Back to Journals » Journal of Pain Research » Volume 15
Best Practices for Postoperative Management of Posterior Sacroiliac Joint Fusion
Authors Buchanan P , Lee DW, Comer A, Hussaini Z , Grillo C, Vodapally S , Strand NH , Sayed D , Deer TR
Received 19 January 2022
Accepted for publication 28 March 2022
Published 19 April 2022 Volume 2022:15 Pages 1149—1162
DOI https://doi.org/10.2147/JPR.S357123
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Michael A Ueberall
Patrick Buchanan,1 David W Lee,2 Ashley Comer,3 Zohra Hussaini,4 Casey Grillo,5 Shashank Vodapally,6 Natalie H Strand,7 Dawood Sayed,4 Timothy R Deer3
1Department of Pain Medicine, Spanish Hills Interventional Pain Specialists, Camarillo, CA, USA; 2Department of Pain Medicine, Fullerton Orthopedic Surgery Medical Group, Fullerton, CA, USA; 3Department of Pain Medicine, The Spine and Nerve Center of the Virginias, Charleston, WV, USA; 4Department of Anesthesiology, Division of Pain Medicine, The University of Kansas Medical Center, Kansas City, KS, USA; 5Department of Pain Medicine, The Spine & Pain Institute of New York, New York, NY, USA; 6Department of Physical Medicine and Rehabilitation, Michigan State University, East Lansing, MI, USA; 7Department of Anesthesiology, Division of Pain Medicine, Mayo Clinic, Phoenix, AZ, USA
Correspondence: Patrick Buchanan, Email [email protected]
Abstract: Sacroiliac joint (SIJ) pain is a common cause of low back pain. Traditionally, treatment for SIJ joint pain and dysfunction has consisted of physical therapy, medication management, SIJ injections, and SIJ ablations. Improved recognition of the SIJ as an etiology for back pain has led to advances in treatment options. Radiofrequency of the lateral sacral branches has been shown to be effective, though evidence is fraught with inconsistent patient selection, study design and procedural technique. It also does not directly address the mechanical dysfunction of the SIJ. In order to create a more enduring approach SIJ fusion has become an attractive option to reduce pain and to improve function. This method of SI joint treatment requires guidance in the perioperative phase of care from both the physicians and advanced practice providers (APP). In order to improve care and outcomes of those undergoing posterior SI joint fusion the American Society of Pain and Neuroscience appointed an expert panel of physicians and advanced practice providers to create a best practice for the post operative care of this approach. As with any best practice, the panel considered current peer reviewed literature and clinical expertise to create guidance today. This is intended to be a living document with modifications as additional evidence comes to light in data publication. The goals of this paper are to focus on (1) wound care, (2) medication use, (3) physical activity and (4) therapeutic exercises.
Keywords: sacroiliac joint, SIJ, low back pain, postoperative care, best practices, review, physical therapy, minimally invasive
Introduction
Low back pain is a major health issue and creates a significant impact on both quality of life and health-care costs. An estimated 70% to 85% of the western population will develop low back pain at least once during their lifetime.1 SIJ pain is a common cause of low back pain accounting for 15% to 30% of all cases.2 It is the most likely source of low back pain in patients having undergone either lumbar or lumbosacral fusion surgery.3 Spinal imaging shows the incidence of SIJ degeneration in patients who have undergone lumbar fusion surgery is 75% at 5 years post-surgery.4 Pain caused by SIJ disorders has also been linked to hip osteoarthritis as well as lumbar disorders.5
Although SIJ pain is common in the general population, it can be difficult to find long-term therapy to treat this problem. Conservative interventional treatment options including intra articular steroid injections and radiofrequency ablation focus on decreasing inflammation and blocking the pain, respectively. These interventions have been shown to have moderate success in the short to intermediate term, but poor long-term resolution of symptoms.6 Surgical fusion, a potentially curative intervention of the SIJ, aims to stabilize the joint to prevent laxity and inflammation. SIJ fusion is appropriate as part of the care algorithm for this disorder requiring a plan that follows more conservative attempts at joint pain resolution. This often includes a period of 6 months or more of non-operative treatment including medication optimization, activity modification and physical therapy.7
Perhaps one of the most difficult aspects of SI joint care is determining that the joint is the cause of the discomfort. The patient often gives the history of pain in the buttock that can be worsened by sitting, standing, crossing their legs, or walking. Physical exam is very important and the examiner should elicit at least three out of the five SIJ provocative maneuvers to reproduce symptoms. Once the history and physical examination suggests this etiology of pain, two fluoroscopically guided blocks of the joint should confirm pain reduction with local anesthetic. Once the patient has met these criteria, a joint fusion can be considered. Prior to moving forward, the care team should rule out tumor and inflammatory rheumatological disease as causes of the pain.
Initially, in the later part of the 20th century, SIJ fusion was a large instrumented surgery and fell out of favor because of the invasiveness and lack of efficacy data. More recently, a lateral screw fusion technique received regulatory clearance and became an option, although limited by risk of vascular and nerve injury and post operative weight bearing restrictions. Miller et al8 analyzed a “post fusion complaints” database from 2009 to 2013 regarding use of the implants in 5319 patients and 96 revision surgeries were performed in 94 patients (revision rate of 1.8%). The complaint rate was 3.8% which included complaints of nerve impingement (0.9%), pain unrelated to nerve impingement (1.3%), improper device placement (1.4%). There were 96 revision surgeries performed in 94 patients. Cher et al9 later reported updated information from this database (n = 11,388), and they found that revision rates fell below 1.8% after 2012 largely due to improved technique and surgical proficiency that avoided vascular and nervous tissue. A posterior SIJ fusion technique was later developed that simultaneously stabilized the joint while limiting these known complications. This method of SIJ treatment requires guidance in the postoperative phase of care from both physicians and advanced practice providers (APP).
SIJ fusion can be most commonly performed using two approaches: lateral or posterior. Classic literature supports the lateral approach in randomized controlled studies.10,11 Evolving studies are showing support for the posterior approach with evidence suggesting lower risks and good efficacy. Additional prospective randomized studies are underway which resemble the design of the previously discussed posterior approach investigations. The posterior approach is a less invasive method to stabilize the SIJ, avoiding critical vascular (ie, superior gluteal artery) and neural tissues (ie, S1, S2 nerve roots) that can be engaged with lateral methods. These techniques utilize one or two cortical allografts that can be placed along the joint by fluoroscopic guidance. There are advantages to fusing the joint posteriorly. Posterior fusion minimizes the risk of neurological complications by avoiding the sacral foramen. The posterior approach passes through less soft tissue including the gluteal musculature and allows for quicker postoperative recovery time and preserved function. The lateral approach typically requires the patient to undergo general anesthesia while the posterior approach can be performed under either general anesthesia or conscious sedation.
Although there has been a development of more substantial evidence supporting SIJ fusion, a standardization of postoperative care has been limited. This article aims to provide postoperative management regarding wound care, medication use, physical activity and therapeutic exercises recommendations based on a review of the current literature.
Posterior SIJ Fusion Technique
The patient is positioned prone on the procedure table. A 22 gauge 3.5” or 5” spinal needle is used to infiltrate the working site with local anesthesia. This may help reduce the amount of intravenous sedation and recovery time after the procedure. Additionally, the needle can be used under fluoroscopic guidance to access the joint prior to incision or guide pin placement. Once the SIJ is optimally viewed, a 1.5 cm incision is made followed by blunt finger dissection down to the joint. A guide pin is then inserted into the SIJ space and confirmed on lateral views (Figures 1 and 2). Next, depending on the system, a dilator is advanced over the guide pin to the posterior cortical line of the sacrum while in the lateral view (Figure 3). Once alignment is confirmed, the dilator is gently tapped on the handle with a mallet until the hard stop on the outer dilator meets the posterior cortical line of the sacrum. Once the dilator is fully seated on the sacrum, the internal dilator and guide pin are removed. The SIJ is then decorticated with a drilling or broaching system. The cortical allograft with demineralized bone matrix is then deployed via an inserter. The outer dilator is removed, and the wound is closed via sutures and covered with dressing (Figure 4).
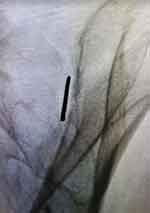 |
Figure 1 Optimal placement of guide pin in the oblique view. |
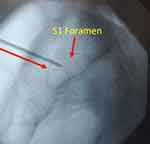 |
Figure 2 Optimal placement of guide pin in lateral view. |
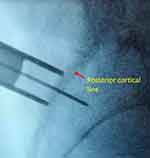 |
Figure 3 Fluoroscopic image of external dilator at the posterior cortical line in the lateral view. |
 |
Figure 4 Closure of the surgical wound. |
Materials and Methods
Although there is evidence to support physical therapy as a treatment for SIJ dysfunction, postoperative guidelines after SIJ fusion are lacking. Therefore, the authors performed a comprehensive literature search of PubMed using key terms such as “sacroiliac joint dysfunction”, “sacroiliac joint pain”, “sacroiliac joint fusion”, “physical therapy and sacroiliac joint fusion”, “postoperative care and sacroiliac joint fusion”. Only journal publications in English were reviewed and referenced. The literature search found no publication directly addressing postoperative guidelines after SIJ fusion.
Anatomy
The SIJ is a large diarthrodial joint that connects the sacrum with the pelvis. It acts as a shock absorber by dissipating the vertical forces of the spine and transmits them to the hips and lower extremities. This joint is supported by several ligaments that limit the rotation. These ligaments include the interosseous SIJ ligament (also known as the short posterior SIJ ligament), the posterior and anterior SIJ ligaments, the sacrotuberous ligament, the sacrospinous ligament, and the iliolumbar ligaments.
There are several large muscles surrounding the SIJ; however, they do not act directly on SIJ movement. These muscle groups play a role in maintaining the stability and function of the joint. Movements of the SIJ are indirectly produced by gravity and muscles acting on the lumbosacral spine, hips, and lower extremities rather than active movements of the sacrum.12 These muscles include the erector spinae, psoas, quadratus lumborum, piriformis, abdominal obliques, gluteals, biceps femoris, and pelvic floor muscles (levator ani and coccygeus muscles). Although these muscles do not specifically move the joint, the health of these muscles can influence the stability and motion of the joint. When these muscles become tight due to inadequate activity (such as a sedentary lifestyle), they become shorter and can cause tension around the SIJ. Adequate stretching strengthening of these muscles allows SIJ flexibility and function without pain.13
Wound Care
Postoperative wound varies significantly with the approach utilized. For the sake of this particular manuscript, the authors focus on the posterior approach. The immediate post-operative period after posterior SI fusion involves days 1 to 7. The objectives during this time closely mimic those of other minimally invasive spinal implants and procedures as it relates to wound care and healing. Goals include facilitating wound healing and joint fusion, monitoring and reducing the risk for infection, and decreasing pain and inflammation. Specific activity restrictions are also imperative to follow during this phase to support healing and joint fusion. Appropriate and thorough patient education is essential to achieve these goals. This education is completed preoperatively and reiterated again on the day of surgery to both the patient and any supporting care giver or companion.
Surgical wounds can be classified into wound types, and healing is either through primary healing or secondary healing. The surgical incision following a posterior SIJ fusion is typically located in near the midline of the lumbosacral region. It is a class 1, clean, non-contaminated surgical wound, with less than 2% infection risk, as classified by the CDC for all types of clean wounds.14 Patients will have a single, small operative wound commonly approximated and closed with either staples or sutures and covered with a basic surgical dressing which will allow for primary healing. Evidence suggests Class 1 clean surgical wounds should remain dry and intact for at least 48 hours. There is no sufficient data to support that a wound dressing beyond 48 hours significantly impacts infection risk positively or negatively, however, a dressing can serve to protect and absorb exudates at the surgical site. Therefore, keeping a dressing on greater than 48 hours can vary by individual provider preference.15 Showering or getting the wound wet should be strictly avoided for the first 48 hours after surgery, and bathing or submerging the wound should be avoided for at least 3 weeks until further tissue healing has occurred. If necessary, only sterile saline should be used within 48 hours to clean the wound. Otherwise, the incision should be left alone, patients should be instructed not to touch or handle the dressing during this 48-hour period. Patients should avoid applying any topical agents, antiseptics, direct heat over the incision, or rubbing and scratching the incision. Patients may wait until the 7–10 day follow-up before they remove the dressing or start showering, though current evidence does not suggest that early showering causes increased surgical site infections.16,17
Monitoring the incision for infection is critical. Patients should be instructed to watch for signs of infection including increased pain, localized swelling, excessive drainage, warmth or redness at the incision site or surrounding skin, as well as fevers or chills. If any of these symptoms occur, patients should contact their provider promptly.
Surgical incision site infections are most likely to arise two to four weeks out of surgery.18 Any condition reducing immune response in the preoperative period can increase risk of a surgical site infection, which can include uncontrolled diabetes, chemotherapy, autoimmune diseases, chronic steroid use and smoking. Attaining an early definitive diagnosis and initiating appropriate management is crucial in mitigating risks and reducing morbidity.
Summary of Best Practices for Wound Care
- Class 1 wound should remain dry for at least 48 hours.
- Bathing or submerging the wound should be avoided for at least 3 weeks.
- Patient may wait 7–10 days prior to showering, though current evidence does not suggest that early showering causes increased surgical site infections.
Medication Usage
Non-Steroidal Anti-Inflammatory (NSAID)
In addition to surgical wound care, the immediate post-operative phase must promote fusion of the posterior SIJ for the procedure to be successful. In novel posterior intra-articular SIJ fusion systems, an allograft is used with demineralized bone matrix (DBM) which creates an osteoinductive environment. Spinal fusion models have confirmed non-steroidal anti-inflammatory drugs (NSAIDS) have an inhibitory effect on healing of a fusion. Although data are limited, it appears this effect is most significant when NSAIDS are administered in the early postoperative period.19 Goodman et al20 conducted a study with non-spinal models suggesting early administration of NSAIDS resulted in greater inhibition of bone formation. One study showed that patients who continued to take NSAIDs for more than 3 months postoperatively showed significantly lower fusion success rates.21,22
The length of the inflammatory phase in humans is not clearly defined. Additionally, the data on NSAIDS inhibitory effect on bone healing are not consistent. Certain animal studies have noted inhibitory effects of NSAIDS during the first 8 weeks, but recommendations for avoidance of NSAIDs in practice may be as long as six months following spinal fusion. The authors recommend a shared physician-patient decision taking into account the risk and benefits of resuming or starting postoperative NSAIDS, although NSAIDS are best avoided when possible.
Tobacco Use
The use of tobacco can increase postoperative complications including impaired wound healing, augmented infection, and delayed and/or impaired arthrodesis.23 One systematic review and meta analysis study demonstrated a relative risk reduction of 41% for prevention of postoperative complications. Each week of cessation increased the magnitude of effect by 19%.24 Therefore, tobacco use should be avoided to decrease the incidence of postoperative complications.
Antibiotics
Antimicrobial prophylaxis (AP) plays an important role in reducing surgical site infections, especially if patient-related risk factors for infection are present. Guidelines for implementing antibiotics for surgical procedures vary by type of surgery, surgical wound classification and patient risk factors and comorbidities. National quality improvement efforts have focused on reduction of surgical site infections and have developed initiatives for improved antimicrobial prophylaxis in surgery.25 As mentioned earlier, the posterior SIJ fusion is considered a class 1, clean, non-contaminated surgical procedure, with less than 2% infection risk, as classified by the CDC.14 Antimicrobial prophylaxis in patients undergoing clean procedures is not well established and generally not recommended, however, there is stronger support and evidence for prophylactic antimicrobial treatment when implanted hardware or foreign materials are involved in orthopedic procedures, or spinal procedures with or without instrumentation or hardware. This is especially true for the intraoperative phase and administration of the prophylactic agent should start within 30 minutes from the surgical incision. Duration of antimicrobial prophylaxis should not exceed 24 hours for most surgical procedures.25 Tan et al26 conducted a systematic review and found that in the presence of intraoperative and pre-incisional antibiotic prophylaxis, postoperative antibiotics for surgical site infection reduction did not show evidence of reduced infection rates in lumbar spinal surgery patients. Therefore, postoperative administration of prophylactic antibiotics after posterior SIJ fusion surgery is not supported.
Anticoagulation
Management of anticoagulation and antiplatelet medications after surgery can be complex, especially given that these patients may often have multiple medical comorbidities. There is evidence that points towards shared decision-making between the patient and the treating physicians (ie, cardiology, neurology, surgeon) and to consider all the appropriate risks associated with continuation or discontinuation of antithrombotic or anticoagulant therapy.27 While ideal, discontinuation of anticoagulation medication may increase the risk of thrombus formation and cardiovascular and/or neurologic issues.
The Neurostimulation Appropriateness Consensus Committee (NACC) and American Society of Regional Anesthesia & Pain Medicine (ASRA) Guidelines provide guidance in addressing use of anticoagulation with spinal cord stimulation procedures.28,29 Epidural hematoma is the most serious concern in not only spinal cord stimulation but any procedure which involves accessing the epidural space. With SIJ fusion, the concern for this is minimal. However, in creating a space for the allograft implant, bone decortication using a box-cutter may result in substantial bleeding within the SIJ itself or the surrounding soft tissue. In a recently published cohort study of 1587 patients, there was no appreciable increase in perioperative morbidities, including bleeding-related complication rates in patients undergoing lumbar minimally invasive spine surgery while continuing to take anticoagulation compared with patients who discontinued anticoagulation therapy.30
Despite these differences, the NACC and ASRA Guidelines may be used as a basis of anticoagulation management for SIJ fusion. Both guidelines recommend discontinuing Warfarin (coumadin) five days before the epidural spinal cord stimulator trial leads are placed. This timeline is based on the pharmacokinetics of the anticoagulant. Considering the half-life of the anticoagulant is key in determining what is the appropriate time period of discontinuing the medication prior to SIJ fusion. There should be laboratory evidence of a normal INR (<1.2) before proceeding. Newer options of anticoagulation allow for shorter periods of discontinuation. Anticoagulation may be resumed 24 hours after completion of the procedure.
Summary of Best Practices for Medication
- A shared physician-patient decision taking into account the risk and benefits of resuming or starting postoperative NSAIDS, although NSAIDS are best avoided when possible.
- Tobacco use should be avoided to decrease the incidence of postoperative complications.
- Postoperative administration of prophylactic antibiotics after posterior SIJ fusion surgery is not supported by the current evidence.
- NACC and ASRA Guidelines may be used as a basis of anticoagulation management for SIJ fusion. Anticoagulation may be resumed 24 hours after completion of the procedure.
Fusion Rates
Data on outcomes for both the lateral transiliac and the posterior approach is becoming more abundant, but questions remain among pain physicians as to how long it takes for the graft to be fixated and when fusion actually takes place. The time and durability of fusion of the SIJ (similar to lumbosacral fusion) has implications for when to initiate certain types of activity and physical therapy exercises.
Looking at literature focusing on the results of patients who underwent distraction arthrodesis of the SIJ, one study showed that 79% of the patients had fusion by 13 months post-procedure.31 A different study evaluating the outcomes of the same procedure at a different location found that at 24 months, CT scans supported SIJ fusion in 31% of patients.32 A third group found that 87% of patients treated with a minimally invasive approach using triangular implants had radiographic evidence of fusion, and this did not decrease during a five year follow-up.33 Additional evidence of successful fusion and durability was shown by Whang et al.34 Independent radiographic analysis showed a high rate (98%) of bone apposition to implants on both the sacral and iliac sides of the SI joint, with a high rate of bony bridging (87%) and a low rate of radiolucencies suggestive of loosening (5%).
Post-Operative Physiologic Precautions
Compared to lateral SIJ fusion, posterior intra-articular SIJ fusion approach allows for full post-operative weight bearing. This is due to the fact that the posterior SIJ fusion avoids major structures like the gluteal muscles and iliac crest needed to mobilize immediately after the procedure. Additionally, unlike other spinal fusion surgeries, posterior approach SIJ fusion does not require use of post-operative orthosis (ie, lumbosacral brace or SIJ belt). As a result, guidance towards activities and movements following the procedure is imperative. There are two main movements of the SIJ, nutation and counternutation. Sturesson et al revealed that nutation occurs when patients load their spine by means of rising from a supine towards a sitting or standing position.35–37 The largest movements within the SIJ occur when changing from standing to lying prone with hyperextension of a leg. The SIJ movements are reduced as additional loads are placed on the joint. While lying, maximal flexion in the hips, using the legs as levers to posteriorly rotate the iliac bones relative to the sacrum, creates nutation. Counternutation normally takes place in unloaded situations, such as lying prone. Understanding these biomechanical forces, the authors recommend that these specific movements be avoided in the immediate post-operative recovery phase; with the rationale that increased stress forces on the SIJ may disrupt allograft placement.
Similarly, SIJ provocative maneuvers as originally proposed by Laslett et al38 provide further guidance as to which body positions to avoid following posterior SIJ fusion. In particular, the Gaenslen Test suggests that hyperflexion of the hip (both contralateral and ipsilateral) should be avoided. The Drop Test suggests that repetitive forces delivered to the ipsilateral heel/foot should be avoided to limit cranial shear forces to the ipsilateral SI joint. Yeoman’s test, which was not included by Laslett but has been adopted in the clinical setting, suggests that forces on the SIJ from hyperextension of the hip should be avoided.
Summary of Best Practices for Post-Operative Precautions
- In general, patients can be advised to walk and resume activities of daily living but with caution to limit strenuous activity including limiting bending or twisting at the waist.39
- Patients should further avoid pushing or pulling activities and should not lift greater than 10 pounds during this phase.
- Negotiating up and down stairs should be taken with caution, holding handrails, and taking one step at a time.
- Sitting for longer than 45 minutes to one hour at a time should be avoided, and patients should be encouraged to take a 10 minute break to get up and move around or lie down before sitting again.
- Driving should be avoided during this phase, but being a passenger in a vehicle for short distances is permissible.
- Further considerations for physical and manual therapy should be reserved after initial healing has started, and is covered in the next section.
Post-Operative Physical Therapy
Admittedly, there is a scarce amount of studies focusing on post-operative physical therapy following SIJ fusion. Consequently, many of the recommendations have been drawn from studies following spinal fusion.
The start and duration of rehabilitation post-SIJ fusion has several variables that should be considered. Patient comorbidities, preoperative deconditioning, age, disability, smoking, secondary orthopedic problems, psychosocial barriers (such as fear avoidance and/or depression), the patient’s commitment and ability to exercise safely and independently, and pain control are factors that all need to be taken into consideration. Often when patients have been impacted by chronic pain, they are hesitant to attempt returning to an active lifestyle. Kinesiophobia, fear of pursuing physical activities that may cause pain to worsen, may often be present. In addition, deconditioning develops over time with chronic pain leading to a non-functional state. Deconditioning may have a negative impact on postoperative recovery. Collaboration with the patient, their family or caregiver, and their physical therapist are crucial to successful activity progression.
Typically, at 6 weeks postoperatively, the wound bed is in the healing process, with near total healing.40 If visual inspection of the wound at 6 weeks suggests wound healing, activity restrictions may be lessened. Working to improve gait mechanics and beginning to focus on core and pelvic strengthening should be introduced and are critical to an optimal outcome (Table 1). Analysis of mechanical body function has shown that the transversus abdominis and the pelvic floor muscles (levator ani and coccygeus muscles) play a major role in SIJ stability as they increase the compression load across the SIJ to resist shear load.41 Deciding long-term activity goals will be patient specific, but the overall optimum goal is to work towards improving pain and function (Figures 567–8).
 |
Table 1 Recommendations for Postoperative Physical Therapy |
 |
Figure 5 Passive hamstring stretch. |
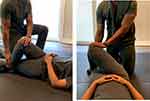 |
Figure 6 Passive external hip rotator stretch. |
 |
Figure 7 (A and B) Both the abdominal draw-in maneuver (A) and quadruped exercise (B) have been found to increase activation of the transversus abdominis muscle as well as recruit other core stabilizers such as erector spinae, multifidus, and oblique abdominal muscles.47 In both exercises the anterior abdominal wall (white dot) should be drawn in toward the spine as depicted by the orange arrow. |
 |
Figure 8 Bridging and clam shell with 30 degrees of hip flexion are a few exercises that have been found to activate the gluteus maximus muscle.48 |
Few studies have examined the timing of initiation of postoperative rehabilitation following fusion surgeries. Dall et al42 recommend initiation of physical therapy within two weeks following posterior minimally invasive SIJ fusion. Initial restrictions include no bending or lifting more than 10 lbs, no twisting at the waist for 12 weeks.
Oestergaard et al randomized two groups of patients who underwent lumbar fusion to start rehabilitation at either 6 or 12 weeks postoperatively.43 The group starting at 12 weeks showed improved outcomes over the 6-week group in pain, activities of daily living, ODI, and Dallas Pain Questionnaire scores, even at 6-month follow-up.
Although current research indicates 12 weeks following surgery as an optimal time-frame for the start of physical therapy, the authors recommend that an initial evaluation for physical therapy be started earlier than that. In a multi-center prospective study performed by Polly et al, 148 patients were randomly assigned to a minimally invasive SIJ fusion group (n = 102) and non-surgical management group (n = 46). The study design had patients begin individualized physical therapy twice a week, which began 1–3 weeks after their procedure for a total of 6 weeks. When compared to the non-intervention group, the intervention group had greater improvement in their Oswestry Disability Index, quality of life scores, and visual analog scale at 24 month follow-up.6 Duhon et al followed the same physical therapy guidelines following minimally invasive SIJ fusion, with a focus on post-intervention activity modification to limit pain, exercises to improve stability and mobility. The outcomes of his study showed significant improvement in function, quality of life and pain compared to pre-surgical assessment.44 These positive outcomes in these studies provide evidence for beginning therapies earlier following minimally invasive SIJ fusion.
Regarding manual therapy, Tullberg et al found that manipulation did not alter the position of the sacrum in relation to the ilium. These results indicate that effective manipulation is not dependent on positional change of the joints.45 However, many provocative maneuvers indicate otherwise (Sacral Thrust, Thigh Thrust, Compression Test, Distraction Test). In general, manual therapy should be avoided directly over surgical areas following a spinal fusion to encourage healing of skin, soft tissue and the fusion itself. Such precautions are reasonable to extrapolate to post-operative therapy following SIJ fusion.
Summary of Best Practices for Post-Operative Physical Therapy
- Physical Therapy can be initiated 6 weeks post operatively focusing on core and pelvic strengthening.
- Initial restrictions include no bending or lifting more than 10 lbs, no twisting at the waist for 12 weeks.
- Current evidence suggests positive outcomes with starting post-operative physical therapy early.
- Manual therapy should be avoided directly.
Postoperative Imaging
There have been studies confirming arthrodesis of the SIJ on radiographic imaging.33,46 Currently, there are no publications recommending radiographic imaging to confirm SIJ fusion postoperatively. However, if there is a complication after the SIJ fusion such as increased pain, infection, or neurological issues, immediate radiographic imaging should be considered.
Conclusion
SIJ fusion is a rapidly growing and evolving therapy with developing evidence to support its safety and efficacy. In the setting of a desired surgical outcome, safety and efficacy can be adversely impacted by poor post-operative care. The American Society of Pain and Neuroscience has created this best practice document in an effort to improve and standardize care in this critical time of patient recovery.
Funding
There is no funding to report.
Disclosure
PB reports personal fees from Abbott and Painteq outside the submitted work and is a consultant for Abbott and PainTEQ. DWL is a consultant for Abbott. AC is a consultant for Abbott, Saluda, PainTeq, SPR, and Vertos.
ZH is a consultant for Nevro, Flowonix, Medtronic, Averitas, Painteq, Spr, and Vertos. CG is a consultant for Abbott. NHS is a consultant for Abbott and Nevro. DS reports personal fees and options from PainTeq outside the submitted work and is a consultant for Abbott, Flowonix, Medtronic, Merit, Nevro, Painteq, SPR, and Vertos. TRD reports personal fees and stock options from and research for PainTeq, personal fees and stock options from Cornerloc, during the conduct of the study; personal fees from Abbott, personal fees and stock options from and research for Vertos, personal fees from Flowonix, personal fees and stock options from SpineThera, personal fees and stock options from Saluda, research for Mainstay, personal fees and stock options from Nalu, personal fees from Ethos, personal fees and stock options from and research for SPR Therapeutic, personal fees from SI Bone, personal fees from Nevro, personal fees from Medtronic, personal fees from and research for Boston Scientific, personal fees from Tissue Tech, and research for Avanos, outside the submitted work; and is a consultant for Abbott, Saluda, Vertiflex, PainTeq, Cornerloc, Spinethera, Nalu, SPR, Vertos, Flowonix, Ethos, SPR Therapeutic, SI Bone, Nevro, Medtronic, Boston Scientific, Tissue Tech. Funded research for Saluda, Abbott, and Vertiflex. In addition, TRD has a patent pending to Abbott & Tim Deer. The authors report no other potential conflicts of interest for this work.
References
1. Andersson GB. Epidemiological features of chronic low-back pain. Lancet. 1999;354(9178):581–585. doi:10.1016/S0140-6736(99
2. Cohen SP, Chen Y, Neufeld NJ. Sacroiliac joint pain: a comprehensive review of epidemiology, diagnosis and treatment. Expert Rev Neurother. 2013;13(1):99–116. doi:10.1586/ern.12.148
3. DePalma MJ, Ketchum JM, Saullo TR. Etiology of chronic low back pain in patients having undergone lumbar fusion. Pain Med. 2011;12(5):732–739. doi:10.1111/j.1526-4637.2011.01098.x
4. Ha KY, Lee JS, Kim KW. Degeneration of sacroiliac joint after instrumented lumbar or lumbosacral fusion: a prospective cohort study over five-year follow-up. Spine (Phila Pa 1976). 2008;33(11):1192–1198. doi:10.1097/BRS.0b013e318170fd35
5. Asada M, Tokunaga D, Arai Y, et al. Degeneration of the sacroiliac joint in hip osteoarthritis patients: a three-dimensional image analysis. J Belg Soc Radiol. 2019;103(1):36. doi:10.5334/jbsr.1648
6. Buchanan P, Mehta A, Gerstman B. Interventional treatments for sacroiliac joint pain. Curr Phys Med Rehabil Rep. 2014;2:66–69. doi:10.1007/s40141-014-0042-5
7. Falowski S, Sayed D, Pope J, et al. A review and algorithm in the diagnosis and treatment of sacroiliac joint pain. J Pain Res. 2020;13:3337–3348. doi:10.2147/JPR.S279390
8. Miller LE, Reckling WC, Block JE. Analysis of postmarket complaints database for the iFuse SI joint fusion system®: a minimally invasive treatment for degenerative sacroiliitis and sacroiliac joint disruption. Med Devices. 2013;6:77–84. doi:10.2147/MDER.S44690
9. Cher DJ, Reckling WC, Capobianco RA. Implant survivorship analysis after minimally invasive sacroiliac joint fusion using the iFuse implant system(®). Med Devices. 2015;8:485–492. doi:10.2147/MDER.S94885
10. Polly DW, Swofford J, Whang PG, et al. Two-year outcomes from a randomized controlled trial of minimally invasive sacroiliac joint fusion vs. non-surgical management for sacroiliac joint dysfunction. Int J Spine Surg. 2016;10:28. doi:10.14444/3028
11. Dengler J, Kools D, Pflugmacher R, et al. Randomized trial of sacroiliac joint arthrodesis compared with conservative management for chronic low back pain attributed to the sacroiliac joint. J Bone Joint Surg Am. 2019;101(5):400–411. doi:10.2106/JBJS.18.00022
12. Kiapour A, Joukar A, Elgafy H, Erbulut DU, Agarwal AK, Goel VK. Biomechanics of the sacroiliac joint: anatomy, function, biomechanics, sexual dimorphism, and causes of pain. Int J Spine Surg. 2020;14(Suppl1):3–13. doi:10.14444/6077
13. Yoo WG. Effects of individual strengthening exercises for the stabilization muscles on the nutation torque of the sacroiliac joint in a sedentary worker with nonspecific sacroiliac joint pain. J Phys Ther Sci. 2015;27(1):313–314. doi:10.1589/jpts.27.313
14. Kolasiński W. Surgical site infections - review of current knowledge, methods of prevention. Pol Przegl Chir. 2018;91(4):41–47. doi:10.5604/01.3001.0012.7253
15. Dumville JC, Gray TA, Walter CJ, et al. Dressings for the prevention of surgical site infection. Cochrane Database Syst Rev. 2016;2016. doi:10.1002/14651858.CD003091.pub4
16. Ban KA, Minei JP, Laronga C, et al. American College of Surgeons and Surgical Infection Society: surgical site infection guidelines, 2016 update. J Am Coll Surg. 2017;224(1):59–74. doi:10.1016/j.jamcollsurg.2016.10.029
17. Toon CD, Sinha S, Davidson BR, Gurusamy KS. Early versus delayed post-operative bathing or showering to prevent wound complications. Cochrane Database Syst Rev. 2013;10:CD010075. doi:10.1002/14651858.CD010075.pub2
18. Chahoud J, Kanafani Z, Kanj SS. Surgical site infections following spine surgery: eliminating the controversies in the diagnosis. Front Med. 2014;1:7. doi:10.3389/fmed.2014.00007
19. Riew KD, Long J, Rhee J, et al. Time-dependent inhibitory effects of indomethacin on spinal fusion. J Bone Joint Surg Am. 2003;85(4):632–634. doi:10.2106/00004623-200304000-00007
20. Goodman S, Ma T, Trindade M, et al. COX-2 selective NSAID decreases bone ingrowth in vivo. J Orthop Res. 2002;20(6):1164–1169. doi:10.1016/S0736-0266(02)00079-7
21. Sivaganesan A, Chotai S, White-Dzuro G, et al. The effect of NSAIDs on spinal fusion: a cross-disciplinary review of biochemical, animal, and human studies. Eur Spine J. 2017;26:2719–2728. doi:10.1007/s00586-017-5021-y
22. Deguchi M, Rapoff AJ, Zdeblick TA. Posterolateral fusion for isthmic spondylolisthesis in adults: analysis of fusion rate and clinical results. J Spinal Disord. 1998;11(6):459–464. doi:10.1097/00002517-199812000-00001
23. Rodriguez-Merchan EC. The importance of smoking in orthopedic surgery. Hosp Pract. 2018;46(4):175–182. doi:10.1080/21548331.2018.1505406
24. Mills E, Eyawo O, Lockhart I, Kelly S, Wu P, Ebbert JO. Smoking cessation reduces postoperative complications: a systematic review and meta-analysis. Am J Med. 2011;124(2):144–154.e8. doi:10.1016/j.amjmed.2010.09.013
25. Bratzler DW, Dellinger EP, Olsen KM, et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am J Health Syst Pharm. 2013;70(3):195–283. doi:10.2146/ajhp120568
26. Tan TL, Shohat N, Rondon AJ, et al. Perioperative antibiotic prophylaxis in total joint arthroplasty: a single dose is as effective as multiple doses. J Bone Joint Surg Am. 2019;101(5):429–437. doi:10.2106/JBJS.18.00336
27. Manchikanti L, Manchikanti L, Novitch MB, et al. Responsible, safe, and effective use of antithrombotics and anticoagulants in patients undergoing interventional techniques: American Society of Interventional Pain Physicians (ASIPP) guidelines. Pain Phy. 2019;22(1S):S75–S128. doi:10.36076/ppj/2019.22.s75
28. Horlocker TT, Vandermeuelen E, Kopp SL, Gogarten W, Leffert LR, Benzon HT. Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy: American Society of Regional Anesthesia and Pain Medicine Evidence-Based Guidelines. Reg Anesth Pain Med. 2018;43(3):263–309. doi:10.1097/AAP.0000000000000763
29. Deer TR, Narouze S, Provenzano DA, et al. The Neurostimulation Appropriateness Consensus Committee (NACC): recommendations on bleeding and coagulation management in neurostimulation devices. Neuromodulation. 2017;20(1):51–62. doi:10.1111/ner.12542
30. Kulkarni AG, Patel J, Khandge A, Mewara N. The practice of continuation of anti-platelet therapy during the perioperative period in lumbar Minimally Invasive Spine Surgery (MISS): how different is the morbidity in this scenario? Spine (Phila Pa 1976). 2020;45(10):673–678. doi:10.1097/BRS.0000000000003357
31. Endres S, Ludwig E. Outcome of distraction interference arthrodesis of the sacroiliac joint for sacroiliac arthritis. Indian J Orthop. 2013;47(5):437–442. doi:10.4103/0019-5413.118197
32. Fuchs V, Ruhl B. Distraction arthrodesis of the sacroiliac joint: 2-year results of a descriptive prospective multi-center cohort study in 171 patients. Eur Spine J. 2018;27:194–204. doi:10.1007/s00586-017-5313-2
33. Rudolf L, Capobianco R. Five-year clinical and radiographic outcomes after minimally invasive sacroiliac joint fusion using triangular implants. Open Orthop J. 2014;8:375–383. PMID: 25352932; PMCID: PMC4209504. doi:10.2174/1874325001408010375
34. Whang P, Cher D, Polly D, et al. Sacroiliac joint fusion using triangular titanium implants vs. non-surgical management: six-month outcomes from a prospective randomized controlled trial. Int J Spine Surg. 2015;9:6. doi:10.14444/2006
35. Sturesson B, Selvik G, Udén A. Movements of the sacroiliac joints. A roentgen stereophotogrammetric analysis. Spine (Phila Pa 1976). 1989;14(2):162–165. doi:10.1097/00007632-198902000-00004
36. Sturesson B, Uden A, Vleeming A. A radiostereometric analysis of movements of the sacroiliac joints during the standing hip flexion test. Spine (Phila Pa 1976). 2000;25(3):364–368. doi:10.1097/00007632-200002010-00018
37. Sturesson B, Uden A, Vleeming A. A radiostereometric analysis of the movements of the sacroiliac joints in the reciprocal straddle position. Spine (Phila Pa 1976). 2000;25(2):214–217. doi:10.1097/00007632-200001150-00012
38. Laslett M, Aprill CN, McDonald B, Young SB. Diagnosis of sacroiliac joint pain: validity of individual provocation tests and composites of tests. Man Ther. 2005;10(3):207–218. doi:10.1016/j.math.2005.01.003
39. Dall B. Post-operative guidelines for sacroiliac joint fusion; 2021. Available from: https://zyga.com/wp-content/uploads/2016/10/Post-op-guidelines_04.pdf.
40. Tekmyster G, Jonely H, Lee DW, et al. Physical therapy considerations and recommendations for patients following spinal cord stimulator implant surgery. Neuromodulation. 2021. doi:10.1111/ner.13391
41. Pel JJ, Spoor CW, Pool-Goudzwaard AL, Hoek van Dijke GA, Snijders CJ. Biomechanical analysis of reducing sacroiliac joint shear load by optimization of pelvic muscle and ligament forces. Ann Biomed Eng. 2008;36(3):415–424. doi:10.1007/s10439-007-9385-8
42. Dall B, Eden S, Rahl M. Surgery for the Painful, Dysfunctional Sacroiliac Joint. Springer; 2015:159–169.
43. Oestergaard LG, Nielsen CV, Bünger CE, Svidt K, Christensen FB. The effect of timing of rehabilitation on physical performance after lumbar spinal fusion: a randomized clinical study. Eur Spine J. 2013;22(8):1884–1890. doi:10.1007/s00586-013-2717-5
44. Duhon BS, Cher DJ, Wine KD, Lockstadt H, Kovalsky D, Soo CL. Safety and 6-month effectiveness of minimally invasive sacroiliac joint fusion: a prospective study. Med Devices. 2013;6:219–229. doi:10.2147/MDER.S55197
45. Tullberg T, Blomberg S, Branth B, Johnsson R. Manipulation does not alter the position of the sacroiliac joint, spine. Spine. 1998;23(10):1124–1128. doi:10.1097/00007632-199805150-00010
46. Buchowski JM, Kebaish KM, Sinkov V, Cohen DB, Sieber AN, Kostuik JP. Functional and radiographic outcome of sacroiliac arthrodesis for the disorders of the sacroiliac joint. Spine J. 2005;5(5):520–529. doi:10.1016/j.spinee.2005.02.022
47. Teyhen DS, Rieger JL, Westrick RB, Miller AC, Molloy JM, Childs JD. Changes in deep abdominal muscle thickness during common trunk-strengthening exercises using ultrasound imaging. J Orthop Sports Phys Ther. 2008;38(10):596–605. doi:10.2519/jospt.2008.2897
48. Reiman MP, Bolgla LA, Loudon JK. A literature review of studies evaluating gluteus maximus and gluteus medius activation during rehabilitation exercises. Physiother Theory Pract. 2012;28(4):257–268. doi:10.3109/09593985.2011.604981
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
