Back to Journals » Drug Design, Development and Therapy » Volume 17
Berberine Alleviates Acute Lung Injury in Septic Mice by Modulating Treg/Th17 Homeostasis and Downregulating NF-κB Signaling
Authors Chen L, Liu X , Wang X, Lu Z , Ye Y
Received 13 December 2022
Accepted for publication 6 April 2023
Published 13 April 2023 Volume 2023:17 Pages 1139—1151
DOI https://doi.org/10.2147/DDDT.S401293
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Georgios Panos
Longwang Chen,1,* Xinyong Liu,2,* Xuetao Wang,3 Zhongqiu Lu,1 Yumei Ye4
1Department of Emergency, the First Affiliated Hospital of Wenzhou Medical University, Wenzhou, Zhejiang, People’s Republic of China; 2Department of Critical Care Medicine, Affiliated Jinhua Hospital, Zhejiang University School of Medicine, Jinhua, Zhejiang, People’s Republic of China; 3Department of Intensive Care Unit, Wenzhou Longwan District First People’s Hospital, Wenzhou, Zhejiang, People’s Republic of China; 4Department of Ultrasound Imaging, the First Affiliated Hospital of Wenzhou Medical University, Wenzhou, Zhejiang, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yumei Ye, Department of Ultrasound Imaging, the First Affiliated Hospital of Wenzhou Medical University, Wenzhou, 325000, People’s Republic of China, Tel +860577-5557-9410, Email [email protected]
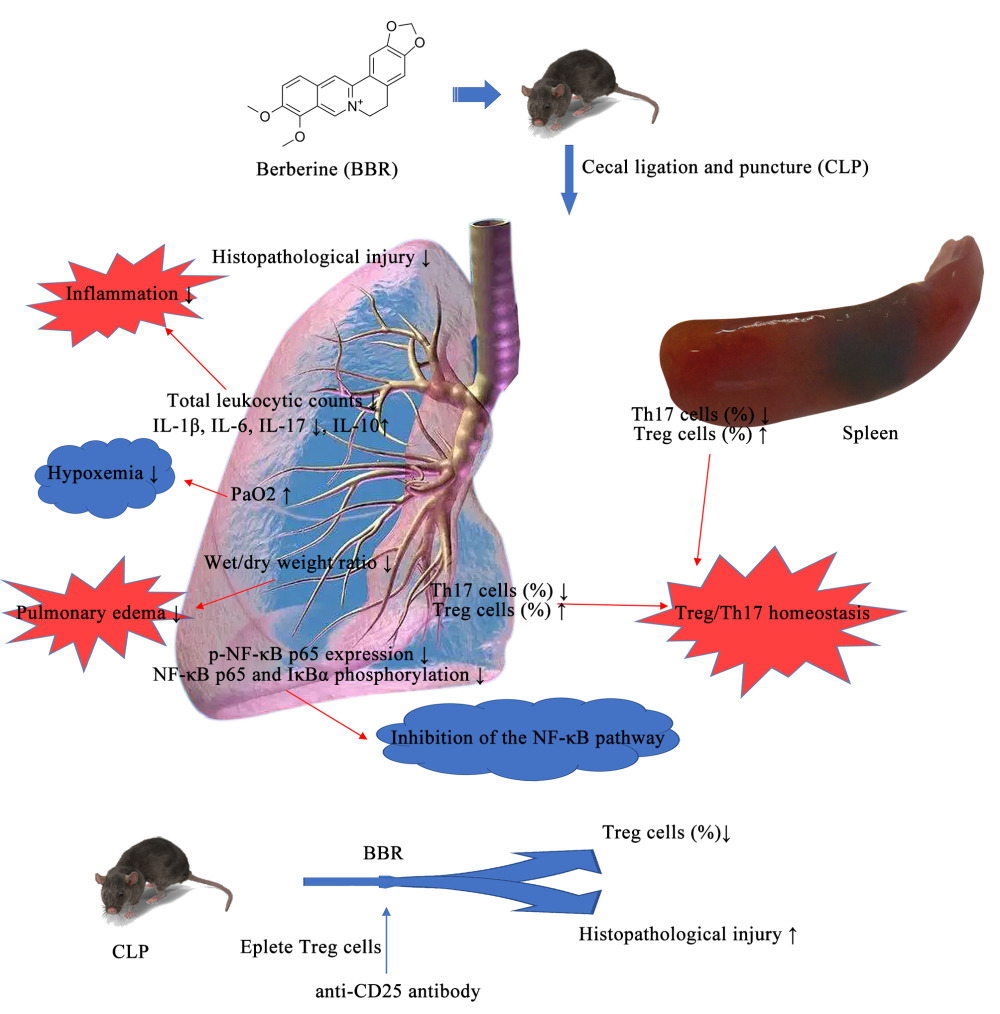
Purpose: A common complication of sepsis is acute lung injury (ALI), which is associated with an acute onset, rapid disease changes, and high mortality. Regulatory T (Treg) and T helper 17 (Th17) cells comprise CD4+ T cell subsets, which strongly influence inflammation during ALI. In this study, we investigated the effect of berberine (BBR), an antioxidant, anti-inflammatory, and immunomodulatory drug, on the inflammatory response and immune state in mice with sepsis.
Methods: A mouse model of cecal ligation and puncture (CLP) was established. The mice were intragastrically administered 50 mg/kg BBR. We used histological techniques to evaluate inflammatory tissue injury and flow cytometry for analyzing Treg/Th17 levels. We also assessed NF-κB signaling pathways by Western blotting assays and immunofluorescence staining. Enzyme-linked immunosorbent assay (ELISA) was performed to measure the content of cytokines.
Results: Treatment with BBR considerably mitigated lung injury while improving survival, post-cecal ligation, and puncture (CLP). Treatment with BBR ameliorated pulmonary edema and hypoxemia in septic mice and inhibited the NF-κB signaling pathway. BBR also increased Treg cells and decreased Th17 proportions in the spleen and lung tissue of CLP-treated mice. Blocking Treg cells weakened the protective effect of BBR on sepsis-associated lung injury.
Conclusion: Overall, these results suggested that BBR is a potential therapeutic agent for sepsis.
Keywords: berberine, sepsis, inflammation, cell differentiation, immunodeficiency, T lymphocyte
Graphical Abstract:
Introduction
Sepsis is a life-threatening form of organ dysfunction related to a disordered response to infection in the host, and it is a main factor leading to mortality globally.1 In 2017, the number of sepsis patients reached 48.9 million globally, and 11 million deaths were reported due to sepsis (one-fifth of the total deaths worldwide).2 The lungs are the most vulnerable among affected organs, and sepsis induces acute lung injury (ALI) in 40% of cases.3 Thus, patient mortality can reach 70–90%.4 Although many therapeutic strategies have been developed, such as mechanical ventilation, fluid management, and hormone application strategies, mortality rates remain high among patients with sepsis and ALI complications.
The characteristics of ALI include inflammation, rapid alveolar damage, neutrophil infiltration, cytokine production, and damage to the lung endothelium and alveolar epithelium. Concerning pathogenic molecular mechanisms, many studies have shown that NF-κB is an important contributor to septic organ damage.5,6 Additionally, the early stages of ALI are associated with an increase in the activation of CD4+ T cells, which is a critical component among adaptive immunocytes, and, specifically, the differentiation of CD4+ T cells into regulatory T cells (Treg)/T helper 17 (Th17) cells.7 Among these, Th17 cells can produce interleukin (IL)-17 to induce the secretion of pro-inflammatory factors (eg, TNF-α, IL-6, and IL-1β).8,9 In contrast, Treg cells maintain immune homeostasis by producing anti-inflammatory factors (like TGF-β and IL-10).10 Accordingly, an imbalance in Th17/Treg cells might be associated with the development and progression of sepsis.11
Berberine (BBR) is an isoquinoline alkaloid that can be isolated from two plants used in Traditional Chinese Medicine, including Coptis chinensis Franch. (Ranunculaceae) and Phellodendron (Rutaceae).12 The extract has anti-inflammatory, antioxidative, antiapoptotic, immunomodulatory, and antitumor effects.13,14 Some studies have shown that BBR can protect against sepsis.15–17 BBR can alleviate sepsis-induced cardiac insufficiency by upregulating the Akt/eNOS pathway in mice.18 Li et al confirmed that BBR could reduce gut-vascular barrier permeability by modulating the ApoM/S1P pathway in sepsis.19 BBR was found to alleviate lipopolysaccharide-induced lung injury by regulating the NF-κB/Nlrp3 (nucleotide-binding domain and leucine-rich repeat protein-3) signaling pathway.20 Wang et al found that BBR, along with yohimbine, can attenuate sepsis-induced neutrophil tissue infiltration and multiorgan dysfunction partly via IL-10-mediated inhibition of CCR2 expression in neutrophils.21 BBR can also regulate immune disorders in sepsis.17,22 Therefore, in this study, we investigated the effects of BBR on immune function and lung injury in septic mice.
Materials and Methods
Reagents and Antibodies
Berberine was purchased from Sigma-Aldrich (St. Louis, MO, USA). Before being added to the medium, BBR was dissolved in DMSO at an appropriate volume. Fetal bovine serum was purchased from Gibco (Grand Island, NY, USA). Rabbit anti-mouse NF-κB p65, phospho-NF-κB p65, phospho-IκB and IκBα, and β-actin antibodies were purchased from Santa Cruz Biotechnology (CA, USA). R700-labeled anti-CD3, BV786-labeled anti-CD4, BB515-labeled anti-CD25, BV421-labeled anti-Foxp3, and BV650-labeled anti-IL-17A antibodies were provided by Becton, Dickinson, and Company (Franklin Lakes, USA). The antibodies against CD25 were purchased from R&D Systems (Minneapolis, MN, USA). The enzyme-linked immunosorbent assay (ELISA) kits were purchased from Biosource (Worcester, MA, USA) for measuring the contents of murine IL-1β, IL-6, IL-10, and IL-17A.
Animals
Male C57BL/6 mice (eight weeks old, 22–25 g) were obtained from Shanghai Slack Laboratory Animal Center [license: SCXK (HU) 2012–0002]. Each mouse was raised in a particular environment with no specific pathogen at an appropriate humidity and temperature for seven days; the animals were allowed to drink water and eat standard chow freely. Our experimental protocols were approved by the Institutional Animal Ethics Committee of Wenzhou Medical University (NO. wydw2020–0457) and were conducted following the Guide for the Care and Use of Laboratory Animals provided by the National Institutes of Health.
Establishment of an Animal Model of Sepsis
We used the cecal ligation and puncture (CLP) approach to induce polymicrobial sepsis, as previously described.23 Clean-grade male C57BL/6 mice were used, with water provided ad libitum and after fasting for one day. The mice were anesthetized via an injection with 2% pentobarbital sodium in the abdomen, and then, a 1–2 cm median incision was made in the abdomen. Next, a 3–0 silk thread was used to ligate 20% of the cecum at the end of the vascular arch. A 20-G needle was used to puncture the intestinal walls on both sides of the cecum at the distal ligation line, causing some overflow of the intestinal content. The cecum was inserted into the abdominal cavity again, and the abdomen was stitched with a 1–0 silk thread, followed by the immediate subcutaneous injection of normal saline (1 mL). The mice in the sham (control) group did not undergo cecal ligation or perforation, although all other aspects of the operation were performed.
Animal Experiments
In total, 24 mice were randomized into four groups, including the sham, BBR, CLP, and CLP+BBR groups. The experiment was repeated at least thrice, and each group included six mice. Each mouse was intragastrically administered 0.1 mL/10 g of distilled water or 50 mg/kg BBR 2 h after the operation. After 24 h, bronchoalveolar lavage fluid (BALF), blood, and lung tissue were sampled.
In a second set of experiments, the mice were injected intraperitoneally with anti-CD25 neutralizing antibodies (25 mg/kg) 1 h before CLP. The depletion of CD25+ cells in the lungs was evaluated by flow cytometry. Then, the animals were sacrificed 24 h after the sham operation or after CLP to obtain the lung samples.
To perform survival analysis, 60 mice were randomized into the sham, BBR, CLP, and CLP+BBR groups. The mice were monitored daily, and their survival was analyzed after seven days. We also randomized 75 mice into the sham, CLP, CLP+anti-CD25, CLP+BBR and CLP+BBR+anti-CD25 groups to record survival.
Bronchoalveolar Lavage Fluid (BALF) Analysis
After 24 h of the CLP operation, the mice were deeply anesthetized. The right main bronchus was ligated, and 2 mL of phosphate-buffered saline (PBS) was administered to the left lung using a tracheal cannula and divided into four instillations of 0.5 mL. The fluid was then slowly recovered, and BALF was collected. The supernatant and cells were collected after centrifuging the BALF at 1000 g for 5 min at 4 °C. The cells were dissolved in 1 mL of PBS, and the supernatant was stored at −70 °C. The cells were stained using the Diff-Quik stain solution, sorted, and counted under a light microscope. The total number of cells was presented as the mean ±SEM/mL for every group.
Enzyme-Linked Immunosorbent Assay (ELISA)
The protein levels of IL-1β, IL-6, IL-10, and IL-17A in the BALF were measured using ELISA kits. The detection limits for IL-1β, IL-6, IL-10, and IL-17A were 1.2 pg/mL, 6.5 pg/mL, 5 pg/mL, and 1.6 pg/mL, respectively. First, the standards and the samples were added to the reaction wells (100 µL/well), and then, a negative control was set up, incubated for 1.5 h, and washed. To each well, horseradish peroxidase (HRP)-labeled streptavidin (diluted 100 times) (100 µL/well) was added, followed by 30 min of incubation and washing. Then, the plate was incubated for 30 min in the dark with the chromogenic solution. After incubation, the termination solution was added to terminate the reaction. Eventually, the OD values of the wells were evaluated using a microplate reader (Beckman, Germany) at 450 nm.
Arterial Blood Gas and Lung W/D Weight Ratio
At 24 h after the animal experiments, the mice were anesthetized. An arterial catheter was implanted into the left carotid artery with the opposite end connected to a 1 mL pre-heparinized syringe used for blood sample collection. Then, 150 µL of arterial blood was collected to immediately measure arterial oxygen partial pressure (PaO2) using the LABOSPECT 008AS platform (Hitachi High-Tech Co., Tokyo, Japan). The wet weight of the upper right lung lobe was measured immediately after excision, followed by incubation for 96 h on sterile non-enzymatic paper at 80 °C to remove moisture. Then, the weight was measured again to determine the W/D weight ratio and assess the extent of edema.
Flow Cytometry
Following euthanasia, lung perfusion was conducted by injecting PBS/0.6 mM ethylenediaminetetraacetic acid (5 mL) into the right ventricular cannula. Later, the lung samples were collected and incubated in a medium containing digestive enzymes (collagenase/DNAse I solution) for 45 min at 37 °C. A mesh strainer (40 µm) was used to filter the digestion solution and collect single-cell suspensions. Finally, 5 × 105 cells per group were used for conducting flow cytometry assays.
The spleen was shredded into small pieces with tweezers in PBS to acquire a suspension which was ground thoroughly with a 200-mesh cell strainer. The cell suspension was transferred to the lymphocyte separation solution and centrifugated at 3000 rpm for 15 min at room temperature. After washing twice, the spleen mononuclear cell suspension was obtained, and 106 cells per group were used for conducting flow cytometry assays.
For surface and nuclear staining, the following antibodies were used: BV421-anti-Foxp3, BB515-conjugated anti-CD25, BV650-conjugated anti-IL-17A, BV786-conjugated anti-CD4, and R700-conjugated anti-CD3. For analyzing Th17 cells, we incubated the cells with R700-anti-CD3 antibodies and BV786-anti-CD4 antibodies. They were fixed and permeabilized with the IL-17A-staining solution and stained using BV650-conjugated anti-IL-17A antibodies. To analyze Treg cells, the cells were stained with anti-mouse CD3-R700 antibodies, CD4-BV786 antibodies, and CD25-BB515 antibodies for 30 min at 4 °C in the dark. The cells were fixed and permeabilized, and then, stained with Foxp3-BV421 antibodies for 30 min. The stained cells were washed twice before analysis via flow cytometry using FACScan (BD Biosciences, USA). The results of the flow cytometry assay were analyzed using the FlowJo software (Ashland, USA).
Immunofluorescence Staining
The single cell suspension (for preparation, see “Flow cytometry”) was fixed with 4% paraformaldehyde for 15 min and permeabilized with PBS containing 0.3% Triton X-100 for 20 min. A PBS blocking solution containing 1% BSA was added to block the cells for 1 h. Then, the cells were incubated with rabbit anti-mouse phospho-NF-κB p65 antibodies (1: 1000 dilution) overnight at 4 °C in a humidified incubator. The cells were sufficiently rinsed with PBS, followed by incubation with Alexa-594-labeled secondary antibodies (1: 1000 dilution) for 1 h under ambient temperature. The nuclei were stained with 4’,6-diamidino-2-phenylindole (DAPI) for 5 min. The samples were mixed with an anti-fluorescence quenching sealing solution. Finally, a laser confocal microscope was used to observe the samples under the corresponding excitation light, and images were acquired.
Western Blotting Assay
The lung tissues were smashed and the total protein was extracted. After transferring 30 µg of proteins from each group onto polyvinylidene fluoride membranes, we used 5% skim milk to block the membranes. Then, the membranes were washed and incubated with primary antibodies, including phospho-NF-κB p65, NF-κB p65, phospho-IκBα, IκBα, and β-actin antibodies (1:1000 dilution for all). Then, they were sequentially incubated for 2 h with HRP-labeled secondary antibodies (1:5000 dilution) under ambient temperature. The X-ray films and enhanced chemiluminescence kits were used for signal detection. Each assay was conducted in triplicate using different samples. The Image J software (NIH Image, Bethesda, MD) was used to analyze protein densitometry.
Pathological Observations
Following treatment, the lung tissues were fixed in 4% paraformaldehyde immediately after collection. Then, they were sliced into thin sections (5 µM) and stained via H&E staining. The histological characteristics were determined under an optical microscope. Then, lung injury was scored on a scale from 0–4 (normal to severe) following a double-blinded evaluation and based on alveolar edema, neutrophil infiltration, and hemorrhage as follows: 0, no injury; 1, injury in < 25% of the field; 2, injury in 25–50% of the field; 3, injury in 50–75% of the field; and 4, injury in > 75% of the field. The sum of these scores (maximum score: 12) was considered to be the overall score.
Statistical Analysis
The results were presented in the form of the mean (M) ±SEM. The Shapiro–Wilk test and the quartile-quartile (Q-Q) plots were used assess data. The results showed that our data were normally distributed. Comparisons among different groups were made by conducting an analysis of variance (ANOVA) followed by Tukey’s multiple comparison tests. Survival analysis and curves were based on Kaplan–Meier tests. The SPSS 22.0 (IBM Corp., Armonk, NY, USA) software was used for conducting the tests, and the results were visualized using Graph Pad Prism 9.0 for Windows (Graph Pad, La Jolla, CA, USA). All differences among and between groups were considered to be statistically significant at P < 0.05.
Results
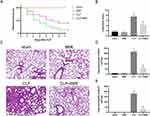
BBR Alleviated Lung Injury in CLP-Treated Mice
The mice in the CLP group showed considerably lower seven-day survival than those in the sham group, but the survival was significantly higher in BBR-treated mice with CLP operation (Figure 1A). The mice in the sham group had minor lung tissue damage, whereas CLP caused severe injury, including bleeding, neutrophil infiltration, and alveolar edema. However, BBR treatment reduced lung injury in septic mice (Figure 1B and C). Total leukocyte and neutrophil counts were considerably higher in the mice of the CLP group than those in the mice of the sham group, but the counts were lower in the CLP+BBR group than those in the mice of the CLP group (Figure 1D and E).
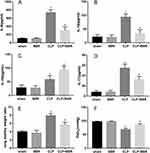
BBR Ameliorated Inflammatory Damage to the Lungs, Pulmonary Edema, and Hypoxemia
The concentrations of IL-6, IL-1β, and IL-17A in BALF were significantly lower in the mice of the BBR-treated group than those in the mice of the CLP group (Figure 2A–D). In contrast, the mice in the CLP+BBR group had substantially higher IL-10 levels in the BALF. Thus, BBR had an anti-inflammatory effect. The mice in the CLP group had a higher lung W/D weight ratio than those in the sham group (Figure 2E and F). The administration of BBR mitigated edema. The PaO2 was lower in CLP-treated mice than that in the mice of the sham group. The PaO2 improved after BBR treatment (Figure 2F). Thus, BBR can also improve hypoxemia in septic mice.
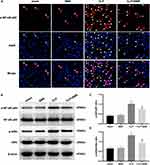
BBR Inhibited the NF-κB Pathway in the Lungs of CLP-Treated Mice
The NF-κB signaling pathway strongly influences the inflammation. Using immunofluorescence, we found that the mice in the CLP group had a significantly higher expression of nuclear p-NF-κB p65 than the mice in the sham group. However, BBR treatment significantly decreased the expression of p-NF-κB p65 compared to the levels in the mice of the CLP group (Figure 3A). Additionally, the results of the Western blotting assays showed that NF-κB p65 and IκBα phosphorylation decreased after BBR treatment (Figure 3B–D). These results indicated that BBR prevented NF-κB activation in septic mice.
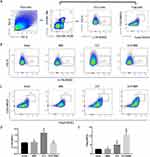
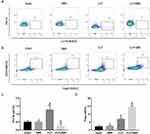
BBR Increased Treg Cells and Decreased Th17 Cells in the Spleen and Lung Tissue of CLP-Treated Mice
The results of the flow cytometry assay showed that the proportion of CD3+CD4+CD25+Foxp3+ Treg cells was high in the lung and spleen tissues of CLP-treated mice, with BBR treatment causing a further increase (Figures 4 and 5). In contrast, the proportion of Th17 cells was higher in the lung and spleen tissues of septic mice, and BBR treatment decreased it.
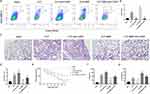
BBR Played a Protective Role in ALI, Which Might Be Linked to Treg Cells
To determine whether BBR protects against sepsis-associated ALI via Treg cells, we blocked CD25 with an anti-CD25 antibody. The results showed that anti-CD25 antibodies decreased the proportion of Tregs in the CLP mice (Figure 6A and B), worsening lung injury (Figure 6C and D). Furthermore, anti-CD25 antibodies diminished the therapeutic effect of BBR on septic lung injury (Figure 6C and D). Moreover, the depletion of CD4+ cells resulted in significantly higher mortality (Figure 6E) and higher IL-6 levels (Figure 6F) in the mice of the CLP+BBR+anti-CD25 group than in the mice of the CLP+BBR group, along with lower IL-10 levels in the BALF (Figure 6G). These outcomes suggested that BBR potentially targets Treg cells to attenuate lung injury.
Discussion
Sepsis is a primary health issue with a high global mortality rate,1 particularly concerning ALI, due to excessive inflammation. Thus, targeting inflammatory responses is essential for the prevention and treatment of sepsis-related ALI. Immune homeostasis is related to the balance of Th17/Treg,24 which influences inflammation during sepsis-induced ALI.25,26 However, most studies regarding immunocytes mainly emphasize middle-to-late stage sepsis, despite changes in immune cell numbers and function during the early stages. Therefore, in this study, we investigated early sepsis, measuring Treg and Th17 cells in the spleen and lung 24 h after CLP. Experimentally induced sepsis significantly increased the proportion of Treg and Th17 cells, corroborating the findings of previous studies.27 We also detected cytokines and the activity related to the NF-κB pathway within the BALF. Overall, we found that sepsis caused lung tissue inflammation, which matched the findings of other studies.27,28
The bioactive plant extract BBR has many pharmacological effects, including antioxidative and anti-inflammatory activities.29,30 The underlying mechanism might involve the suppression of the activation of HMGB1/TLR4/NF-κB signaling.31 Some studies have shown that administering BBR modulates excessive inflammation, significantly attenuating lung tissue damage while improving the survival of septic mice. Additionally, BBR inhibits the expression of tumor necrosis factor-α (TNF-α), IL-6, and IL-1β by inhibiting the activation of the NF-κB pathway.15 A study on rats also found that BBR attenuates septic cardiomyopathy by inhibiting TLR4/NF-κB signaling32 and acts on Toll-like receptor pathways to prevent intestinal mucosal barrier damage during early sepsis.33 In this study, we applied 50 mg/kg of BBR to determine its effects on ALI in septic mice. A study found that 50 mg/kg of BBR significantly inhibited HMGB1 release and NF-κB nuclear translocation31 besides exerting anti-inflammatory effects by activating the mTORC1 pathway.34 Our results showed that BBR reduces sepsis-induced ALI and decreases mortality in mice. We also provided evidence on the mechanism of action by detecting pro-inflammatory and anti-inflammatory factors in alveolar lavage fluid. We concluded that BBR acts by inhibiting the NF-κB signaling pathway.
Inflammation and the immune response are closely related and interact with each other, and they show dynamic changes in sepsis.35 Also, the enhancement of immunosuppressive functions can reduce inflammatory reactions. Since the proportion of Treg/Th17 cells reflects the immunosuppressive state, we also evaluated the ratio of Treg/Th17 cells. The result showed that BBR increased the proportion of Tregs in the spleen and lungs of CLP-treated mice and reduced the proportion of Th17 cells. Our results contributed to previous findings of the effect of BBR on Th17/Treg cells. For example, a study investigated the therapeutic potential of BBR concerning the autophagy of adjuvant-mediated arthritic fibroblast-like synoviocytes induced by IL-21/IL-21R via PI3K/Akt signaling and imbalances in Th17/Treg cells.36 The authors found that BBR inhibited Th17 cell proliferation by downregulating RORγt, depending on its dose. BBR can also enhance Treg cell differentiation by activating Foxp3 through aryl hydrocarbon receptors and the upregulation of cytochrome P450 family 1, subfamily A, and polypeptide 1 (CYP1A1) expression.36 In a mouse model, BBR prevented ulcerative colitis by modifying the gut microbiota and regulating Treg/Th17 homeostasis.37 BBR also ameliorates non-alcoholic steatohepatitis, which is associated with the restoration of the Treg/Th17 ratio.38 In this study, we examined the effect of BBR on Treg/Th17 cells in sepsis. Additionally, by blocking CD25 with a CD25 antibody, we confirmed the involvement of Treg cells in BBR activity, as lung injury worsened when CD25 was neutralized.
Traditional Chinese medicine (TCM) is extensively used to treat inflammatory diseases. Many studies have suggested the importance of TCM for modulating the Th17/Treg balance. Xuanbai Chengqi decoction (XBCQ; a TCM) can be used to treat acute exacerbation of chronic obstructive pulmonary disease (COPD). XBCQ decoction effectively restores the Th17/Treg balance in mice with COPD by suppressing Th17 accumulation while mitigating Treg cell insufficiency.39 Moreover, juglone, mainly extracted from Juglans mandshurica (green walnut) husks, has antitumor and anti-inflammatory effects. Treatment using Juglone suppresses Th17 cell production while increasing Treg cells, which helps in restoring the Th17/Treg balance in ulcerative colitis.40 However, in autoimmune uveitis rats, Longdan Xiegan Decoction effectively suppresses the differentiation of Th17 cells and downregulates the levels of inflammatory factors. It can also reverse the Th17/Treg and CD4+/CD8+ balance by suppressing the activation of the Notch pathway, thus protecting the eye tissue architecture and mitigating eye inflammation efficiently, as well as, favorably modulating the immune state in the entire body and the intraocular microenvironment.41 These results indicate that some Chinese medicines only affect the Treg cell lineage, whereas some only affect the Th17 cell lineage, while some Chinese medicines affect both. Such agents can effectively protect against various disorders by modulating the balance of Treg/Th17 cells.
Conclusion
In this study, we identified two mechanisms underlying the protective effects of BBR against sepsis-induced lung injury. The first mechanism involves a reduction in the inflammatory response in lung tissue through the modulation of the NF-kB signaling pathway. The second mechanism involves the regulation of the Treg/Th17 balance. Although several limitations need to be addressed before its clinical application is feasible, our findings indicated that BBR is a promising therapeutic drug for the treatment of sepsis.
Acknowledgments
This work was supported by the Wenzhou Municipal Science and Technology Project [grant numbers Y2020094], Natural Science Foundation of Zhejiang Province [grant numbers LQ22H150003], and National Key R&D Program of China (No. 2018YFC2000305).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Freund Y, Lemachatti N, Krastinova E, et al. Prognostic accuracy of sepsis-3 criteria for in-hospital mortality among patients with suspected infection presenting to the emergency department. JAMA. 2017;317(3):301–308. doi:10.1001/jama.2016.20329
2. Rudd KE, Johnson SC, Agesa KM, et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the Global Burden of Disease Study. Lancet. 2020;395:200–211.
3. Iscimen R, Cartin-Ceba R, Yilmaz M, et al. Risk factors for the development of acute lung injury in patients with septic shock: an observational cohort study. Crit Care Med. 2008;36:1518–1522.
4. Haitsma JJ, Lachmann B. Lung protective ventilation in ARDS: the open lung maneuver. Minerva Anestesiol. 2006;72:117–132.
5. Wang YM, Ji R, Chen WW, et al. Paclitaxel alleviated sepsis-induced acute lung injury by activating MUC1 and suppressing TLR-4/NF-κB pathway. Drug Des Devel Ther. 2019;13:3391–3404.
6. Li J, Ma J, Li M, et al. GYY4137 alleviates sepsis-induced acute lung injury in mice by inhibiting the PDGFRβ/Akt/NF-κB/NLRP3 pathway. Life Sci. 2021;271:119192.
7. Martin-Loeches I, Levy MM, Artigas A. Management of severe sepsis: advances, challenges, and current status. Drug Des Devel Ther. 2015;9:2079–2088.
8. Liang SC, Tan XY, Luxenberg DP, et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–2279.
9. Miossec P, Kolls JK. Targeting IL-17 and TH17 cells in chronic inflammation. Nat Rev Drug Discov. 2012;11:763–776.
10. Nagler-Anderson C, Bhan AK, Podolsky DK, Terhorst C. Control freaks: immune regulatory cells. Nat Immunol. 2004;5:119–122.
11. Xia H, Wang F, Wang M, et al. Maresin1 ameliorates acute lung injury induced by sepsis through regulating Th17/Treg balance. Life Sci. 2020;254:117773.
12. Imenshahidi M, Hosseinzadeh H. Berberine and barberry (Berberis vulgaris): a clinical review. Phytother Res. 2019;33:504–523.
13. Hou Q, He WJ, Wu YS, Hao HJ, Xie XY, Fu XB. Berberine: a Traditional Natural Product With Novel Biological Activities. Altern Ther Health Med. 2020;26:20–27.
14. Song D, Hao J, Fan D. Biological properties and clinical applications of berberine. Front Med. 2020;14:564–582.
15. Wang Y, Du P, Jiang D. Berberine functions as a negative regulator in lipopolysaccharide -induced sepsis by suppressing NF-κB and IL-6 mediated STAT3 activation. Pathog Dis. 2020;78.
16. Li B, Niu S, Geng H, Yang C, Zhao C. Berberine Attenuates Neonatal Sepsis in Mice By Inhibiting FOXA1 and NF-κB Signal Transduction Via the Induction of MiR-132-3p. Inflammation. 2021;44:2395–2406.
17. Pierpaoli E, Cirioni O, Simonetti O, et al. Potential application of berberine in the treatment of Escherichia coli sepsis. Nat Prod Res. 2021;35:4779–4784.
18. Zhang H, Wu X, Tao Y, Lu G. Berberine attenuates sepsis-induced cardiac dysfunction by upregulating the Akt/eNOS pathway in mice. Exp Ther Med. 2022;23:371.
19. Li Y, Zhou J, Qiu J, et al. Berberine reduces gut-vascular barrier permeability via modulation of ApoM/S1P pathway in a model of polymicrobial sepsis. Life Sci. 2020;261:118460.
20. Chen J, Huang Y, Bian X, He Y. Berberine Ameliorates Inflammation in Acute Lung Injury via NF-κB/Nlrp3 Signaling Pathway. Front Nutr. 2022;9:851255.
21. Wang Y, Wang F, Yang D, et al. Berberine in combination with yohimbine attenuates sepsis-induced neutrophil tissue infiltration and multiorgan dysfunction partly via IL-10-mediated inhibition of CCR2 expression in neutrophils. Int Immunopharmacol. 2016;35:217–225.
22. Shin JS, Choi HE, Seo S, Choi JH, Baek NI, Lee KT. Berberine Decreased Inducible Nitric Oxide Synthase mRNA Stability through Negative Regulation of Human Antigen R in Lipopolysaccharide-Induced Macrophages. J Pharmacol Exp Ther. 2016;358:3–13.
23. Rittirsch D, Huber-Lang MS, Flierl MA, Ward PA. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat Protoc. 2009;4:31–36.
24. Gupta DL, Bhoi S, Mohan T, Galwnkar S, Rao DN. Coexistence of Th1/Th2 and Th17/Treg imbalances in patients with post traumatic sepsis. Cytokine. 2016;88:214–221.
25. Nadeem A, Ahmad SF, Al-Harbi NO, et al. Inhibition of spleen tyrosine kinase signaling protects against acute lung injury through blockade of NADPH oxidase and IL-17A in neutrophils and γδ T cells respectively in mice. Int Immunopharmacol. 2019;68:39–47.
26. Nadeem A, Al-Harbi NO, Ahmad SF, et al. Blockade of interleukin-2-inducible T-cell kinase signaling attenuates acute lung injury in mice through adjustment of pulmonary Th17/Treg immune responses and reduction of oxidative stress. Int Immunopharmacol. 2020;83:106369.
27. Xia H, Ge Y, Wang F, et al. Protectin DX ameliorates inflammation in sepsis-induced acute lung injury through mediating PPARγ/NF-κB pathway. Immunol Res. 2020;68:280–288.
28. Li H, Hao Y, Yang LL, et al. MCTR1 alleviates lipopolysaccharide-induced acute lung injury by protecting lung endothelial glycocalyx. J Cell Physiol. 2020;235:7283–7294.
29. Siow YL, Sarna L, Karmin O. Redox regulation in health and disease — therapeutic potential of berberine. Food Res Int. 2011;44:2409–2417.
30. Huang SX, Qiu G, Cheng FR, et al. Berberine Protects Secondary Injury in Mice with Traumatic Brain Injury Through Anti-oxidative and Anti-inflammatory Modulation. Neurochem Res. 2018;43:1814–1825.
31. Zhu JR, Lu HD, Guo C, et al. Berberine attenuates ischemia-reperfusion injury through inhibiting HMGB1 release and NF-κB nuclear translocation. Acta Pharmacol Sin. 2018;39:1706–1715.
32. Chen H, Liu Q, Liu X, Jin J. Berberine attenuates septic cardiomyopathy by inhibiting TLR4/NF-κB signalling in rats. Pharm Biol. 2021;59:121–128.
33. Li GX, Wang XM, Jiang T, Gong JF, Niu LY, Li N. Berberine Prevents Intestinal Mucosal Barrier Damage During Early Phase of Sepsis in Rat through the Toll-Like Receptors Signaling Pathway. Korean J Physiol Pharmacol. 2015;19:1–7.
34. Li Q, Qu X, Pang X, et al. Berberine Protects Mice Against Dextran Sulfate Sodium-Induced Colitis by Activating mTORC1 Pathway. Front Pharmacol. 2019;10:786.
35. Nadeem A, Al-Harbi NO, Alfardan AS, Ahmad SF, AlAsmari AF, Al-Harbi MM. IL-17A-induced neutrophilic airway inflammation is mediated by oxidant-antioxidant imbalance and inflammatory cytokines in mice. Biomed Pharmacother. 2018;107:1196–1204.
36. Dinesh P, Rasool M. Berberine mitigates IL-21/IL-21R mediated autophagic influx in fibroblast-like synoviocytes and regulates Th17/Treg imbalance in rheumatoid arthritis. Apoptosis. 2019;24:644–661.
37. Cui H, Cai Y, Wang L, et al. Berberine Regulates Treg/Th17 Balance to Treat Ulcerative Colitis Through Modulating the Gut Microbiota in the Colon. Front Pharmacol. 2018;9:571.
38. Lu Z, Lu F, Wu L, He B, Chen Z, Yan M. Berberine attenuates non-alcoholic steatohepatitis by regulating chemerin/CMKLR1 signalling pathway and Treg/Th17 ratio. Naunyn Schmiedebergs Arch Pharmacol. 2021;394:383–390.
39. Wang Y, Li N, Li Q, et al. Xuanbai Chengqi Decoction Ameliorates Pulmonary Inflammation via Reshaping Gut Microbiota and Rectifying Th17/Treg Imbalance in a Murine Model of Chronic Obstructive Pulmonary Disease. Int J Chron Obstruct Pulmon Dis. 2021;16:3317–3335.
40. Hua Y, Liu R, Lu M, et al. Juglone regulates gut microbiota and Th17/Treg balance in DSS-induced ulcerative colitis. Int Immunopharmacol. 2021;97:107683.
41. Yin X, Qiu Y, Li Z, et al. Longdan Xiegan Decoction alleviates experimental autoimmune uveitis in rats by inhibiting Notch signaling pathway activation and Th17 cell differentiation. Biomed Pharmacother. 2021;136:111291.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.