Back to Journals » Journal of Inflammation Research » Volume 14
Biotite-Calx Based Traditional Indian Medicine Sahastraputi-Abhrak-Bhasma Prophylactically Mitigates Allergic Airway Inflammation in a Mouse Model of Asthma by Amending Cytokine Responses
Authors Balkrishna A, Solleti SK, Singh H, Singh R, Sharma N, Varshney A
Received 7 April 2021
Accepted for publication 25 August 2021
Published 17 September 2021 Volume 2021:14 Pages 4743—4760
DOI https://doi.org/10.2147/JIR.S313955
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
Acharya Balkrishna,1– 3 Siva Kumar Solleti,1,* Hoshiyar Singh,1,* Rani Singh,1 Niti Sharma,1 Anurag Varshney1,2,4
1Drug Discovery and Development Division, Patanjali Research Institute, Haridwar, Uttarakhand, India; 2Department of Allied and Applied Sciences, University of Patanjali, Patanjali Yog Peeth, Haridwar, Uttarakhand, India; 3Patanjali UK Trust, Glasgow, UK; 4Special Centre for Systems Medicine, Jawaharlal Nehru University, New Delhi, India
*These authors contributed equally to this work
Correspondence: Anurag Varshney
Drug Discovery and Development, Patanjali Research Institute, NH-58, Near Bahadrabad, Haridwar, 249405, UttraKhand, India
Tel +91 1334-244107 x7458
Fax +91 1334 244805
Email [email protected]
Purpose: Asthma is a heterogeneous airway inflammatory disease with limited therapeutic options. Traditional medicine is extensively used for treating various ailments including asthma. Sahastraputi-Abhrak-Bhasma (SPAB) is a biotite-calx based Indian medicine.
Methods: We have tested for the anti-inflammatory and anti-asthmatic properties of SPAB, using a mouse model of ovalbumin-induced allergic asthma in-vivo and cell-based assays in-vitro. Histological analysis, qPCR and ELISA were performed to assess the pathology. SEM, EDX and XRD-analysis were performed to characterize the SPAB particles.
Results: SEM, EDX and XRD-analysis identified the presence of SPAB particle of 100 nm–∼ 1μm diameter and contains annite-1M, aluminium silicate, kyanite, aluminium oxide, magnesium silicate, and maghemite in the samples. Ova-challenge resulted in severe inflammatory responses, airway remodelling and increased oxidative burden in lungs. Importantly, prophylactic treatment with SPAB significantly attenuated allergen induced leukocyte infiltration specifically eosinophils, lymphocytes, macrophages and neutrophils in BALF. Ova-induced mucus hypersecretion, peri-bronchial collagen deposition, inflammatory cell infiltration and bronchial epithelial thickening were significantly abrogated upon SPAB treatment. qPCR and ELISA analysis identified that allergen induced increases in IL-5, IL-13, IL-33, IFN-γ and IL-1β cytokines mRNA in whole lungs and the levels of IL-6, IL-1β and TNF-α proteins in BALF were significantly attenuated upon oral SPAB treatment. SPAB restored allergen induced decreases in anti-oxidant markers in lungs. In-vitro, SPAB attenuated the secretion of IL-6, and TNF-α from human bronchial epithelial cells and modestly inhibited NF-kB/AP-1 pathway in HEK cells.
Conclusion: Taken together, our results experimentally validated the prophylactic ameliorative potential of the Indian classical medicine Sahastraputi-Abhrak-Bhasma against asthma associated airway inflammation.
Keywords: Sahastraputi-Abhrak-Bhasma, asthma, physicochemical analysis, lung inflammation, cytokine, mica-medicine
Introduction
Asthma is a common heterogeneous disease of the airways, affecting millions of people of all age groups globally and its incidence is rising.1,2 It is characterized by inflamed lung airways, wheezing, coughing and shortness of breath.3 Allergic asthma is more prevalent in developing countries and is associated with Th2 cell responses,2 including secretion of Th2 related cytokine, such as interleukin (IL)-4, IL-5 and IL-13.3 This in turn contributes to eosinophilic airway inflammation, airway hyper responsiveness (AHR), mucus hyper secretion and airway wall remodelling.4 AHR leads to obstruction of airflow and the long term use of steroidal anti-inflammatory drugs for asthma in clinical practice can cause potential complications, such as pneumonia and hyperglycaemia.5
Involvement of various cells including, eosinophils, mast cells, dendritic cells, lymphocytes, macrophages and neutrophils is obvious in asthma pathology; and interaction between immune cells and structural cells like epithelial cells and mesenchymal cells plays a crucial role in progression and severity of the disease.3,6 Even though extensive advances in management of asthma and allergy, in nearly 5–10% of people, this condition is persistently severe and ineffectively controlled.5 Currently, there is no established method to avert asthma and allergy. Hence, identification of new therapeutic strategies for effective treatment are obligatory.
The chronological significance of traditional medicine as remedy in the management of asthma is irrefutable7 and 60–70% population with moderate and severe asthma rely upon traditional medicine as supplement.7 In traditional Indian Ayurveda, Tibetan medicine and traditional Chinese medicine, use of herbo-metallic or herbo-mineral remedies for asthma has been in practice.8 Rasa-shastra, of ancient Indian Ayurveda deals with the harnessing the therapeutic potential of various herbs, mineral, metals and non-metals by applying various incineration procedures that will convert them into non-toxic, bio-acceptable forms called bhasma,9,10 this has been recently defined by WHO.11 However, appropriate scientific validation of the therapeutic potentials of Bhasma is lacking.
Abhrak bhasma, is a biotite-calx based traditional Indian medicine prepared by a process called puta (incineration in a closed earthen vessel) to harness its unique attributes.12,13 Calx is defined as the ashy or oxide material which remains after metals and minerals have been methodically incinerated. Shataputi Abhrak Bhasma and Sahastraputi-Abhrak-Bhasma (SPAB) are two unique preparations where mica is titrated with distinct herbals and subjected to incineration 100 times and 1000 times respectively followed by treating with cow ghee,12 resulting in mica/biotite-calx. Abhrak bhasma is classically prescribed for the handling anaemia and tropical sprue, treating chronic cough12 bronchitis and asthma.14 It protects from various types of cancers,13 CCl4 induced hepatitis15 and improves kidney and liver function16 and exerts diuretic activity.17
Shataputi abhrak bhasma and SPAB are considered to be finest form of biotite-calx (mica based) bhasma14 and contains immunomodulatory properties.18 SPAB has been shown to protect from heat-induced oligozoospermia and azoospermia, possibly by modulating p53 and Fas mediated apoptotic pathways and ameliorates germinal epithelial proliferation.19 The aim of the present study is to scientifically validate the anti-inflammatory and anti-asthmatic properties of SPAB using a mouse model of Ovalbumin (Ova)-induced allergic asthma and analyses its chemical composition.
Materials and Methods
Physicochemical Analysis of the SPAB
Sahastraputi-Abhrak-Bhasma (SPAB) was sourced from Divya Pharmacy, Haridwar, India. Scanning electron microscope (SEM; LEO-438 VP) coupled with electron dispersive X-ray analysis system (Carl Zeiss, Germany) was used for analysing the morphological and elemental characteristics of SPAB powder. SEM imaging was carried out at 10 kV accelerated voltage. EDX analysis was done by mapping and spot-based analysis. X-ray diffraction (XRD) analysis of SPAB powder was carried out using a Rigaku D-Max 2200 X-ray diffractometer (XRD) applying Cu-Kα radiation at 40 kV/40 mA. Scanning was performed at a step width of 0.02° over an angular range of 5° to 80° and a scanning rate of 0.5° min−1.20 Qualitative analysis of the XRD spectra of SPAB was performed using QualX (Version 2.24).21
Animals
Specific Pathogen Free (SPF), female BALB/c mice weighing 20–22 g at eight weeks’ age were maintained in vivarium under 12:12-h light-dark cycle. Mice were procured from Charles River Laboratory licensed animal supplier, Hylasco Biotechnology Pvt. Ltd, Hyderabad, India and nourish with standard pellet diet (Purina Lab Diet, St. Louis, MO, USA) and sterile water ad-libitum. Animal experiments and treatment procedures were approved by Institutional Animal Ethics Committee of Patanjali Research Institute (IAEC approval number- PRIAS/LAF/IAEC-078; approval date: 17th July, 2019) in adherence to the guidelines from Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Government of India.
Calculation of in-vivo Experimental Doses
For in-vivo experiments, the mouse equivalent doses of SPAB were calculated based on body surface area of mice. The recommended human therapeutic dose of SPAB was 500 mg/day and the mouse equivalent doses (mg/kg) was calculated by multiplying human equivalent dose (mg/kg) by factor of 12.3.22 The calculated therapeutic equivalent dose for mouse was 102 mg/kg and considered as mid dose (1x) and its 1/3x and 3x doses were regarded as low-dose and high-dose, for pharmacological studies (34 mg/kg, 102 mg/kg and 306 mg/kg).
Establishment of Allergic Asthma Model and Drug Treatment
Mice were allocated into six different treatment groups: (1) Normal Control (NC); (2) Disease Control (DC); (3) Dexamethasone (DM)- 2 mg/kg; (4) SPAB - 34 mg/kg; (5) SPAB- 102 mg/kg; and (6) SPAB-306 mg/kg. For establishing asthma model, (mice in groups 2 to 6 were intraperitoneally (i.p.) sensitized with Ovalbumin (Ova) (25 μg) and Aluminum hydroxide (2 mg) in 0.2 mL of saline (PBS) on days 0, 7 and 14, as described previously23. On day 21, 23, 25, and 27 mice were challenged with 100 μg Ova- in PBS (25 μL) by intranasal instillation under mild anesthetic. Mice in NC group received only PBS during sensitization and challenge. While NC and DC received 0.5% Carboxy methyl cellulose (CMC), three different doses of SPAB daily were given orally in 0.5% CMC from day −7 prophylactically. DM group received 2 mg/kg DM by i.p., 2 h before Ova-challenge. Ovalbumin (Ova) and Dexamethasone (DM) was sourced from Sigma Aldrich (St Louis, MO, USA).
Broncho-Alveolar Lavage (BAL) Phenotyping and Harvesting Lungs
BAL fluid was collected and lobes of lungs were resected as described previously23–25 followed by calculation of total and differential cell counts manually. Briefly, mice were sacrificed, and the left lung was ligated and the right lung was lavaged with 3 vol (1 mL/25 g) of ice-cold PBS by inserting a catheter in the trachea.24 The resulting broncho-alveolar lavage (BAL) fluid was centrifuged (3000 rpm for 3 min at 4°C), and the supernatant was retained for further analysis. 1% acetic acid was used for lysing the red blood cells in cell pellets, and BAL cells were re-suspended in PBS. The total cell counts were measured, and cyto-smear preparation was performed and stained with Wright’s staining.24 Subsequently, the differential leukocyte counts of 300 cells was performed using standard morphological criteria.
Histopathological Assessment of Lungs
Histological examinations were performed as described previously.23,25 Briefly, left lung lobes were sectioned and stained with Hematoxylin and Eosin (H&E) for gross pathological analysis. Mucus secretion was identified using Periodic-Acid-Schiff (PAS) staining and peri-bronchial collagen deposition was assessed using Mason-Trichrome (MT) staining. The severity of histopathological alterations were determined using 5-point score methods26,27 as follows: 0= No Abnormality Detected (NAD), 1= Minimal, 2= Mild, 3= Moderate, 4= Moderately Severe, 5= Severe; and distribution was noted as focal, multifocal and diffused.
RNA Isolation and Real-Time Quantitative RT-PCR of Lung Tissue
Total RNA from lung tissue were isolated using TRIzol reagent (Invitrogen, USA) and cDNA synthesis was performed using the Verso cDNA synthesis kit (Thermo Fisher Scientific, USA) as described previously,23,25 RNA was rendered DNA-free and re-purified using the RNeasy mini kit (Qiagen, USA) and RNAs were reverse-transcribed using Verso cDNA synthesis kit (Thermo Fisher Scientific, USA). Quantitative real-time PCR was performed using Biometra TProfessional thermocycler (Analytik Jena, Germany) and SYBR green master mix (Applied Biosystems, USA). Gene expression levels were calculated relative to Ppia (cyclophilin A) using 2−∆∆Ct as described previously24,28 using the primers sequences derived from MGH primer bank.
Measurement of Oxidative Stress Biochemical Markers in Lungs
DTNB reagent was used to measure Glutathione peroxidase (GPX) activity at 420 nm as per Rotruck et al 197329 in a 96 well format. Goth 199130 was adapted for measuring the Catalase (CAT) activity at 405 nm. The Super oxide dismutase (SOD) activity was measured using NBT31 at 560 nm. The Glutathione (GSH) and Glutathione disulfide (GSSG) levels were measured by the protocol of Hissin & Hilf, 197632 fluorometrically at Ex 350/Em 420 nm. Malondialdehyde (MDA) levels were measured as per Heath & Packer, 196833 and the absorbance was measured at 532 nm. For measuring Myeloperoxidase (MPO)/ Eosinophil peroxidase (EPO) activity, 3,3ʹ,5,5ʹ-Tetramethylbenzidine substrate34,35 was used and absorbance was measured at 450 nm as described.36 The absorbance or fluorescence intensity was measured using Envision microplate reader (Perkin Elmer, USA) and expressed as U/mg or µM/mg weight of lung tissue or U/mL BAL fluid.
Pro-Inflammatory Cytokine Analysis
Enzyme-linked immunosorbent assay (ELISA) of BALF was carried out for measuring the levels of mouse IL-6, IL-1β and TNF-α (BioLegend Inc., San Diego, USA) and the secretion of cytokines into culture supernatants by BEAS2B were measured using human IL-6 and TNF-α ELISA (BD Bioscience, USA) as per the manufacturer’s instructions and the plates were read at 450 nm using Envision microplate reader (Perkin Elmer, USA).
In-vitro Cell Culture
The cell lines used in this study includes, BEAS2B cells (kindly provided by Dr. Rajarshi Pal, India, and approved for use by the Institutional Biosafety Committee of Patanjali Research Institute) and HEK-Blue TNF-α cells (InvivoGen, USA). BEAS2B were maintained in RPMI-1640 (Invitrogen, USA) containing 10% heat-inactivated FBS, L-glutamine (4 mM) and antibiotics. HEK-Blue TNF-α cells (stably expressing secreted embryonic alkaline phosphatase (SEAP) reporter gene fused with NF-κB/AP-1 elements) were cultured in DMEM containing 10% heat inactivated FBS, 100 μg/mL Normocin, L-Glutamine (2 mM), 100 μg/mL Streptomycin and 100 U/mL Penicillin and cells were kept in a humidified incubator at 5% CO2 at 37°C.
Cell Viability and Anti-Inflammatory Activity Assay of SPAB in-vitro
BEAS2B cells were seeded at 1×105 cell/mL in a 96 well plate and treated with various doses of SPAB (1 µg/mL - 100 µg/mL) for 24 hr upon reaching 70% confluence. Cells were washed and incubated with 10 µL of Alamar blue (0.15 mg/mL) (Hi Media, India) in serum free medium for 1 hr and fluorescence at Ex. 530 nm/Em. 590 nm was measured and expressed as percentage cell viability. BEAS2B cells were pre-treated for 24 hr with various concentrations of SPAB (1–30 µg/mL) followed by co-treatment with SPAB + 500 ng/mL lipopolysaccharide (LPS) for another 24 hr. Cell culture supernatants were used for measuring cytokine secretion.
NF-kB/AP-1-SEAP Reporter Assay
HEK-Blue TNF-α cells were seeded at 3×105 cells/mL density in 96 well plates and treated with different concentrations of SPAB (1 μg/mL to 10 μg/mL) for 6 hr followed by addition of 500 pg/mL of human TNF-α. Post 24 hr incubation, alkaline phosphatase activity was measured at 630 nm as per the manufacturers (InvivoGen, San Diego, CA, USA) instruction using QUANTI-Blue substrate.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism version 7.0 software (GraphPad Software, San Diego, CA, USA). A one-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison test was used to calculate the statistical difference. All data were expressed as the Mean ± Standard Error of Means (SEM). Differences in mean values were considered significant at p < 0.05.
Results
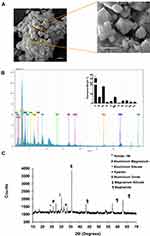
Physicochemical Characterization of Sahastraputi Abhrak Bhasma (SPAB) Particles
Scanning electron microscope (SEM) analysis of the SPAB powder revealed heterogeneous nature of SPAB in shape and size (Figure 1A) of diameters of 100 nm to ~1 µm (Figure 1A). Electron dispersive X-ray (EDX) quantitative analysis of SPAB revealed the presence of oxygen (O) (55.5 ± 2.9%); Silicon (Si) (6.8 ± 0.4%); Iron (Fe) (18.1 ± 1.1%); Aluminium (Al) (1.3 ± 0.09%); Potassium (K) (2.2 ± 0.1%); Magnesium (Mg) (6.7 ± 0.4%); Chlorine (Cl) (0.43 ± 0.02%); Nickel (Ni) (2.7 ± 0.1%); Chromium (Cr) (2.9 ± 0.1%); Sodium (Na) (1.6 ± 0.1%); Calcium (Ca) (1.4 ± 0.09%) elements on the surface of the SPAB particles (Figure 1B). X-ray diffraction (XRD) analysis of SPAB discovered the presence of various silica rich compounds and aluminium, Iron and magnesium compounds such as, Annite-1M (KFe32+AlSi3O10(OH,F)2), Aluminium Magnesium (Al-Mg), Aluminium Silicate (Al2SiO5), Kyanite (Al2SiO5), Aluminium Oxide (Al2O3), Magnesium Silicate (MgO3Si), and Maghemite (γ-Fe2O3) in the samples (Figure 1C).
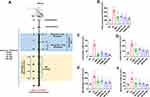
SPAB Alleviates Allergen-Induced Leukocyte Influx in Asthmatic Lungs
To determine if SPAB could alleviate Allergen induced airway inflammatory responses, as described in the Materials and Methods section (Figure 2A), we first collected BALF and tested for the total and differential leukocyte recruitment in lungs. The results indicated a significant upsurge in the total number of inflammatory cells in DC lungs upon Ova sensitization and challenge (DC vs NC, p<0.001) (Figure 2B), mirroring the clinical presentation of human. Conversely, SPAB at all the three doses, significantly blocked the inflammatory cell recruitment (DC vs SPAB 34–306, p<0.001) (Figure 2B), similar to DM (DC vs DM-2, p<0.001) (Figure 2B) treatment group.
We next tested for the differential cell counts and found a significant increase (DC vs NC, p<0.001) in the numbers of eosinophils, lymphocytes, macrophages and neutrophils in DC (Figure 2C–F). Notably, a dose dependent decline in the above mentioned cell numbers were noticed upon daily oral SPAB administration (DC vs SPAB 34–306, p<0.001) (Figure 2C–F) of allergic mice, similar to DM (DC vs DM, p<0.001). These results advocate the beneficial role of SPAB in regulating the allergen induced leukocyte influx into the lungs.
SPAB Improves Allergen-Induced Airway Remodelling and Whole Lung Pathology
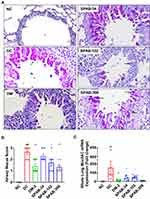
Upon confirmation of inhibitory effect of SPAB on leukocyte recruitment in lungs, we next tested for the lung histopathology. PAS staining of the lung tissue sections (Figure 3) for the mucus secretion identified a marked increase in the allergen-induced mucus accumulation in DC compared to NC (Figure 3A, NC & DC), increasing semi quantitative airway mucus score (Figure 3B). Conversely, allergic mice receiving three different doses of SPAB (Figure 3A, SPAB-34, −102 and −306) or DM (Figure 3A, DM-2) showed a considerable decrease in mucus accumulation and airway mucus score (Figure 3B). In line with mucus accumulation, mRNA expression analysis confirmed SPAB mediated significant diminution of Muc5AC gene expression (Figure 3C) in allergic mice (DC vs SPAB 34–306, p<0.05), parallel to DM treated mice (DC vs DM-2, p<0.001).
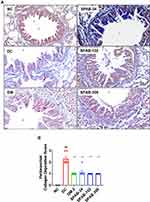
In addition, Masson Trichrome staining of lung tissue section identified a robust increase in the peri-bronchial collagen deposition in allergic DC mice, compared to unchallenged NC mice (Figure 4A, NC & DC). This resulted in the significant increase in collagen deposition score, measured semi-quantitatively (DC vs NC, p<0.001) (Figure 4B). Whereas, either treatment with DM (Figure 4A, DM-2) or SPAB (Figure 4A, SPAB-34, −102 and −306) inhibited collagen deposition, and significantly decreased collagen deposition scores (DC vs SPAB 34–306, p<0.001) (Figure 4B).
We next evaluated lung inflammation using H&E staining and identified peribronchial and perivascular leukocyte cell aggregates in allergen challenged DC compared to NC mice (Figure 5A, NC & DC) and leukocyte recruitment in lungs was also significantly increased (DC vs NC, p<0.001) (Figure 5B). Conversely, treatment with DM (Figure 5A, DM-2) and SPAB (Figure 5A, SPAB-34, −102 and −306) significantly repressed (p<0.05) inflammatory cell infiltration into (Figure 5B) lung airspaces and decreased leukocyte aggregates. Furthermore, allergen induced thickening of bronchial epithelium was significantly decreased upon SPAB (DC vs SPAB 34–306, p<0.05) (Figure 5C) and DM (DC vs DM-2, p<0.05) (Figure 5C) treatment. Collectively, Ova allergen induced increases in the total pathological score (p<0.001) (Figure 5D) was significantly abrogated upon oral SPAB treatment (p<0.001).
Oral SPAB Treatment Mitigates Allergen-Induced Pro-Inflammatory Gene Expression
Upon confirmation of SPAB mediated reversal of lung histopathological responses, we tested for the mRNA expression of asthma associated pro-inflammatory genes. The results indicated that, compared to unchallenged NC allergen challenge in DC resulted in significant (DC vs NC, p<0.001) multi fold upregulation of expression of Interleukin (IL)-5 (p<0.001) (Figure 6A), IL-13 (p<0.001) (Figure 6B), IL-33 (p<0.001) (Figure 6C), Interferon (IFN)-ɣ (Figure 6D) and IL-1β (p<0.001) (Figure 6E). Therapeutic administration of SPAB significantly attenuated the Ova induced expression of IL-5 (p<0.05) (Figure 6A), IL-13 (p<0.05) (Figure 6B), IL-33 (p<0.001) (Figure 6C), IFN-ɣ (Figure 6D) at mid and high doses or at its highest dose IL-1β (p<0.05) (Figure 6E). Similar attenuation of pro-inflammatory gene expression was attained with DM treatment (Figure 6A–D) for the above genes except IL-1β (NS) (Figure 6E).
BAL fluid analysis for the cytokine protein secretion indicated a substantial increase in the BALF levels of IL-1β (Figure 6F), tumour necrosis factor (TNF)-α (Figure 6G) and IL-6 (Figure 6H) upon Ova-challenge (DC vs NC, p<0.001). Treatment with SPAB (DC vs SPAB 34–306, p<0.001) or DM (DC vs DM, p<0.001) treatment significantly abrogate the Ova-induced secretion of IL-1β, TNF-α and IL-6 at all doses tested (6F-H).
Oral Administration of SPAB Restores Allergen Induced Alterations in Oxidative Stress Biochemical Markers
We next tested if SPAB could modulate allergen-induced oxidative stress biochemical parameters (GPX, CAT, GSH, GSSG, SOD, MDA and EPO) in the allergic lungs. Test for the activity of GPX (DC vs NC, p<0.05) (Figure 7A), CAT (DC vs NC, p<0.05) (Figure 7B) and SOD (DC vs NC, p<0.001) (Figure 7C) identified significant decrease in enzyme activates upon allergen challenge in DC. Treatment with three different doses of SPAB restored GPX (DC vs SPAB-306, p<0.05) (Figure 7A) and CAT (DC vs SPAB-306, p<0.05) (Figure 7B) activity at its highest dose. Similar observations were made in DM treatment group (7A-C).
Levels of GSH and GSSG indicated that allergen induced decreases in GSH levels (DC vs NC, p<0.001) (Figure 7D) and simultaneous increase in GSSG levels (DC vs NC, p<0.001) (Figure 7E) in DC. SPAB treatment restored the GSH levels and highest dose (DC vs SPAB-306, p<0.05) (Figure 7D). Conversely, GSSG levels were declined to normal levels at all the three doses of SPAB (DC vs SPAB-34, −102 and −306, p<0.001) (Figure 7E). MDA estimation indicated a significant increase in its levels upon Ova-challenge (DC vs NC, p<0.001) (Figure 7F). Whereas, either treatment with DM (DC vs DM, p<0.001) or SPAB (DC vs SPAB-34, −102 and −306, p<0.001) (Figure 7F) significantly diminished its levels to normal.
Test for the peroxidase activity in whole lung and BAL indicated a significant increase in MPO/EPO in Ova-allergen challenged DC (DC vs NC, p<0.001) (Figure 7G and H). Treatment with SPAB dose dependently decreased allergen induced MPO/EPO activity at mid and high dose in lungs (DC vs SPAB-102 and −306, p<0.001) (Figure 7G) and in BAL fluid (DC vs SPAB-102 and −306, p<0.05) (Figure 7H).
SPAB Attenuates Endotoxin Induced Pro-Inflammatory Cytokine Secretion from Human Bronchial Epithelial Cells and Suppresses TNF-α Induced NF-kB/AP-1 Transcriptional Activity in Reporter Cells in-vitro
To recapitulate in-vivo anti-inflammatory responses of SPAB, human bronchial epithelial cell line BEAS2B were first treated with SPAB to test for the cyto-safety (Figure 8A). The results indicated that treatment of BEAS2B cells with various doses of SPAB for 48 hrs resulted in 20–25% cell death up to 30 µg/mL SPAB. At 100 µg/mL, 37% cell death was noticed. Hence 30 µg/mL was considered as cyto-safe.
Compared to NC, challenge of bronchial epithelial cells with LPS induced a significant increase in the secretion of IL-6 (p<0.001) (Figure 8B) and TNF-α (p<0.001) (Figure 8C) cytokine proteins into cell culture supernatant. Pre-treatment of cells with SPAB dose dependently abrogated IL-6 (SPAB, 3 µg/mL - 30 µg/mL, p<0.001) (Figure 8B) secretin and supressed TNF-α (SPAB, 1 µg/mL - 30 µg/mL, p<0.001) (Figure 8C) release.
Next, to identify the molecular pathway involved in SPAB mediated suppression of the pro-inflammatory cytokine levels, HEK-Blue TNF-α reporter cells were used. Treatment of HEK-Blue cells with TNF-α significantly induced NF-kB/AP-1 transcriptional activation by 5.5-fold (p<0.001) (Figure 8D). Conversely, pre-treatment of cells with SPAB modestly supressed TNF-α induced transcriptional activation (SPAB, 3 µg/mL – 10 µg/mL, 22–25%) (p<0.001) (Figure 8D). Collectively, in-vitro findings suggest the probable involvement of NF-kB/AP-1 pathway in SPAB mediated suppression of pro-inflammatory responses. Apparently, contribution of NF-kB signaling towards SPAB mediated amelioration of asthma pathology is limited. Therefore, the efficacy of SPAB in assuaging asthma associated inflammation is likely to be taking effect through other pathways as well.
Discussion
Use of traditional medicines is emerging as a new paradigm for treating respiratory illnesses.37 Asthma is a chronic airway disease with limited therapeutic avenues. Use of traditional medicine as complementary in asthma treatment could help a subset of asthmatics. In the present study we have successfully evaluated the anti-asthmatic, anti-inflammatory and anti-oxidant properties of an Indian traditional mica/biotite-calx based medicine, Sahastraputi-Abhrak-Bhasma (SPAB). We have demonstrated that in lungs, SPAB could ameliorate Ova-allergen induced inflammatory cell accumulation, peri-bronchial collagen deposition, mucus hyper secretion, epithelial thinking and allergen induced oxidative stress in-vivo; and pro-inflammatory cytokine expression and secretion in-vitro. Further, chemical analysis identified the presence of various silica rich compounds of therapeutic value in SPAB. Use of bhasma based traditional medicines for the asthma treatment have been well studied.23,38
Long term use of most popular corticosteroids and bronchodilators as therapeutic for reducing asthma symptoms comes at a long enduring side effects.5 These include immune suppression and susceptibility to co-morbidities and lung infections.39 Hence, alternative asthma therapeutics that can mitigate asthma symptoms without immune suppression are needed. Use of traditional herbal medicines,40 metal based medicines8 for various respiratory diseases including asthma and COPD are in extensive use as complementary medicine. Bhasma are biologically processed ancient nano-medicines.9 SPAB is a mica (Biotite)-calx based medicine. When used directly, unprocessed mica minerals may exert harmful effect on human body due to the presence of high quantities of trace-elements.41 SPAB was processed thermally and chemically for purification, detoxification and particle size reduction by incinerating a thousand times. In the present investigation, use of SPAB significantly abrogated various human endo-points of asthma in our mouse model of disease suggesting the therapeutic potential and experimental validation of SPAB.
Allergen induced leukocyte infiltration into lungs, specifically, eosinophils and lymphocytes, mucus hypersecretion, airway remodelling and sub epithelial fibrosis results in morbidity and mortality in asthma.3,42,43 Previous reports loosely indicate that abhrak bhasma comprises anti-inflammatory properties and offers relief from chronic cough and asthma.44 In the present investigation treatment of allergic mice orally with SPAB protected from above mentioned asthmatic endpoints, indicating the ameliorative potential of SPAB against asthma-associated airway inflammation. In line with our results, shatputi abhrak bhasma exerts immune modulatory properties by stimulating the phagocytic activity in leucocytes.18 In patient with TB, shatputi abhrak bhasma treatment provided better outcome by modulating cough, fever, dyspnoea, mucus, haemoptysis.45
Nano-particle based medicines are used for treating respiratory illnesses.37 Chemical fingerprinting of SPAB identified the presence of various silica rich, aluminium, iron and magnesium containing compounds. Rats fed with 2000 mg/kg Abhrak bhasma did not induce any DNA damage and did not show any signs of genotoxicity and cytotoxicity.46 Further, intravenous injection of Biotite mica did not induce any changes in the cytokine levels of tumour necrosis factor alpha (TNF‐α), interleukin (IL)‐6, IL‐12 and interferon gamma (IFN‐γ) indicating the immunological safety of Biotite.47 Dietary supplementation of mouse with germanium biotite, rich in Aluminium Silicate, boosted lymphocyte proliferation and augmented the cytotoxic T-lymphocyte (CTL) percentage.48 Aluminium Silicate exerted anti-viral effects by decreasing the titers of porcine reproductive and respiratory syndrome virus (PRRSV) in lungs and lymphoid tissues and promoted clearance of PRRSV probably by increasing the CTL.48
In the present investigation, whole lung and BAL analysis identified that Ova-allergen induced the expression of cytokines, IL-5, IL-13, IL-33, IFN-ɣ and IL-1β in lungs, and IL-1β, TNF-α and IL-6 were significantly abrogated upon oral administration of SPAB. Th2 asthma is characterised by the increases in the levels of type 2 inflammation which is prominently mediated by eosinophils, mast cells, T-helper 2 cells and basophils.49 Increased levels of IL-5 have been associated with eosinophilia.50 In our study, increases in the levels of IL-5 parallels the increases in the eosinophils. Th2 inflammation is also associated with increased IL-13 levels.49 Increases in the levels of IL-13 induces sub-epithelial fibrosis mucus hypersecretion.51 Allergen challenge in the present study increased the levels of IL-13 and subsequent airway remodelling. IL-33 acts as alarm during endothelial and epithelial cell damage.52 In allergic inflammation, IL-33 plays a crucial role in type-2 innate immunity by activating, mast cells, eosinophils, basophils and macrophages.52 IFN-ɣ plays an important role in chronic stable asthma and acute severe asthma.53 Use of blocking antibodies for asthma treatment have limited effect as they are inadequate to prevent the complex instrumentation of allergic inflammation and clinical concerns in severe asthma.50 Importantly, SPAB treatment significantly abrogated the airway remodelling, eosinophilia and the levels of associated cytokines, indicating the therapeutic value of SPAB. Previous reports indicated that by increasing the expression of Th1 cytokines, IFN‐γ and IL‐12, Biotite exerted anti-bacterial activity54 and decreased the LPS induced TNF-α levels.55 Conversely, dietary supplementation of biotite boosts the expression of TNF-α, IFN‐γ against foot-and-mouth disease virus vaccine, improving its immune-stimulatory capacity in pigs56 and cattle via the induction of humoral and cellular immune responses.57 Our in-vitro results are in line with in-vivo findings, which indicate that SPAB reduced cytokine secretion from lung airway epithelial cells at least in part via modulation of NF-kB/AP-1 transcriptional activity. The partial effect of SPAB on NF-kB/AP-1 transcriptional activity leaves room for assuming involvement of other pathways in the process. Nevertheless, collectively, these results indicate the anti-inflammatory and immune modulatory properties of SPAB.
Maghemite (γ-Fe2O3), is emerging as promising nanotheranostic material due to its bio-compatibility, bio-degradability and magnetic properties.58 Previous reports indicate that γ-Fe2O3 experts anti-inflammatory properties by inhibiting macrophage M1 polarisation, secretion of LPS induced TNF-α, IL-1β and iNOS expression.59 In rat alveolar macrophages, iron oxides inhibited the secretion of IL-6 by upregulating prostaglandin E2 levels.60 In osteoblasts, γ-Fe2O3 was capable of inducing the expression of integrin-α3 and α5 levels.59 In osteoclasts, it inhibited the expression of MMP9, cathepsin K.59 Decrease in the levels of TNF-α, IL-1β and iNOS and increases in the prostaglandin E2 levels indicates the anti-inflammatory potential of maghamite in SPAB and possible role in the present anti-inflammatory and anti-asthmatic property of SPAB.
Oxidative stress is an important confounding factor in aggravating asthma pathobiology61,62 and the cellular non-catalytic machinery such as glutathione system63 is the first line of defence against scavenging free radicals being generated under normal as well as pathological conditions non-specifically. The catalytic antioxidant system such as SOD, peroxidase and catalase targets specific free radicals apart from acting as signalling intermediates for regulating cellular stress during asthma.61 In the present work, SPAB was capable of restoring Ova-alteration in the activity of GPX, CAT, MPO/EPO, and levels of GSH, GSSG and MDA. The anti-oxidant properties of abhrak bhasma were evaluated in rats using CCl4 induced hepatotoxicity,64 testicular hyperthermia.65 Single dose of abhrak bhasma was capable of restoring GSH64 and MDA66 levels in liver and GSH, GSSG, GSH/GSSG ratio in testes.65 Conversely, in Drosophila system, SPAB was capable of suppressing anti-oxidant components without any adverse effect on general free radical scavenging capability.67 Thus, the ingredients present in SPAB could be compensating for decreased anti-oxidant capacity.67
Non-dose dependent and non-linear pharmacological outcomes of SPAB are the apparent limitations of this study, which could be due to two plausible reasons. Firstly, it could be due to pleiotropic nature of SPAB that could be acting on several target molecules within the living organisms. Drug-receptor bindings have been shown to be extremely complex process, with varying degree of functional outputs that depends on several variables. It is therefore understandable to observe this type of non-dose dependent effects.68,69 In the case of in vivo studies, such effects are common, since the total observable effects depend on a milieu of physiological mediators of inflammatory homeostasis. In fact, similar non-dose dependent effects were observed for various other herbal drugs.70 Secondly, it is also possible that the dose of SPAB may provoke several physiological response mechanisms which may alter its effective concentration or several other factors too numerous to mention and thereby modulate its effect by compensatory mechanisms to maintain homeostasis. All in all, the specific purpose of undertaking this study to validate the prophylactic anti-inflammatory effect and ameliorative potential of SPAB against asthma associated airway inflammation, has been satisfyingly fulfilled. It is duly acknowledged that with the observations from the current study, conclusions on the curative effect of SPAB cannot be drawn. A separated detailed study on this aspect is fittingly warranted that is quite apparent to become the most obvious spin-off in nearer future.
Conclusions
The significance of traditional medicine as a remedy in the management of asthma is undisputed. Identification of safe, reliable and affordable therapeutic options are needed as whole or complementary therapy for asthma management. We have experimentally validated the anti-inflammatory properties of traditional Indian-medicine, Sahastraputi-Abhrak-Bhasma (SPAB) and demonstrated that SPAB could reduce the classic signs of asthma associated airway inflammation, such as allergen induced inflammation, mucus hypersecretion, collagen deposition and oxidative stress in-vivo and cytokine secretion in-vitro. Hence, this study provides strong implications on the suitability of including the biotite-calx based medicine, Sahastraputi-Abhrak-Bhasma as an effective prophylactic adjunct therapy in asthma management.
Data Sharing Statement
All data generated during study has been included in the manuscript.
Acknowledgments
Authors acknowledge Dr. L. P. Singh and colleagues at Central Building Research Institute, Roorkee, India for their support with the Scanning Electron Microscope, Electron Dispersive X-Ray, and XRD analysis; Dr. Vinamra Sharma and Mr. Pradeep Nain for physicochemical analysis supports; Ms. Deepika Mehra and Mr. Kamal Joshi for histopathology support; Mr. Vipin, Mr. Pushpender, Mr. Sonit, Dr. Sachin Sakat and Dr. GC Sar for vivarium helps, and Dr. Swati Haldar with her help with manuscript editing. We extend our gratitude to Mr. Brij Kishore, Ms. Priyanka Kandpal, Mr. Tarun Rajput, Mr. Gagan Kumar and Mr. Lalit Mohan for their swift administrative supports. Sahastraputi-abhrak-bhasma (SPAB) is a classical Ayurvedic medicine.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflicts of interest.
References
1. Initiative Global. Global initiative for asthma: global strategy for asthma management and prevention (Updated 2020). Rev Fr d’Allergologie d’Immunologie Clin. 2020;36(6):685–704. https://ginasthma.org/
2. Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med. 2012;18:716–725. doi:10.1038/nm.2678
3. Lambrecht BN, Hammad H. The immunology of asthma. Nat Immunol. 2015;16(1):45–56. doi:10.1038/ni.3049
4. Lloyd CM, Saglani S. T cells in asthma: influences of genetics, environment, and T-cell plasticity. J Allergy Clin Immunol. 2013;131:1267–1274. doi:10.1016/j.jaci.2013.02.016
5. Akdis CA. Therapies for allergic inflammation: refining strategies to induce tolerance. Nat Med. 2012;18:736–749. doi:10.1038/nm.2754
6. Holtzman MJ, Byers DE, Alexander-Brett J, Wang X. The role of airway epithelial cells and innate immune cells in chronic respiratory disease. Nat Rev Immunol. 2014;14(10):686–698. doi:10.1038/nri3739
7. Huntley A. Herbal medicines for asthma: a systematic review. Thorax. 2000;55(11):925–929. doi:10.1136/thorax.55.11.925
8. Liu J, Zhang F, Ravikanth V, Olajide OA, Li C, Wei L-X. Chemical compositions of metals in Bhasmas and Tibetan Zuotai are a major determinant of their therapeutic effects and toxicity. Evidence Based Complement Altern Med. 2019;2019:1–13. doi:10.1155/2019/1697804
9. Pal D, Sahu CK, Haldar A. Bhasma: the ancient Indian nanomedicine. J Adv Pharm Technol Res. 2014;5(1):4–12. doi:10.4103/2231-4040.126980
10. Kumar A, Nair AGC, Reddy AVR, Garg AN. Bhasmas: unique ayurvedic metallic–herbal preparations, chemical characterization. Biol Trace Elem Res. 2006;109(3):231–254. doi:10.1385/BTER:109:3:231
11. World Health Organization (WHO). WHO Traditional Medicine Strategy 2014–2023. World Health Organization; 2013.
12. Kantak S, Rajurkar N, Adhyapak P. Synthesis and characterization of Abhraka (mica) bhasma by two different methods. J Ayurveda Integr Med. 2020;11:236–242. doi:10.1016/j.jaim.2018.11.003
13. Tamhankar YL, Gharote AP. Effect of Puta on in vitro anticancer activity of Shataputi Abhrak Bhasma on lung, leukemia and prostate cancer cell lines. J Ayurveda Integr Med. 2020;11(2):118–123. doi:10.1016/j.jaim.2017.07.007
14. Subedi RP, Vartak RR, Kale PG. Study of general properties of Abhrak Bhasma: a nanomedicine. Int J Pharm Sci Rev Res. 2017;44(2):238–242. https://globalresearchonline.net/journalcontents/v44-2/47.pdf
15. Buwa S, Patil S, Kulkarni PH, Kanase A. Hepatoprotective action of abhrak bhasma, an ayurvedic drug in albino rats against hepatitis induced by CCl4. Indian J Exp Biol. 2001;39(10):1022–1027. http://www.ncbi.nlm.nih.gov/pubmed/11883510
16. Teli P, Chougule P, Jadhav J, Kanase A. Abhrak bhasma mediated alterations in liver and kidney functions in male albino rats during carbon tetrachloride induced toxicity. Int J Res Ayurveda Pharm. 2013;4(5):696–700. doi:10.7897/2277-4343.04514
17. Kumar RCS, Ilango K, Kumar RM, Vasanth K, Kumar RVM, Mitti JG. Evaluation of physico chemical properties and diuretic activity of biotite calx (abhraka bhasma) processed with Tribulus terrestris l.: an experimental study. Int J Res Ayurveda Pharm. 2015;6(3):303–309. doi:10.7897/2277-4343.06361
18. Laxman TY, Deodatta BD, Tryambaklal MM, Nilesh S, Ekta T. Screening of immunomodulatory effect of Abhrak Bhasma- Ayurveda’s Rasayan. Int J Ayurveda Pharma Res. 2015. https://ijapr.in/index.php/ijapr/article/view/4
19. Bhatia B, Daoo J, Kale P, Panchal P. Abhraka Bhasma treatment ameliorates proliferation of germinal epithelium after heat exposure in rats. Anc Sci Life. 2012;31:171. doi:10.4103/0257-7941.107350
20. Balkrishna A, Rustagi Y, Bhattacharya K, Varshney A. Application of zebrafish model in the suppression of drug-induced cardiac hypertrophy by traditional Indian medicine yogendra ras. Biomolecules. 2020;10(4):600. doi:10.3390/biom10040600
21. Altomare A, Corriero N, Cuocci C, Falcicchio A, Moliterni A, Rizzi R. QUALX2.0: a qualitative phase analysis software using the freely available database POW-COD. J Appl Crystallogr. 2015;48(2):598–603. doi:10.1107/S1600576715002319
22. Nair A, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016;7(2):27–31. doi:10.4103/0976-0105.177703
23. Balkrishna A, Solleti SK, Singh H, Tomer M, Sharma N, Varshney A. Calcio-herbal formulation, Divya-Swasari-Ras, alleviates chronic inflammation and suppresses airway remodelling in mouse model of allergic asthma by modulating pro-inflammatory cytokine response. Biomed Pharmacother. 2020;126:110063. doi:10.1016/j.biopha.2020.110063
24. Solleti SK, Simon DM, Srisuma S, et al. Airway epithelial cell PPARγ modulates cigarette smoke-induced chemokine expression and emphysema susceptibility in mice. Am J Physiol. 2015;309(3):L293–L304. doi:10.1152/ajplung.00287.2014
25. Balkrishna A, Solleti SK, Singh H, et al. Herbal decoction Divya-Swasari-Kwath attenuates airway inflammation and remodeling through Nrf-2 mediated antioxidant lung defence in mouse model of allergic asthma. Phytomedicine. 2020;78:153295. doi:10.1016/j.phymed.2020.153295
26. Shackelford C, Long G, Wolf J, Okerberg C, Herbert R. Qualitative and quantitative analysis of nonneoplastic lesions in toxicology studies. Toxicol Pathol. 2002;30(1):93–96. doi:10.1080/01926230252824761
27. Mann PC, Vahle J, Keenan CM, et al. International harmonization of toxicologic pathology nomenclature: an overview and review of basic principles. Toxicol Pathol. 2012;40(4):7S–13S. doi:10.1177/0192623312438738
28. Solleti SK, Srisuma S, Bhattacharya S, et al. Serpine2 deficiency results in lung lymphocyte accumulation and bronchus-associated lymphoid tissue formation. FASEB J. 2016;30(7):2615–2626. doi:10.1096/fj.201500159R
29. Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG. Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973;179(4073):588–590. doi:10.1126/science.179.4073.588
30. Góth L. A simple method for determination of serum catalase activity and revision of reference range. Clin Chim Acta. 1991;196(2–3):143–151. doi:10.1016/0009-8981(91)90067-M
31. Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971;44(1):276–287. doi:10.1016/0003-2697(71)90370-8
32. Hissin PJ, Hilf R. A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal Biochem. 1976;74(1):214–226. doi:10.1016/0003-2697(76)90326-2
33. Heath RL, Packer L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys. 1968;125(1):189–198. doi:10.1016/0003-9861(68)90654-1
34. Menegazzi R, Zabucchi G, Knowles A, Cramer R, Patriarca P. A new, one-step assay on whole cell suspensions for peroxidase secretion by human neutrophils and eosinophils. J Leukoc Biol. 1992;52(6):619–624. doi:10.1002/jlb.52.6.619
35. Bozeman PM, Learn DB, Thomas EL. Assay of the human leukocyte enzymes myeloperoxidase and eosinophil peroxidase. J Immunol Methods. 1990;126(1):125–133. doi:10.1016/0022-1759(90)90020-V
36. Suzuki K, Ota H, Sasagawa S, Sakatani T, Fujikura T. Assay method for myeloperoxidase in human polymorphonuclear leukocytes. Anal Biochem. 1983;132(2):345–352. doi:10.1016/0003-2697(83)90019-2
37. Da Silva AL, Santos RS, Xisto DG, Alonso SDV, Morales MM, Rocco PRM. Nanoparticle-based therapy for respiratory diseases. An Acad Bras Cienc. 2013;85:137–146. doi:10.1590/S0001-37652013005000018
38. Sarda K, Sarda K, Pandit V, Dawane J, Deshmane G. Study mechanism of action of Krishnavajrabhraka Bhasma in asthma. Nat Preced. 2010;1. doi:10.1038/npre.2010.54221
39. Barnes PJ. Severe asthma: advances in current management and future therapy. J Allergy Clin Immunol. 2012;129(1):48–59. doi:10.1016/j.jaci.2011.11.006
40. Clarke R, Lundy FT, McGarvey L. Herbal treatment in asthma and COPD – current evidence. Clin Phytosci. 2015;1(1):4. doi:10.1186/s40816-015-0005-0
41. Wijenayake A, Pitawala A, Bandara R, Abayasekara C. The role of herbometallic preparations in traditional medicine – a review on mica drug processing and pharmaceutical applications. J Ethnopharmacol. 2014;155(2):1001–1010. doi:10.1016/j.jep.2014.06.051
42. Kumar RK, Herbert C, Yang M, Koskinen AML, McKenzie ANJ, Foster PS. Role of interleukin-13 in eosinophil accumulation and airway remodelling in a mouse model of chronic asthma. Clin Exp Allergy. 2002;32(7):1104–1111. doi:10.1046/j.1365-2222.2002.01420.x
43. Curran DR, Cohn L. Advances in mucous cell metaplasia: a plug for mucus as a therapeutic focus in chronic airway disease. Am J Respir Cell Mol Biol. 2010;42(3):268–275. doi:10.1165/rcmb.2009-0151TR
44. Vaidya R. Ayurveda Sar Sangrah. Allahabad, India: Shri Baidyanath Ayurveda Bhawan Ltd; 2009:90–91.
45. Kokane VT, Tathed P, Pawar V. Comparative clinical assessment of efficacy of Shataputi Abhrak Bhasma along with chausasti pippalias an adjuvant to AKT in pulmonary TB. Int J Res Indian Med. 2019;3(4):1–12. https://www.ayurline.in/index.php/ayurline/article/view/275
46. Vardhini NV, Sathya TN, Balakrishna Murthy P. Assessment of genotoxic potential of herbomineral preparations - bhasmas. Curr Sci. 2010;99(8):1096–1000. https://www.jstor.org/stable/24066119
47. Ji X, Kang Y, Ouyang J, et al. Synthesis of ultrathin biotite nanosheets as an intelligent theranostic platform for combination cancer therapy. Adv Sci. 2019;6(19):1901211. doi:10.1002/advs.201901211
48. Jung BG, Lee JA, Lee BJ. Antiviral effect of dietary germanium biotite supplementation in pigs experimentally infected with porcine reproductive and respiratory syndrome virus. J Vet Sci. 2013;14(2):135. doi:10.4142/jvs.2013.14.2.135
49. Fahy JV. Type 2 inflammation in asthma-present in most, absent in many. Nat Rev Immunol. 2015;15(1):57–65. doi:10.1038/nri3786
50. Lambrecht BN, Hammad H, Fahy JV. The cytokines of asthma. Immunity. 2019;50(4):975–991. doi:10.1016/j.immuni.2019.03.018
51. Zhu Z, Homer RJ, Wang Z, et al. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin Invest. 1999;103:779–788. doi:10.1172/JCI5909
52. Chan BCL, Lam CWK, Tam LS, Wong CK. IL33: roles in allergic inflammation and therapeutic perspectives. Front Immunol. 2019;10:364. doi:10.3389/fimmu.2019.00364.
53. Kumar R, Webb D, Herbert C, Foster P. Interferon-gamma as a possible target in chronic asthma. Inflamm Allerg. 2006;5(4):253–256. doi:10.2174/187152806779010909
54. Lee J-A, Jung B-G, Kim T-H, Kim Y-M, Koh H-B, Lee B-J. Improvement of bacterial clearance and relief of clinical signs of Salmonella enterica serovar Typhimurium infection in pigs through upregulation of Th 1-specific responses by administration of a combination of two silicate minerals, biotite and be. J Vet Med Sci. 2015;77(9):1087–1094. doi:10.1292/jvms.14-0362
55. Guo L, Liu Y, Han J, Zhu H, Wang X. Effects of Biotite V supplementation on growth performance and the immunological responses of weaned pigs after an Escherichia coli lipopolysaccharide challenge. Livest Sci. 2017;195:112–117. doi:10.1016/j.livsci.2016.12.003
56. Lee J-A, Jung B-G, Jung M, Kim T-H, Yoo HS, Lee B-J. Dietary germanium biotite supplementation enhances the induction of antibody responses to foot-and-mouth disease virus vaccine in pigs. J Vet Sci. 2014;15(3):443. doi:10.4142/jvs.2014.15.3.443
57. Jung M, Shin M-K, Cha S-B, et al. Supplementation of dietary germanium biotite enhances induction of the immune responses by foot-and-mouth disease vaccine in cattle. BMC Vet Res. 2014;10(1):179. doi:10.1186/s12917-014-0179-6
58. Sharma SK, Shrivastava N, Rossi F, Tung LD, Thanh NTK. Nanoparticles-based magnetic and photo induced hyperthermia for cancer treatment. Nano Today. 2019;29:100795. doi:10.1016/j.nantod.2019.100795
59. Marycz K, Sobierajska P, Roecken M, et al. Iron oxides nanoparticles (IOs) exposed to magnetic field promote expression of osteogenic markers in osteoblasts through integrin alpha-3 (INTa-3) activation, inhibits osteoclasts activity and exerts anti-inflammatory action. J Nanobiotechnology. 2020;18(1):1–24. doi:10.1186/s12951-020-00590-w
60. Beck-Speier I, Kreyling WG, Maier KL, et al. Soluble iron modulates iron oxide particle-induced inflammatory responses via prostaglandin E2 synthesis: in vitro and in vivo studies. Part Fibre Toxicol. 2009;6(1):34. doi:10.1186/1743-8977-6-34
61. Erzurum SC. New insights in oxidant biology in asthma. Ann Am Thorac Soc. 2016;13:S35–S39. doi:10.1513/AnnalsATS.201506-385MG
62. Sahiner UM, Birben E, Erzurum S, Sackesen C, Kalayci O. Oxidative stress in asthma. World Allergy Organ J. 2011;4(10):151–158. doi:10.1097/WOX.0b013e318232389e
63. Meister A. Selective modification of glutathione metabolism. Science. 1983;220(4596):472–477. doi:10.1126/science.6836290
64. Teli P, Jadhav J, Kanase A. Effect of abhrak bhasma and silicon dioxide on hepatic and renal glutathione status in rats: hepatoprotection testing against single dose carbon tetrachloride induced hepatotoxicity. Int J Pharmacol Toxicol. 2014;2(2):92. doi:10.14419/ijpt.v2i2.3288
65. Bhatia B, Kale PG, Daoo JV, Meshram P. Testicular oxidative stress protective effects of Abhraka Bhasma in male Wistar rats after heat exposure. Int J Pharm Pharm Sci. 2013;5:472–477. https://innovareacademics.in/journal/ijpps/Vol5Issue2/6632.pdf
66. Teli P, Jadhav J, Kanase A. Comparison of abhrak bhasma and silicon dioxide efficacy against single dose of carbon tetrachloride induced hepatotoxicity in rat by evaluation of lipid peroxidation. Int J Pharm Sci Res. 2014;2(7):186–196. http://ajphr.com/archive/volume-2/july-2014-issue-7#
67. Subedi RP, Vartak RR, Kale PG. Modulation of oxidative stress by abhrak bhasma in drosophila melanogaster. Asian J Pharm Clin Res. 2018;11(5):247. doi:10.22159/ajpcr.2018.v11i5.24472
68. Spedding M. Resolution of controversies in drug/receptor interactions by protein structure. Limitations and pharmacological solutions. Neuropharmacology. 2011;60:3–6. doi:10.1016/j.neuropharm.2010.08.002
69. Wenthur CJ, Gentry PR, Mathews TP, Lindsley CW. Drugs for allosteric sites on receptors. Annu Rev Pharmacol Toxicol. 2014;54:165–184. doi:10.1146/annurev-pharmtox-010611-134525
70. Doğruer Akan B, Demir Özkay Ü. The antinociceptive effects of some piperazine alkanol derivatives. Cukurova Med J. 2019;44:729–744. doi:10.17826/cumj.490690
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.