutropenia, and thrombopenia. In many patients, Abs against therapeutic BsAbs appear in the blood during treatment. Most adverse events occur during the beginning of therapy, and in most cases side effects normalize under continued treatment. The majority of data on therapeutic BsAb adverse effects are available on blinatumomab and catumaxomab, since these drugs have undergone numerous clinical trials. A common side effect of blinatumomab and catumaxomab therapy is “cytokine storm”, elevation of cytokine levels. Cytokine release-related symptoms are general side effects of many therapeutic mAbs, and occur due to specific mechanisms of action: use of cytotoxic T cells as effectors. Minimizing cytokine-release syndrome is possible with a low initial dose of the drug in combination with subsequent high doses,54 as well as corticosteroid (dexamethasone)40 and antihistamine55 premedication.
The most common side effects during blinatumomab treatment are hepatotoxicity, leukopenia, lymphopenia, thrombocytopenia, CRP increase, chills, fever, pyrexia, nausea, and vomiting.44 There are also noted neurological and psychiatric side effects, which are completely reversible after the end of therapy.56 In catumaxomab treatment, the most frequent adverse events are cytokine release-related symptoms, which may have some predictive value for treatment efficacy. Some disorders of liver parameters and white blood cells are often observed, but usually these changes are reversible.40 Adverse events in cases of Mus110 (BiTEs BsAb anti-EpCAM and anti-CD3) are in general due to activation of T cells. During treatment with MEDI565 (AMG211, MT111: BiTEs BsAb anti-CEA and anti-CD3), the most common side effects are nausea, vomiting, abdominal pain, and fatigue. Significant increases in antidrug Abs are detected in the blood of half the patients.57 The main adverse event during TF2 (anti-CEA Fabs and anti-histamine–succinyl–glycine Fab) therapy is bone-marrow toxicity.57
History and methods of bispecific antibody generation
The first work on BsAb generation was published in 1961 and described the production of chimeric BsAbs containing simultaneously two different antigen-binding sites from a mixture of two monospecific Abs. Such BsAbs simultaneously precipitated both antigens specific to the original Abs.58 In 1983–2007, it was shown that IgG4 molecules stochastically exchanged HL fragments; this posttranslational modification results in a generation of chimeric bispecific molecules.59,60 In 2012–2016, it was shown that the bispecific IgG molecules of all four subclasses are present in blood,61 placenta,62 and milk (IgG and sIgA)63,64 of healthy donors.
The explosive growth of publications on BsAb generation happened in the 1980s and 1990s, when preparations of mAbs obtained by hybridoma technology became available. In 1983, hybrid hybridomas, called quadromas, expressing two types of light and heavy chains simultaneously and producing BsAbs were described.65 In 1985, bispecific Fab2 was obtained by chemical cross-linking.66 In 1987, Fab molecules were first cross-linked with thioesters.67
In 1984, mAbs against the T-lymphocyte receptor and leukemia cell-line antigen were cross-linked with a heterobifunctional linker and it was shown that generated BsAbs attract T-lymphocytes and stimulate T-cell mediated cytotoxicity.68 In 1985, a bispecific Fab2 was constructed from HL fragments against human CD3 receptors and HL fragments against H2k mouse protein; the resulting construct directed cytotoxic human T-lymphocytes to murine tumor cells with surface KK antigen and destroyed them by an Ab-dependent cytotoxicity mechanism.69 In 1987, it was shown that BsAbs containing HL fragments, anti-T-cell antigen receptors, and antihemagglutinin of influenza virus demonstrate aspecific cytotoxic T-lymphocyte-mediated lysis of infected cells.70 A detailed review of BsAb development history has been published.71
The first papers on perspectives on BsAbs in clinical use were published in the 1990s. In 1992, BsAbs were shown to direct monocytes (carriers of FcγRI) against CD15+ breast carcinoma cells PM81, acute myeloid leukemia, and small-cell lung and intestinal carcinoma.72 In 1995, MDX210 BsAbs against FcγR1 on the surface of monocytes and macrophages and against HER2+ cells were described; the construct was clinically active in breast and ovarian tumors.73,74 Clinical studies of 2B1 specific against tumor cells expressing c-erbB-2 and cells bearing FcyRIII showed the possibility of BsAb-mediated targeted lysis of HER2+ cells (breast, intestine, lung, kidney, prostate cancer) by natural killers and phagocytes.75
The first generation of BsAbs was obtained by chemical cross-linking or from hybridomas. Now, most BsAbs are generated by three methods: by chemical conjugation with cross-linkers, by somatic fusion of two hybridoma lines (quadroma), and by genetic (protein/cell) engineering. Large pharmacological and biotechnological companies are developing new techniques for therapeutic BsAb generation, and more than 60 different technological platforms have been developed in the last 15 years.16 Depending on the production method and structure, BsAbs vary in the number of antigen-binding sites, geometry, half-life in the blood serum, and effector functions. According to the mechanism of action, most modern BsAbs undergoing preclinical and clinical studies are classified into four formats: BiTEs, dual-affinity retargeting Abs, homodimeric “knob-in-hole” Abs, and trifunctional BsAbs.
Chemical conjugation and covalent attachment of fragments
BsAbs can be generated by the attachment of light or heavy chains, single-domain Abs, single-chain variable fragment (scFv), or other genetic engineering structures with additional antigen-binding sites to the amino or carboxyl ends of monospecific IgG molecules.2 The most widely format used is IgG with dual-variable domains (DVD-Ig),76 in which a variable part of HL fragment is added to another variable part of another Ab with a short peptide linker. The resulting molecules are bispecific and bivalent with regard to each antigen.77 For example, tetravalent tetraspecific Abs binding EGFR, HER2, HER3, and VEGF are constructed by combining DVD-Ig technology with other methods.78 One of the advantages of BsAbs designed with DVD-Ig technology is their ability simultaneously to bind antigens with all variable domains. This is especially true in cases of binding cytokines and other proteins present in the blood in low concentrations. Also, this allows less frequent administration of these BsAbs.79
Chemical conjugation for the BsAb generation was used for the first time in 1985: two Fab2 obtained by pepsinolysis of rabbit IgG were reduced and then oxidized, resulting in bispecific Fab2.66 Subsequently, homo- and heterobifunctional reagents interacting with cysteine residues67 and Fab obtained by genetic engineering80 were used. CovX-Body technology is the most modern approach for the preparation of BsAbs based on the site-specific attachment of low-molecular-weight ligands to IgG.81 The half-life of low-molecular-weight drugs increases significantly after attachment to HL fragments. CVX241 BsAbs were produced by the addition of two short peptides that inhibited VEGF or angiopoietin 2 with a branched linker and then with the Abs.82 However, the clinical trials of CVX241 were terminated ahead of schedule, due to a lack of pharmacological effect.2
Chemical conjugation of Abs against CD3 and CD20 (rituximab) was used to obtain T cells with BsAb-coated surfaces.83 Autologous polyclonal activated T lymphocytes were generated by the surface localization of antigen-binding sites against a CD20 receptor. Such cells in the first phase of clinical trials were administered after high-dose chemotherapy and transplantation of peripheral blood stem cells. Injections increased natural and specific cellular response in refractory non-Hodgkin’s lymphoma.84
IMCgp100, developed by ImmTAC technology, contains a single-chain anti-CD3 mAb with an attached mature T-cell receptor that recognizes peptides of human leukocyte antigen. The drug is now undergoing clinical trials against metastatic melanoma.85 IMCgp100 directs and activates CD8 and CD4+ effector cells and memory cells,86 and then after the death of melanoma cells, its antigens are presented by dendritic cells.87
The dock-and-lock method allows the production of polyvalent, multispecific, and multifunctional constructs.88,89 The linker covalently bonds the first Fab to the dimerization and docking domain of cAMP-dependent protein kinase (contains a sulfhydryl group), and the second Fab bonds to the anchoring domain of A-kinase (contains two sulfhydryl groups). The interaction of the two cAMP-dependent protein-kinase domains results in the dimerization of the structures carrying Fab, and then the resulting fragment binds to the protein A-kinase domain bearing the third Fab. Further, the formation of disulfide bonds covalently stabilizes the triple construction.88,90 The trifunctional constructs containing four cytokine IFNα2b molecules linked to the anti-CD20 Ab (veltuzumab) are effective in non-Hodgkin’s lymphoma and multiple myeloma.91,92 The hexavalent construct containing BsAbs against CD20 and CD22 (veltuzumab and epratuzumab) penetrates lipid rafts, stimulates apoptosis, and inhibits tumor growth.90 A hexavalent construct composed of anti-CD22 and anti-CD19 BsAbs (epratuzumab, hA19) enhances the formation of the immunological synapse and trogocytosis (transmission of antigens) of CD19, CD20, CD21, and CD22 between B lymphocytes and antigen-presenting cells and represents a candidate molecule for the treatment of autoimmune diseases.93 Another construct containing a single-chain anti-CD3 Ab covalently bounds the antitumor Fab dimer; bispecific binding of the BsAb to tumor cells and further to T cells results in antitumor T-cell cytotoxicity.94
Therapeutic Ab fragments (scFv, diabody) may be fused with albumin95 or proteins that bind albumin,96 which increases the half-life of the drug in the blood up to five to six times. The construction of such molecules gives unpredictable results, thereby BsAbs generated as the result of different Ab-fragment fusion or binding of Abs to other proteins have limited application in research and development of new therapeutic molecules. Formats of BsAb generation based on Ab or Ab-fragment conjugation today are not used, because of the possibility of stable fusion recombinant protein generation.2
Protein, cellular, and genetic engineering
Coexpression of two heavy- and two light-chain genes in one cell may result in expression of IgG-like molecules. This method allows the creation of a preparation of monoclonal bispecific anti-CD3/anti-EpCAM Abs (catumaxomab).97 Quadroma technology implies fusion of two cell lines producing Abs, generating a hybrid hybridoma. In such cells, heavy and light chains of two different Abs combine, resulting in BsAbs with conventional IgG-like structure, which retain effector functions mediated by Fc. In cases of quadromas, BsAb constant regions of heavy and light chains can be of the same or different isotypes or even from different species (Triomab).6 Several variants of quadroma-producing cell selection have been described. The first method uses two cell lines producing mAbs resistant to different antibiotics. In this case, hybrid cells carrying resistance markers to both antibiotics are selected.98 One of the advantages of this method is the functional stability of fused nuclei in cell hybrids. Another method uses fluorescence-activated cell sorting: one hybridoma cell line is modified with fluorescein isothiocyanate, another with tetramethyl rhodamine isothiocyanate.99 After fusion, hybrid cells carrying two fluorescent markers are selected. An electrofusion technique, which is innocuous and alternative to polyethylene glycol fusion, is also used.100
The main problem of two-Ab coexpression (particularly quadromas) is the formation of up to nine variants of undesirable chimeric Abs along with the target BsAbs. This problem originates due to the homodimerization of heavy chains (instead of heterodimerization) and random binding of light chains to heavy chains. As a result, the significant disadvantage of this method is very low yield of target BsAbs.2 The yield of heterodimers from two different heavy chains can be increased using the knob-in-hole method, in which one heavy chain with the T366W mutation (“knob”: replacement with a sterically bulky amino acid) joins with the second heavy chain with mutations T366S, L368A, or Y407V (“hole”: replacement by a smaller amino acid); formation of heterodimers is thermodynamically favorable.101,102 A variant of method using structural similarity of IgG and IgA CH3 domains ensures the formation of heterodimers of heavy chains.103 Several methods of BsAb production use just one type of light chain.56 However, the most widely used method of BsAb production today is expression of monospecific mAbs in two different cell lines, isolation and subsequent combination in vitro.104,105 The advantage of this approach is using well-characterized Abs, but the significant disadvantage is the high cost and difficulty of obtaining such BsAbs.2
BsAbs containing a paratope recognizing two different antigens have been demonstrated for an anti-HER2 Ab binding VEGF106 and anti-HER3 Ab binding EGFR.107 CrossMAb technology, developed by Roche, made it possible to generate tetraspecific Abs binding EGFR, HER2, HER3, and VEGF.78 DutaMab technology (Creative Biolabs and Roche) uses three complementarity-determining regions (CDRs) in each antigen-binding site to bind one and three other CDRs to bind the second antigen, thus forming two paratopes. This technology allows BsAbs to be produced using methods typical for monospecific Abs, but the drawback of this approach is its nonuniversality: it is not possible for each pair of antigens to match the paratopes within a single antigen-binding site.2
BsAbs that do not contain constant regions have been described, with the most popular and demanded formats diabody and BiTE. Such BsAbs are expressed in one cell, where the fragments of heavy and light chains are connected by short peptide sequences. ScFvs are widely used for generation of such BsAbs. For diabody generation, sequences encoding two different scFvs are combined into one construct in which heavy chains are expressed in a single polypeptide and then joined with the corresponding light chains. The first described diabodies were bivalent BsAbs.108 Heterodimeric constructs were later obtained by the knob-in-hole method109 and by single-chain diabody generation.110,111 Diabody technology was used to construct the first BiTEs in eukaryotic cells. BiTEs are small scFv molecules tandem connected by flexible peptide linkers. BiTEs usually contain an antigen-binding site against CD3 and another against a high-affinity surface antigen of tumor cells.44,112,113 A disadvantage of such molecules is their short life span in blood serum, which is associated with smallness and lack of Fc. The advantage of this BsAb format is extremely highly specific antitumor activity at concentrations up to 10 pg/mL in cell culture.114 According to some data, one BiTE molecule can be involved several times in elimination of tumor cells by cytotoxic T lymphocytes.115
Single-domain Ab fragments obtained from mouse and human libraries by a phage display are also used to construct BsAbs.116,117 Nanobodies derived from llamas and camels contain only heavy chains; for the production of BsAbs, nanobodies are readily connected by short peptide linkers.118 The advantages of using single-domain Ab fragments are their smallness, easy penetration into cells, and access to antigens hidden for IgG. A significant disadvantage of such small structures is their low half-life in the blood, which requires frequent injection of the drug.2 The most successful representative of the BiTE family is blinatumomab. Amgen has also developed other BiTEs that bind EpCAM, HER2, CEA, EphA2, MCSP, and CD33, some of which are currently undergoing clinical trials.49
The combination of two VL and VH domains in one polypeptide makes it possible to obtain tetravalent TandAb molecules. TandAb AFM13 combines antigen-binding sites against CD16A and CD30, enhancing the response of natural killers in Hodgkin’s lymphoma. Two antigen-binding sites against each of the antigens and the lack of Fc increase the molecular weight and in vivo stability of these BsAbs.119
Perspectives
Blinatumomab and catumaxomab, approved for use as medicines, are designed to treat oncological diseases. In perspective, one can expect the construction of new platforms that will allow development of full processes from BsAb expression to preclinical tests. To develop new antitumor drugs, it is necessary to search for new combinations of BsAb targets to increase the therapeutic effect and reduce side effects; specific features of tumors also should be taken into account. BsAbs can be used in combination with other medications, eg, drugs controlling the cell cycle, indoleamine dioxygenase inhibitors, and vaccines. There is no doubt that continuous development of new approaches to BsAb production is required to control oncological diseases.
The design of new BsAbs will likely include the ability to bind two or more tumor antigens in combination with the attraction of T lymphocytes and assistant cells into the immunosynapse. Of particular importance is the increase in BsAb specificity and sensitivity, as well as reducing cytotoxicity to nontumor cells. Other important tasks are increasing BsAb yield from hybridomas and decreasing the cost of the drugs.
A promising work on short nonviral minicircle DNA for the synthesis of BsAbs in vivo was recently published. BsAbs against CD3 and CD20 efficiently stimulated T-cell cytotoxicity against CD20+ lymphoma cell lines. Introduction of 5 μg plasmid DNA was sufficient for 1 month’s BsAb expression in mice.120 An oncolytic adenovirus expressing a bispecific single-chain Ab in tumor cells has been constructed. The secreted Abs format is BiTE and BsAbs bind EpCAM on a tumor cell and activate CD4+ and CD8+ T-cell cytotoxicity by directing to CD3.121
A relatively universal method of BsAb preparation is the exchange of IgG HL fragments, which occurs stochastically between natural IgG4,60 IgG1 with a mutation in the CH3 domain,105 and IgG2 through disulfide linkers.122 According to the literature, the HL-fragment exchange occurs in human blood, milk, and placenta between IgG of all subclasses, resulting in polyreactive BsAbs.61–64 However, the exchange of HL fragments between the therapeutic molecules of bispecific IgG4 and the patient’s IgG4 leads to the formation of BsAbs that do not possess the original properties,123 so this imposes significant limitations on this method.
In a short time, one may expect the completion of clinical trials and approval of BsAbs directed to treat autoimmune and other diseases. For example, promising results can be obtained in anti-HIV BsAbs. It has been shown that BsAbs directed against the CD4- and V3-binding sites on the Env (gp120) protein exhibit a synergistic effect in vivo and in vitro. Other variants of anti-HIV BsAbs combine antigen-binding sites against gp41, CD4, or CCR5 protein, and can be used to prevent HIV infection.124,125
The design of bispecific molecules demands analysis of the required BsAb properties (affinity to target molecules, pharmacokinetics in blood, and near target cells) and mechanisms of action. The increasing number of therapeutic BsAbs entering clinical trials and results of BsAb use in clinical medicine may improve understanding of their pharmacokinetics in the near future. The ideal therapeutic BsAbs are expected to have a long half-life in human blood, distribution among organs, and sufficient penetration of tissue.126
The use of BsAbs in diagnostic tools is very promising, since BsAbs simplify the detection of target antigens. BsAbs are used in sensitive immunoassays developed for simple and rapid detection of bacterial and viral infectious diseases and in cancer diagnostics.127 BsAbs significantly enhance the quality and reliability of in vivo cancer-diagnostic imaging by positron-emission tomography. The use of BsAbs and 131I-labeled haptens allows pretargeting human prostate cancer xenografts in severe combined immunodeficient mice. With minimal signal background and high sensitivity and specificity, the use of BsAbs in this method is superior to mAbs.128 Lipoarabinomannan is present in the blood of tuberculosis patients and is considered as a disease marker. The BsAb specific to lipoarabinomannan and horseradish peroxidase was developed with quadroma technology. The use of immunoswabs has shown 100% specificity and 64% sensitivity compared with bacterial cultures. Results were obtained within 2 hours of sample collection, which is very competitive compared to the standard laboratory-culture method, where results are obtained 2–6 weeks after sampling.129 The use of anti-HBsAg × antihuman erythrocyte BsAbs allows detection of hepatitis B with 100% specificity and 97.7% sensitivity. The method can be used in actively infected patients since the serum HBsAg detection level ranges from 5 ng/mL to 600 μg/mL.130
The quadroma expressing BsAbs against Escherichia coli lipopolysaccharide and whole bacteria in combination with horseradish peroxidase was used for rapid one-step sandwich enzyme-linked immunosorbent-assay detection of the E. coli strain O157:H7. The sensitivity of the detection was 100 CFU/mL and specific, since it did not detect Salmonella, Pseudomonas, or nonpathogenic E. coli.131 The same approach was used for Bordetella pertussis detection. A quadroma producing anti-B. pertussis lipopolysaccharide and anti-horseradish peroxidase BsAbs was constructed for ultrasensitive immunoassay.132 A similar method was developed for BsAbs detection of Staphylococcus aureus. The assay is highly sensitive and specific, due to release of a bound fluorescent reporter from a BsAb-active center after binding to the thermonuclease-specific antigen of S. aureus.133 BsAbs recognizing several epitopes of the NP antigen of SARS coronavirus (causing severe acute respiratory syndrome) and binding to horseradish peroxidase were used for immunoswab assays, with sensitivity of NP detection 10 pg/mL and high specificity.134
Conclusion
The development of new methods of BsAb generation made it possible to obtain various variants of promising Ab derivatives for use in therapy. The resulting BsAbs differ from natural IgG by pharmacokinetics, blood serum half-life, ability to penetrate tumors, size, valence, and presence of Fc. Simultaneous blocking of several biological pathways allows BsAbs to exhibit a synergistic effect unachievable with a mixture of monospecific Abs. The results of recent years indicate that in the close future, by combinations of methods developed earlier, new BsAbs directed against a variety of diseases in which simultaneous binding of several specific antigens can play a key role will be generated. BsAbs may be used for the development of diagnostic devices of the “next generation”. The potential of simultaneous detection of several antigens or combining antigen-binding sites with assay markers makes BsAbs an important object of further research in biomedicine, pharmacology, and diagnostics.
Acknowledgments
This study was funded by the Russian Foundation for Basic Research, according to the research projects 16-34-60066-mol_a_dk, 16-15-10103-a, and 16-04-00603-a, and with a grant from the Ministry of Education and Science (MK-410.2017.4).
Disclosure
The authors report no conflicts of interest in this work.
References
Redman JM, Hill EM, AlDeghaither D, Weiner LM. Mechanisms of action of therapeutic antibodies for cancer. Mol Immunol. 2015;67(2 Pt A):28–45. | ||
Spiess C, Zhai Q, Carter PJ. Alternative molecular formats and therapeutic applications for bispecific antibodies. Mol Immunol. 2015;67(2 Pt A):95–106. | ||
Florio M, Gunasekaran K, Stolina M, et al. A bispecific antibody targeting sclerostin and DKK-1 promotes bone mass accrual and fracture repair. Nat Commun. 2016;7:11505. | ||
Shima M, Hanabusa H, Taki M, et al. Factor VIII-mimetic function of humanized bispecific antibody in hemophilia A. N Engl J Med. 2016;374(21):2044–2053. | ||
Yu YJ, Atwal JK, Zhang Y, et al. Therapeutic bispecific antibodies cross the blood-brain barrier in nonhuman primates. Sci Transl Med. 2014;6(261):261ra154. | ||
Brinkmann U, Kontermann RE. The making of bispecific antibodies. MAbs. 2017;9(2):182–212. | ||
Genovese MC, Cohen S, Moreland L, et al. Combination therapy with etanercept and anakinra in the treatment of patients with rheumatoid arthritis who have been treated unsuccessfully with methotrexate. Arthritis Rheum. 2004;50(5):1412–1419. | ||
Baker KF, Isaacs JD. Novel therapies for immune-mediated inflammatory diseases: what can we learn from their use in rheumatoid arthritis, spondyloarthritis, systemic lupus erythematosus, psoriasis, Crohn’s disease and ulcerative colitis? Ann Rheum Dis. Epub 2017 Aug 1. | ||
Torres T, Romanelli M, Chiricozzi A. A revolutionary therapeutic approach for psoriasis: bispecific biological agents. Expert Opin Investig Drugs. 2016;25(7):751–754. | ||
Khatri A, Goss S, Jiang P, Mansikka H, Othman AA. Pharmacokinetics of ABT-122, a TNF-α- and IL-17A-targeted dual-variable domain immunoglobulin, in healthy subjects and patients with rheumatoid arthritis: results from three phase I trials. Clin Pharmacokinet. Epub 2017 July 25. | ||
Silacci M, Lembke W, Woods R, et al. Discovery and characterization of COVA322, a clinical-stage bispecific TNF/IL-17A inhibitor for the treatment of inflammatory diseases. MAbs. 2016;8(1):141–149. | ||
Kosloski MP, Goss S, Wang SX, et al. Pharmacokinetics and tolerability of a dual variable domain immunoglobulin ABT-981 against IL-1α and IL-1β in healthy subjects and patients with osteoarthritis of the knee. J Clin Pharmacol. 2016;56(12):1582–1590. | ||
Zhang X, Yang Y, Fan D, Xiong D. The development of bispecific antibodies and their applications in tumor immune escape. Exp Hematol Oncol. 2017;6:12. | ||
Kontermann RE. Dual targeting strategies with bispecific antibodies. MAbs. 2012;4(2):182–197. | ||
[No authors listed]. Bispecific antibody therapeutics market (3rd edition), 2017–2030. 2014. Available from: http://www.reportlinker.com/p02530816-summary/bispecific-antibody-therapeutics-market-edition.html. Accessed November 27, 2017. | ||
Thakur A, Lum LG. “NextGen” biologics: bispecific antibodies and emerging clinical results. Expert Opin Biol Ther. 2016;16(5):675–688. | ||
Ruf P. Induction of a long-lasting antitumor immunity by a trifunctional bispecific antibody. Blood. 2001;98(8):2526–2534. | ||
Suryadevara CM, Gedeon PC, Sanchez-Perez L, et al. Are BiTEs the “missing link” in cancer therapy? Oncoimmunology. 2015;4(6):e1008339. | ||
Bargou R, Leo E, Zugmaier G, et al. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science. 2008;321(5891):974–977. | ||
Topp MS, Kufer P, Gökbuget N, et al. Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J Clin Oncol. 2011;29(18):2493–2498. | ||
Topp MS, Gökbuget N, Zugmaier G, et al. Long-term follow-up of hematologic relapse-free survival in a phase 2 study of blinatumomab in patients with MRD in B-lineage ALL. Blood. 2012;120(26):5185–5187. | ||
Topp MS, Gökbuget N, Zugmaier G, et al. Phase II trial of the anti-CD19 bispecific T cell-engager blinatumomab shows hematologic and molecular remissions in patients with relapsed or refractory B-precursor acute lymphoblastic leukemia. J Clin Oncol. 2014;32(36):4134–4140. | ||
Topp MS, Gökbuget N, Stein AS, et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol. 2015;16(1):57–66. | ||
Nuñez-Prado N, Compte M, Harwood S, et al. The coming of age of engineered multivalent antibodies. Drug Discov Today. 2015;20(5):588–594. | ||
Löffler A, Kufer P, Lutterbüse R, et al. A recombinant bispecific single-chain antibody, CD19×CD3, induces rapid and high lymphoma-directed cytotoxicity by unstimulated T lymphocytes. Blood. 2000;95(6):2098–2103. | ||
Wu J, Fu J, Zhang M, Liu D. Blinatumomab: a bispecific T cell engager (BiTE) antibody against CD19/CD3 for refractory acute lymphoid leukemia. J Hematol Oncol. 2015;8:104. | ||
Goebeler ME, Bargou R. Blinatumomab: a CD19/CD3 bispecific T cell engager (BiTE®) with unique anti-tumor efficacy. Leuk Lymphoma. 2016;57(5):1021–1032. | ||
Segal DM, Weiner GJ, Weiner LM. Bispecific antibodies in cancer therapy. Curr Opin Immunol. 1999;11(5):558–562. | ||
Aldoss I, Song J, Stiller T, et al. Correlates of resistance and relapse during blinatumomab therapy for relapsed/refractory acute lymphoblastic leukemia. Am J Hematol. 2017;92(9):858–865. | ||
Klinger M, Brandl C, Zugmaier G, et al. Immunopharmacologic response of patients with B-lineage acute lymphoblastic leukemia to continuous infusion of T cell-engaging CD19/CD3-bispecific BiTE antibody blinatumomab. Blood. 2012;119(26):6226–6233. | ||
Reusch U, Duell J, Ellwanger K, et al. A tetravalent bispecific TandAb (CD19/CD3), AFM11, efficiently recruits T cells for the potent lysis of CD19+ tumor cells. MAbs. 2015;7(3):584–604. | ||
Wu J, Fu J, Zhang M, Liu D. AFM13: a first-in-class tetravalent bispecific anti-CD30/CD16A antibody for NK cell-mediated immunotherapy. J Hematol Oncol. 2015;8:96. | ||
Brentjens RJ, Davila ML, Riviere I, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med. 2013;5(177):177ra38. | ||
Frankel SR, Baeuerle PA. Targeting T cells to tumor cells using bispecific antibodies. Curr Opin Chem Biol. 2013;17(3):385–392. | ||
Chames P, Van Regenmortel M, Weiss E, Baty D. Therapeutic antibodies: successes, limitations and hopes for the future. Br J Pharmacol. 2009;157(2):220–233. | ||
Scott AM, Wolchok JD, Old LJ. Antibody therapy of cancer. Nat Rev Cancer. 2012;12(4):278–287. | ||
Linke R, Klein A, Seimetz D. Catumaxomab: clinical development and future directions. MAbs. 2010;2(2):129–136. | ||
Sebastian M, Passlick B, Friccius-Quecke H, et al. Treatment of non-small cell lung cancer patients with the trifunctional monoclonal antibody catumaxomab (anti-EpCAM × anti-CD3): a phase I study. Cancer Immunol Immunother. 2007;56(10):1637–1644. | ||
Heiss MM, Murawa P, Koralewski P, et al. The trifunctional antibody catumaxomab for the treatment of malignant ascites due to epithelial cancer: results of a prospective randomized phase II/III trial. Int J Cancer. 2010;127(9):2209–2221. | ||
Seimetz D, Lindhofer H, Bokemeyer C. Development and approval of the trifunctional antibody catumaxomab (anti-EpCAM × anti-CD3) as a targeted cancer immunotherapy. Cancer Treat Rev. 2010;36(6):458–467. | ||
Ströhlein MA, Lordick F, Rüttinger D, et al. Immunotherapy of peritoneal carcinomatosis with the antibody catumaxomab in colon, gastric, or pancreatic cancer: an open-label, multicenter, phase I/II trial. Onkologie. 2011;34(3):101–108. | ||
Mau-Sørensen M, Dittrich C, Dienstmann R, et al. A phase I trial of intravenous catumaxomab: a bispecific monoclonal antibody targeting EpCAM and the T cell coreceptor CD3. Cancer Chemother Pharmacol. 2015;75(5):1065–1073. | ||
Lindhofer H, Mocikat R, Steipe B, Thierfelder S. Preferential species-restricted heavy/light chain pairing in rat/mouse quadromas: implications for a single-step purification of bispecific antibodies. J Immunol. 1995;155(1):219–225. | ||
Baeuerle PA, Reinhardt C. Bispecific T-cell engaging antibodies for cancer therapy. Cancer Res. 2009;69(12):4941–4944. | ||
Goere D, Flament C, Rusakiewicz S, et al. Potent immunomodulatory effects of the trifunctional antibody catumaxomab. Cancer Res. 2013;73(15):4663–4673. | ||
Ruf P, Lindhofer H. Induction of a long-lasting antitumor immunity by a trifunctional bispecific antibody. Blood. 2001;98(8):2526–2534. | ||
Fossati M, Buzzonetti A, Monego G, et al. Immunological changes in the ascites of cancer patients after intraperitoneal administration of the bispecific antibody catumaxomab (anti-EpCAM × anti-CD3). Gynecol Oncol. 2015;138(2):343–351. | ||
Walsh G. Biopharmaceutical benchmarks 2010. Nat Biotechnol. 2010;28(9):917–924. | ||
Kontermann RE, Brinkmann U. Bispecific antibodies. Drug Discov Today. 2015;20(7):838–847. | ||
Ott MG, Marmé F, Moldenhauer G, et al. Humoral response to catumaxomab correlates with clinical outcome: results of the pivotal phase II/III study in patients with malignant ascites. Int J Cancer. 2012;130(9):2195–2203. | ||
Trivedi A, Stienen S, Zhu M, et al. Clinical pharmacology and translational aspects of bispecific antibodies. Clin Transl Sci. 2017;10(3):147–162. | ||
Yuraszeck T, Kasichayanula S, Benjamin JE. Translation and clinical development of bispecific T cell engaging antibodies for cancer treatment. Clin Pharmacol Ther. 2017;101(5):634–645. | ||
Yang F, Wen W, Qin W. Bispecific antibodies as a development platform for new concepts and treatment strategies. Int J Mol Sci. 2017;18(1):E48. | ||
Zhu M, Wu B, Brandl C, et al. Blinatumomab, a bispecific T-cell engager (BiTE) for CD-19 targeted cancer immunotherapy: clinical pharmacology and its implications. Clin Pharmacokinet. 2016;55(10):1271–1288. | ||
Chames P, Baty D. Bispecific antibodies for cancer therapy: the light at the end of the tunnel? MAbs. 2009;1(6):539–547. | ||
Dhimolea E, Reichert JM. World Bispecific Antibody Summit, September 27–28, 2011, Boston, MA. MAbs. 2012;4(1):4–13. | ||
Yu S, Li A, Liu Q, et al. Recent advances of bispecific antibodies in solid tumors. J Hematol Oncol. 2017;10(1):155. | ||
Nisonoff A, Rivers MM. Recombination of a mixture of univalent antibody fragments of different specificity. Arch Biochem Biophys. 1961;93(2):460–462. | ||
Aalberse RC, van der Gaag R, van Leeuwen J. Serologic aspects of IgG4 antibodies – I: prolonged immunization results in an IgG4-restricted response. J Immunol. 1983;130(2):722–726. | ||
Kolfschoten MN, Schuurman J, Losen M, et al. Anti-inflammatory activity of human IgG4 antibodies by dynamic Fab arm exchange. Science. 2007;317(5844):1554–1557. | ||
Sedykh SE, Lekchnov EA, Prince VV, Buneva VN, Nevinsky GA. Half molecular exchange of IgGs in the blood of healthy humans: chimeric lambda-kappa-immunoglobulins containing HL fragments of antibodies of different subclasses (IgG1-IgG4). Mol Biosyst. 2016;12(10):3186–3195. | ||
Lekchnov EA, Sedykh SE, Dmitrenok PS, Buneva VN, Nevinsky GA. Human placenta: relative content of antibodies of different classes and subclasses (IgG1-IgG4) containing lambda- and kappa-light chains and chimeric lambda-kappa-immunoglobulins. Int Immunol. 2015;27(6):297–306. | ||
Sedykh SE, Buneva VN, Nevinsky GA. Human milk IgGs contain various combinations of different antigen-binding sites resulting in multiple variants of their bispecificity. PLoS One. 2012;7(8):e42942. | ||
Sedykh SE, Buneva VN, Nevinsky GA. Human milk sIgA molecules contain various combinations of different antigen-binding sites resulting in a multiple binding specificity of antibodies and enzymatic activities of abzymes. PLoS One. 2012;7(11):e48756. doi:10.1371/journal.pone.0048756. | ||
Milstein C, Cuello AC. Hybrid hybridomas and their use in immunohistochemistry. Nature. 1983;305(5934):537–540. | ||
Brennan M, Davison PF, Paulus H. Preparation of bispecific antibodies by chemical recombination of monoclonal immunoglobulin G1 fragments. Science. 1985;229(4708):81–83. | ||
Glennie MJ, McBride HM, Worth AT, Stevenson GT. Preparation and performance of bispecific F(ab’ gamma)2 antibody containing thioether-linked Fab’ gamma fragments. J Immunol. 1987;139(7):2367–2375. | ||
Staerz UD, Kanagawa O, Bevan MJ. Hybrid antibodies can target sites for attack by T cells. Nature. 1985;314(6012):628–631. | ||
Perez P, Hoffman RW, Shaw S, Bluestone JA, Segal DM. Specific targeting of cytotoxic T cells by anti-T3 linked to anti-target cell antibody. Nature. 1985;316(6026):354–356. | ||
Staerz UD, Yewdell JW, Bevan MJ. Hybrid antibody-mediated lysis of virus-infected cells. Eur J Immunol. 1987;17(4):571–574. | ||
Scott AM, Allison JP, Wolchok JD. Monoclonal antibodies in cancer therapy. Cancer Immun. 2012;12:14. http://www.ncbi.nlm.nih.gov/pubmed/22896759 | ||
Ball ED, Guyre PM, Mills L, Fisher J, Dinces NB, Fanger MW. Initial trial of bispecific antibody-mediated immunotherapy of CD15-bearing tumors: cytotoxicity of human tumor cells using a bispecific antibody comprised of anti-CD15 (MoAb PM81) and anti-CD64/FcγRI (MoAb 32). J Hematother. 1992;1(1):85–94. | ||
Valone FH, Kaufman PA, Guyre PM, et al. Clinical trials of bispecific antibody MDX-210 in women with advanced breast or ovarian cancer that overexpresses HER-2/neu. J Hematother. 1995;4(5):471–475. | ||
Valone FH, Kaufman PA, Guyre PM, et al. Phase IA/IB trial of bispecific antibody MDX-210 in patients with advanced breast or ovarian cancer that overexpresses the proto-oncogene HER-2/neu. J Clin Oncol. 1995;13(9):2281–2292. | ||
Weiner LM, Clark JI, Davey M, et al. Phase I trial of 2B1, a bispecific monoclonal antibody targeting c-erbB-2 and FcγRIII. Cancer Res. 1995;55(20):4586–4593. | ||
Wu C, Ying H, Grinnell C, et al. Simultaneous targeting of multiple disease mediators by a dual-variable-domain immunoglobulin. Nat Biotechnol. 2007;25(11):1290–1297. | ||
Jakob CG, Edalji R, Judge RA, et al. Structure reveals function of the dual variable domain immunoglobulin (DVD-Ig) molecule. MAbs. 2013;5(3):358–363. | ||
Hu S, Fu W, Xu W, et al. Four-in-one antibodies have superior cancer inhibitory activity against EGFR, HER2, HER3, and VEGF through disruption of HER/MET crosstalk. Cancer Res. 2015;75(1):159–170. | ||
Correia I, Sung J, Burton R, et al. The structure of dual-variable-domain immunoglobulin molecules alone and bound to antigen. MAbs. 2013;5(3):364–372. | ||
Shalaby MR, Shepard HM, Presta L, et al. Development of humanized bispecific antibodies reactive with cytotoxic lymphocytes and tumor cells overexpressing the HER2 protooncogene. J Exp Med. 1992;175(1):217–225. | ||
Doppalapudi VR, Tryder N, Li L, et al. Chemically programmed antibodies: endothelin receptor targeting CovX-Bodies. Bioorg Med Chem Lett. 2007;17(2):501–506. | ||
Doppalapudi VR, Huang J, Liu D, et al. Chemical generation of bispecific antibodies. Proc Natl Acad Sci U S A. 2010;107(52):22611–22616. | ||
Gall JM, Davol PA, Grabert RC, Deaver M, Lum LG. T cells armed with anti-CD3 × anti-CD20 bispecific antibody enhance killing of CD20+ malignant B cells and bypass complement-mediated rituximab resistance in vitro. Exp Hematol. 2005;33(4):452–459. | ||
Lum LG, Thakur A, Liu Q, et al. CD20-targeted T cells after stem cell transplantation for high risk and refractory non-Hodgkin’s lymphoma. Biol Blood Marrow Transplant. 2013;19(6):925–933. | ||
Iams WT, Sosman JA, Chandra S. Novel targeted therapies for metastatic melanoma. Cancer J. 2017;23(1):54–58. | ||
Boudousquie C, Bossi G, Hurst JM, Rygiel KA, Jakobsen BK, Hassan NJ. Polyfunctional response by ImmTAC (IMCgp100) redirected CD8+ and CD4+ T cells. Immunology. 2017;152(3):425–438. | ||
Bossi G, Buisson S, Oates J, Jakobsen BK, Hassan NJ. ImmTAC-redirected tumour cell killing induces and potentiates antigen cross-presentation by dendritic cells. Cancer Immunol Immunother. 2014;63(5):437–448. | ||
Rossi EA, Goldenberg DM, Cardillo TM, McBride WJ, Sharkey RM, Chang CH. Stably tethered multifunctional structures of defined composition made by the dock and lock method for use in cancer targeting. Proc Natl Acad Sci U S A. 2006;103(18):6841–6846. | ||
Chang CH, Rossi EA, Goldenberg DM. The dock and lock method: a novel platform technology for building multivalent, multifunctional structures of defined composition with retained bioactivity. Clin Cancer Res. 2007;13(18):5586S–5591S. | ||
Rossi EA, Goldenberg DM, Cardillo TM, Stein R, Chang CH. Hexavalent bispecific antibodies represent a new class of anticancer therapeutics – 1: properties of anti-CD20/CD22 antibodies in lymphoma. Blood. 2009;113(24):6161–6171. | ||
Rossi EA, Goldenberg DM, Cardillo TM, Stein R, Chang CH. CD20-targeted tetrameric interferon-α, a novel and potent immunocytokine for the therapy of B-cell lymphomas. Blood. 2009;114(18):3864–3871. | ||
Rossi EA, Rossi DL, Stein R, Goldenberg DM, Chang CH. A bispecific antibody-IFNα2B immunocytokine targeting CD20 and HLA-DR is highly toxic to human lymphoma and multiple myeloma cells. Cancer Res. 2010;70(19):7600–7609. | ||
Rossi EA, Chang CH, Goldenberg DM. Anti-CD22/CD20 bispecific antibody with enhanced trogocytosis for treatment of lupus. PLoS One. 2014;9(5):e98315. | ||
Rossi DL, Rossi EA, Cardillo TM, Goldenberg DM, Chang CH. A new class of bispecific antibodies to redirect T cells for cancer immunotherapy. MAbs. 2014;6(2):381–391. | ||
Müller D, Karle A, Meissburger B, Höfig I, Stork R, Kontermann RE. Improved pharmacokinetics of recombinant bispecific antibody molecules by fusion to human serum albumin. J Biol Chem. 2007;282(17):12650–12660. | ||
Stork R, Müller D, Kontermann RE. A novel tri-functional antibody fusion protein with improved pharmacokinetic properties generated by fusing a bispecific single-chain diabody with an albumin-binding domain from streptococcal protein G. Protein Eng Des Sel. 2007;20(11):569–576. | ||
Chelius D, Ruf P, Gruber P, et al. Structural and functional characterization of the trifunctional antibody catumaxomab. MAbs. 2010;2(3):309–319. | ||
Bos R, Nieuwenhuizen W. Enhanced transfection of a bacterial plasmid into hybridoma cells by electroporation: application for the selection of hybrid hybridoma (quadroma) cell lines. Hybridoma. 1992;11(1):41–51. | ||
Karawajew L, Micheel B, Behrsing O, Gaestel M. Bispecific antibody-producing hybrid hybridomas selected by a fluorescence activated cell sorter. J Immunol Methods. 1987;96(2):265–270. | ||
Cao Y, Suresh MR. Bispecific antibodies as novel bioconjugates. Bioconjug Chem. 1998;9(6):635–644. | ||
Ridgway JB, Presta LG, Carter P. “Knobs-into-holes” engineering of antibody CH3 domains for heavy chain heterodimerization. Protein Eng. 1996;9(7):617–621. | ||
Atwell S, Ridgway JB, Wells JA, Carter P. Stable heterodimers from remodeling the domain interface of a homodimer using a phage display library. J Mol Biol. 1997;270(1):26–35. | ||
Davis JH, Aperlo C, Li Y, et al. SEED bodies: fusion proteins based on strand-exchange engineered domain (SEED) CH3 heterodimers in an Fc analogue platform for asymmetric binders or immunofusions and bispecific antibodies†. Protein Eng Des Sel. 2010;23(4):195–202. | ||
Strop P, Ho WH, Boustany LM, et al. Generating bispecific human IgG1 and IgG2 antibodies from any antibody pair. J Mol Biol. 2012;420(3):204–219. | ||
Labrijn AF, Meesters JI, de Goeij BE, et al. Efficient generation of stable bispecific IgG1 by controlled Fab-arm exchange. Proc Natl Acad Sci U S A. 2013;110(13):5145–5150. | ||
Bostrom J, Yu SF, Kan D, et al. Variants of the antibody herceptin that interact with HER2 and VEGF at the antigen binding site. Science. 2009;323(5921):1610–1614. | ||
Schaefer G, Haber L, Crocker LM, et al. A two-in-one antibody against HER3 and EGFR has superior inhibitory activity compared with monospecific antibodies. Cancer Cell. 2011;20(4):472–486. | ||
Holliger P, Prospero T, Winter G. “Diabodies”: small bivalent and bispecific antibody fragments. Proc Natl Acad Sci U S A. 1993;90(14):6444–6448. | ||
Zhu Z, Presta LG, Zapata G, Carter P. Remodeling domain interfaces to enhance heterodimer formation. Protein Sci. 2008;6(4):781–788. | ||
Kipriyanov SM, Moldenhauer G, Schuhmacher J, et al. Bispecific tandem diabody for tumor therapy with improved antigen binding and pharmacokinetics. J Mol Biol. 1999;293(1):41–56. | ||
Alt M, Müller R, Kontermann RE. Novel tetravalent and bispecific IgG-like antibody molecules combining single-chain diabodies with the immunoglobulin γ1 Fc or CH3 region. FEBS Lett. 1999;454(1–2):90–94. | ||
Mack M, Riethmüller G, Kufer P. A small bispecific antibody construct expressed as a functional single-chain molecule with high tumor cell cytotoxicity. Proc Natl Acad Sci U S A. 1995;92(15):7021–7025. | ||
Baeuerle PA, Kufer P, Bargou R. BiTE: teaching antibodies to engage T-cells for cancer therapy. Curr Opin Mol Ther. 2009;11(1):22–30. | ||
Dreier T, Lorenczewski G, Brandl C, et al. Extremely potent, rapid and costimulation-independent cytotoxic T-cell response against lymphoma cells catalyzed by a single-chain bispecific antibody. Int J Cancer. 2002;100(6):690–697. | ||
Haas C, Krinner E, Brischwein K, et al. Mode of cytotoxic action of T cell-engaging BiTE antibody MT110. Immunobiology. 2009;214(6):441–453. | ||
Ward ES, Güssow D, Griffiths AD, Jones PT, Winter G. Binding activities of a repertoire of single immunoglobulin variable domains secreted from Escherichia coli. Nature. 1989;341(6242):544–546. | ||
Davies J, Riechmann L. Antibody VH domains as small recognition units. Biotechnology (N Y). 1995;13(5):475–479. | ||
Conrath KE, Lauwereys M, Wyns L, Muyldermans S. Camel single-domain antibodies as modular building units in bispecific and bivalent antibody constructs. J Biol Chem. 2001;276(10):7346–7350. | ||
Rothe A, Sasse S, Topp MS, et al. A phase 1 study of the bispecific anti-CD30/CD16A antibody construct AFM13 in patients with relapsed or refractory Hodgkin lymphoma. Blood. 2015;125(26):4024–4031. | ||
Pang X, Ma F, Zhang P, et al. Treatment of human B-cell lymphomas using minicircle DNA vector expressing anti-CD3/CD20 in a mouse model. Hum Gene Ther. 2017;28(2):216–225. | ||
Freedman JD, Hagel J, Scott EM, et al. Oncolytic adenovirus expressing bispecific antibody targets T-cell cytotoxicity in cancer biopsies. EMBO Mol Med. 2017;9(8):1067–1087. | ||
Patterson JT, Gros E, Zhou H, et al. Chemically generated IgG2 bispecific antibodies through disulfide bridging. Bioorg Med Chem Lett. 2017;27(16):3647–3652. | ||
Labrijn AF, Buijsse AO, van den Bremer ET, et al. Therapeutic IgG4 antibodies engage in Fab-arm exchange with endogenous human IgG4 in vivo. Nat Biotechnol. 2009;27(8):767–771. | ||
Huang Y, Yu J, Lanzi A, et al. Engineered bispecific antibodies with exquisite HIV-1-neutralizing activity. Cell. 2016;165(7):1621–1631. | ||
Bournazos S, Gazumyan A, Seaman MS, Nussenzweig MC, Ravetch JV. Bispecific anti-HIV-1 antibodies with enhanced breadth and potency in brief bispecific anti-HIV-1 antibodies with enhanced breadth and potency. Cell. 2016;165(7):1609–1620. | ||
Chen Y, Xu Y. Pharmacokinetics of bispecific antibody. Curr Pharm Rep. 2017;3(3):126–137. | ||
Byrne H, Conroy PJ, Whisstock JC, O’Kennedy RJ. A tale of two specificities: bispecific antibodies for therapeutic and diagnostic applications. Trends Biotechnol. 2013;31(11):621–632. | ||
McBride WJ, Zanzonico P, Sharkey RM, et al. Bispecific antibody pretargeting PET (immunoPET) with an 124I-labeled hapten-peptide. J Nucl Med. 2006;47(10):1678–1688. | ||
Sarkar S, Tang XL, Das D, Spencer JS, Lowary TL, Suresh MR. A bispecific antibody based assay shows potential for detecting tuberculosis in resource constrained laboratory settings. PLoS One. 2012;7(2):e32340. | ||
Chen YP, Qiao YY, Zhao XH, Chen HS, Wang Y, Wang Z. Rapid detection of hepatitis B virus surface antigen by an agglutination assay mediated by a bispecific diabody against both human erythrocytes and hepatitis B virus surface antigen. Clin Vaccine Immunol. 2007;14(6):720–725. | ||
Guttikonda S, Tang XL, Yang BM, Armstrong GD, Suresh MR. Monospecific and bispecific antibodies against E. coli O157 for diagnostics. J Immunol Methods. 2007;327(1–2):1–9. | ||
Tang XL, Peppler MS, Irvin RT, Suresh MR. Use of bispecific antibodies in molecular velcro assays whose specificity approaches the theoretical limit of immunodetection for Bordetella pertussis. Clin Vaccine Immunol. 2004;11(4):752–757. | ||
Wagstaffe SJ, Hill KE, Williams DW, et al. Bispecific antibody-mediated detection of the Staphylococcus aureus thermonuclease. Anal Chem. 2012;84(14):5876–5884. | ||
Sunwoo HH, Palaniyappan A, Ganguly A, et al. Quantitative and sensitive detection of the SARS-CoV spike protein using bispecific monoclonal antibody-based enzyme-linked immunoassay. J Virol Methods. 2013;187(1):72–78. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
