Back to Journals » Journal of Inflammation Research » Volume 14
B-Cell Deficiency Exacerbates Inflammation and Bone Loss in Ligature-Induced Experimental Periodontitis in Mice
Authors Zeng W, Liu G, Luan Q, Yang C, Li S , Yu X , Su L
Received 2 August 2021
Accepted for publication 16 September 2021
Published 15 October 2021 Volume 2021:14 Pages 5367—5380
DOI https://doi.org/10.2147/JIR.S330875
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
Wenmin Zeng,1 Guojing Liu,1 Qingxian Luan,1 Chunyu Yang,1 Shiyi Li,1 Xiaoqian Yu,1 Li Su2
1Department of Periodontology, Peking University School and Hospital of Stomatology, Beijing, People’s Republic of China; 2Center of Medical and Health Analysis, Peking University, Beijing, People’s Republic of China
Correspondence: Xiaoqian Yu
Department of Periodontology, Peking University School and Hospital of Stomatology, Beijing, 100081, People’s Republic of China
Email [email protected]
Li Su
Center of Medical and Health Analysis, Peking University, Beijing, 100191, People’s Republic of China
Email [email protected]
Objective: Periodontitis, one of the most prevalent chronic oral infectious diseases in humans, is induced by the breakdown in the balance between the biofilm and host immune system. Previous studies have shown the presence of large numbers of B cells in periodontitis lesions, implicating that B lymphocytes play a predominant role during the pathogenesis of periodontitis. This study aimed to investigate the role of all B cells in the initiation of periodontitis.
Methods: Experimental periodontitis was induced in B cell-deficient (CD19Cre) mice and wild-type (WT) control mice by 5-0 silk ligation around the maxillary second molar. Four weeks after ligation, alveolar bone loss was determined by micro-computed tomography. The levels of inflammatory cytokines and receptor activator of NF-κB ligand (RANKL)/osteoprotegerin in periodontal lesions were analyzed using real-time quantitative polymerase chain reaction, enzyme-linked immunosorbent assay, and immunohistochemistry. Lymphocyte populations in the cervical lymph nodes and spleen and among the peripheral blood mononuclear cells were detected by flow cytometry.
Results: B-cell deficiency resulted in increased severity of alveolar bone loss in mouse experimental periodontitis, which was associated with increased osteoclast activity and upregulated RANKL expression in the periodontal lesions. In addition, gingiva cytokine expression profiles were shifted to T helper type 1 (Th1) and Th17 in the CD19Cre mice with ligature-induced periodontitis compared with WT mice. In addition, a reduced CD4+/CD8+ T cell ratio was observed in the CD19Cre mice.
Conclusion: B-cell deficiency exacerbates the inflammation and alveolar bone loss in ligature-induced experimental periodontitis in mice, implicating that B cells may overall play a protective role in the initiation of periodontitis.
Keywords: periodontitis, experimental periodontitis, alveolar bone loss, B lymphocytes
Introduction
Periodontitis is a chronic inflammatory disease, mediated by a complex interplay between a dysbiotic microbiota and the host immune-inflammatory response, which may result in destruction of the tooth-supporting structures (gingiva, alveolar bone, periodontal ligament, and cementum), and even tooth loss.1 The hallmark of established and advanced lesions in periodontitis is the large number of B cells in these lesions. In periodontitis lesions, plasma cells are the prevailing cell type and represent approximately 50% of all the infiltrated leukocytes, while B cells comprise approximately 18%.2
B cells perform a variety of immune functions, including antibody secretion, antigen presentation, cytokine production, and regulation of T effector cell differentiation.3 Abnormalities of these B-cell functions could contribute to periodontitis induction or development. Accumulating evidence suggests that B cells play multiple critical roles in the immune pathogenesis of periodontitis. Researchers reported that anti-B lymphocyte therapy (rituximab) improved periodontitis in rheumatoid arthritis patients, suggesting a destructive role of B cells in this disease.4 B cells could destroy the periodontal soft and hard tissues by secreting pro-inflammatory cytokines, receptor activator of NF-κB ligand (RANKL), secreted osteoclastogenic factor of activated T cells, matrix metalloproteinases, and auto-antibodies (Abs).5–7 By contrast, recent studies have confirmed a newly identified subset of B cells known as regulatory B (Breg) cells, which mediate negative regulation of immunity through the expression of the anti-inflammatory cytokine interleukin-10 (IL-10), transforming growth factor-β (TGF-β), and IL-35.8,9 Previous research has indicated that Breg cells have protective and potentially therapeutic effects on allergic and autoimmune diseases, cancer, infection, and transplant rejection.9–13 Similarly, recent studies have shown that Breg cells could inhibit periodontal inflammation and bone loss in murine experimental periodontitis.14–19
It was demonstrated that B-cell deficiency increased the severity of multiple diseases, including experimental autoimmune encephalomyelitis, contact hypersensitivity, chronic intestinal inflammation, and arthritis.20–22 Regarding periodontitis, few studies have investigated the role of B cells and the results were inconsistent. Anti-μ treatment aggravated the periodontal resorption in rats.23 However, μMT mice were protected from Porphyromonas gingivalis-induced alveolar bone loss in another study.24 B cells are a functionally heterogeneous population; thus, the overall role of B cells and furthermore of the different B-cell subsets in periodontitis need to be clarified.
Materials and Methods
Animals
Healthy 6–10-week-old male C57BL/6J (Wild-Type [WT]) mice were purchased from the Charles River Laboratory (Beijing, China). B6.129P2(C)-Cd19 tm1(cre)Cgn/J (CD19Cre) mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). All the mice used in this study were maintained under pathogen-free conditions in laminar flow cabinets. The experimental protocols were approved by the Animal Welfare Ethics of Peking University Biomedical Ethics Committee (LA201406) and followed the US National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Mouse Model of Experimental Periodontitis
To induce experimental periodontitis in mice, a ligature-induced experimental periodontitis model was used as previously described.25 Mice were randomly assigned to the following four groups (n = 10 animals/group): (i) Wild-Type ligation group (WT ligation); (ii) Wild-Type non-ligation group (WT control); (iii) CD19Cre ligation group (CD19Cre ligation); (iv) CD19Cre non-ligation group (CD19Cre control). At 6 weeks of age, the mice were anesthetized with sodium pentobarbital (6 mg/100 g, intraperitoneal injection), and for the ligation groups, 5-0 silk ligatures were tied gently around the maxillary second molars on day 0 and retained in place for 4 weeks.
Tissue Collection and Preparation
Four weeks after ligation, 800–1000 µL blood was collected from each animal under general anesthesia by heart punctures in 1.5 mL EP tubes with 100 μL 1% heparin sodium saline. Subsequently, the animal was euthanized by anesthesia overdose. The spleen and cervical lymph nodes (LN) were harvested and placed in ice-cold Roswell Park Memorial Institute (RPMI) 1640. The maxilla was removed from each mouse. Gingival tissues surrounding the left maxillary molars were dissected from the left-side maxilla as previously described.26 Subsequently, the collected gingival tissue was homogenized in 1 mL phosphate-buffered saline (PBS) using a tissue homogenizer (Qiagen, Hilden, Germany) at 30 m/s for 3 min. Half of the homogenized gingival tissue was subjected to RNA isolation to determine the mRNA expression of cytokines by real-time quantitative polymerase chain reaction (RT-qPCR). The other half was used in enzyme-linked immunosorbent assay (ELISA). Both the left and right maxillae were fixed in paraformaldehyde for 24 h.
Alveolar Bone Loss Assessment by Micro-Computed Tomography (Micro-CT) Analysis
The paraformaldehyde-fixed left maxilla was scanned using a high‐resolution Micro-CT system (Siemens Medical Solutions USA, Inc., Malvern, PA, USA) at 8.89 μm voxel resolution using a source voltage of 60 kV and a current of 220 μA. The obtained images of teeth were rotated 360° along their long axes and one absorption image was recorded every 1° of rotation. Following scanning, the raw images were reconstructed into a three-dimensional (3D) image and the teeth were aligned and measured in 3D using the Inveon Research Workshop software (Siemens Medical Solutions USA, Inc.).
Linear measurement— Alveolar bone height loss as measured (in mm) from the alveolar bone crest (ABC) of the jaw to the cementoenamel junction (CEJ) on the tooth were obtained at six different sites (mesio-buccal, buccal, disto-buccal, mesio-palatal, palatal, and disto-palatal) of each molar by an independent, blinded investigator as previously described.27 The measurements were repeated three times per site and the mean values were obtained.
Volumetric measurement—Volumetric measurements followed the procedure reported by Park et al and Liu et al.28,29 Two-dimensional (2D) contours were drawn along the roots of the second molar using the landmarks of the most distal root of M1 (d-M1) and the most medial root of M3 (m-M3) from the roof of the furcation to the root apex at regular intervals (every five data planes), Subsequently, a 3D region of interest (ROI) was generated by the software based on the resultant 2D contours. The bone volume fraction (BVF) in the selected ROI images was assessed. The BVF value indicates the ratio of the residual bone volume to total volume. One independent, blinded observer analyzed all images three times, and then mean values were averaged.
RT-qPCR
Total RNA was extracted from homogenized gingival tissues using a TaKaRa Mini BEST Universal RNA Extraction Kit (TaKaRa, Kusatsu, Shiga, Japan) according to the manufacturer’s instructions. Isolated mRNA (0.5 μg each) was reverse transcribed into cDNA using the Prime Script™ RT Master Mix (TaKaRa, Kusatsu, Shiga, Japan). RT-qPCR was performed with a 10-μL reaction mixture using Power SYBR Green PCR Master Mix (Roche, Indianapolis, IN, USA) with a 7500 Real-Time PCR Detection System (Applied Biosystems, Foster City, CA, USA). The amplification conditions were as follows: 95°C for 10 min, followed by 40 cycles of 95°C for 15 s, 60°C for 60 s, and 72°C for 30 s. The primers are listed in Table 1. Results were assessed using the comparative 2ΔΔCt method and presented as fold changes relative to the value for the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) reference.
 |
Table 1 Primers and Sequences Used for Real-Time qPCR |
ELISA
The homogenized gingival tissues were centrifuged at 12,000 rpm and 4°C for 30 min to eliminate unground gingiva fragments. The supernatants were retrieved and kept at −80°C for subsequent ELISA analysis. The total protein level in the supernatants was determined using a BCA Protein Assay Kit (Thermo Fisher Scientific, Rockford, IL Campus, USA). The IL-1β, IL-10, tumor necrosis factor-α (TNF-α), osteoprotegerin (OPG), and RANKL protein concentrations in the supernatants were determined by ELISA following the methods as described in the ELISA kit (Beijing Qisong Biotech Company, Beijing, China) protocol. Results were standardized to the total amount of protein in the supernatant, and presented as the amount of cytokine per mg of total protein in the supernatant.
Tissue Histological Analysis
For each animal, the paraformaldehyde-fixed right maxilla was decalcified in 10% ethylenediaminetetraacetic acid (pH 7.4) for 1 month at 37°C with agitation. Specimens were embedded in paraffin and 4 μm sections were cut along the long axis of the molars. Sections were deparaffinized and rehydrated before staining.
Immunohistochemistry
Sections were incubated with 3% hydrogen peroxide for 20 min at room temperature to inactivate endogenous peroxidase, followed by heat-induced antigen retrieval in 10 mM sodium citrate buffer (pH 6.0) for 15 min at 62°C. The samples were blocked with 5% bovine serum albumin (BSA) for 30 min at room temperature, incubated with rabbit anti-mouse Abs against IL-1β, RANKL, or TNF-α overnight at 4°C, and were washed three times with PBS. All the Abs were from Abcam (Cambridge, England). A rabbit-specific immunohistochemistry kit (Zhong shan Golden Bridge Biotechnology, Beijing, China) and a DAB detection kit (Zhong shan Golden Bridge Biotechnology) were used to visualize the antigen location.
Tartrate-Resistant Acid Phosphatase (TRAP) Staining
Sections were stained using an acid phosphatase kit (Sigma-Aldrich, St Louis, MO, USA) according to the manufacturer’s instruction.
Images were captured on a light microscope (Olympus, Tokyo, Japan). A 4×3 mm2 area including the periodontal soft and hard tissues between the first and second molars was selected as the ROI. The number of cells positive for multinucleated TRAP along the alveolar bone surface and for RANKL, IL-1β or TNF-α in the ROI from each section was counted at ×200 magnification.
Flow Cytometry Analysis
Peripheral blood mononuclear cells (PBMC) were purified with Mouse Lymphocyte Separation Medium (Solarbio, Beijing, China), counted, and re-suspended in RPMI1640.
Spleen and cervical LN cells were collected by mashing the samples through a 70-µm cell strainer using the thumb-piece of a plunger removed from a 1-mL syringe. Red blood cells were lysed with 3 mL 1× Red Blood Cell Lysis Buffer (Biolegend, San Diego, CA, USA), incubated for 2 min at room temperature, and washed with PBS+2% fetal calf serum. Cells were filtered through another 70-µm cell strainer, counted, and re-suspended in RPMI1640.
For flow cytometry, 1×106 cells from each sample were stained with combinations of fluorescence-conjugated monoclonal Abs, including APC/Cyanine7 anti-mouse CD3, PerCP/Cyanine5.5 anti-mouse CD4, FITC anti-mouse CD8a, and APC anti-mouse CD19. All the Abs were from Biolegend. Samples were subjected to flow cytometry analysis using Beckman Coulter Gallios flow cytometer (Beckman Coulter, Inc., USA) and data were analyzed using FLOWJO (Tree Star, Inc., San Carlos, CA, USA).
Statistical Analysis
All data are presented as the mean and standard error of the mean (mean ± SEM). Statistical analyses were performed using one-way analysis of variance (ANOVA) and Turkey’s tests with GraphPad Prism 8 software (La Jolla, CA, USA). A value of P < 0.05 was considered to be statistically significant.
Results
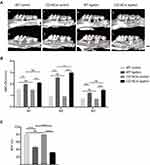
B-Cell Deficiency Exacerbated Alveolar Bone Loss in Mice with Ligature-Induced Periodontitis
Experimental periodontitis was established successfully 4 weeks after ligation, as indicated by the ligature-induced alveolar bone loss around the maxillary molars (Figure 1A). In the non-ligation groups, the ABC-CEJ distances and the BVF between d-M1 and m-M3 were comparable in the CD19Cre and WT groups (P > 0.05; Figure 1B), indicating that B-cell deficiency alone did not induce alveolar bone loss. In the ligation groups, varying degrees of alveolar bone loss around the three molars are induced four weeks after ligation of the second molar. For M2, the ABC-CEJ distances were significantly increased in the CD19Cre mice compared with the WT mice (3.00 ± 0.10 mm vs 2.56 ± 0.09 mm, respectively, P < 0.01). For M1 and M3, the ABC-CEJ distances were increased slightly in CD19Cre mice compared with the WT mice (2.12 ± 0.06 mm vs 1.99 ± 0.05 mm, P = 0.39; 1.48 ± 0.10 mm vs 1.24 ± 0.04 mm, P = 0.06; respectively). Additionally, the BVF was significantly smaller for the ligation group than for the non-ligation group (P < 0.01). And about 14.39% of the decrease in the BVF was observed in CD19Cre ligation group compared with that in the WT ligation group (31.98 ± 1.64% vs 46.37 ± 6.60%, P < 0.0001, Figure 1C).
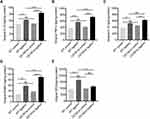
B-Cell Deficiency Promoted mRNA Expression of Th1 and Th17 Cytokines and RANKL in the Gingiva of Periodontitis Mice
RANKL and OPG, as well as inflammatory cytokines in gingiva were examined by RT-qPCR (Figure 2). In the non-ligation groups, the mRNA expression of the inflammatory cytokines IL-1β, IL-6, IL-8, IL-10, IL-12, IL-17, TNF-α, and gamma interferon (IFN-γ) were comparable, although the mean values were slightly higher in the CD19Cre group (P > 0.05). In the ligation groups, the mRNA expression of RANKL, OPG, and inflammatory cytokines increased significantly in the gingiva (P < 0.05). Notably, in the CD19Cre ligation group, IL-1β, IL-12, TNF-α, IFN-γ, and IL-17 mRNA expressions were significantly higher than in WT mice (1.48, 1.4, 1.38, 1.67, and 1.52-fold, respectively). However, no differences were observed in IL-4, IL-6, IL-10, IL-8, or TGF-β expression between the CD19Cre and WT groups (P > 0.05). Taken together, IL-1β, TNF-α, T helper type 1 (Th1) and Th17 cytokine mRNA expressions were selectively upregulated in the CD19Cre mice with periodontitis. Additionally, RANKL mRNA expression was significantly elevated in the CD19Cre ligation group compared with WT ligation control, whereas the OPG expression was decreased (P < 0.0001). This led to an increased RANKL/OPG mRNA ratio in the CD19Cre mice with ligature-induced periodontitis, which was nearly 2.32-fold higher than that in the WT periodontitis mice (P < 0.0001).
B-Cell Deficiency Elevated Th1 Cytokine and RANKL Levels in Gingiva in Periodontitis
IL-1β, TNF-α, IL-10, RANKL, and OPG quantification in the gingiva was performed using ELISA (Figure 3). Similar to the mRNA levels, for the non-ligation groups, inflammatory cytokine IL-1β and TNF-α levels were comparable. In the two ligation groups, IL-1β, TNF-α, IL-10, and RANKL were upregulated (P < 0.05). Notably, IL-1β, TNF-α, and RANKL were significantly increased and OPG decreased in the CD19Cre ligation group compared with WT ligation control (P < 0.05). There was no significant difference in the IL-10 level between the two ligation groups.
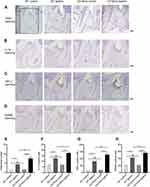
B-Cell Deficiency Increased TRAP (+), RANKL (+), IL-1β (+) and TNF-α (+) Cells in Periodontal Tissues in Periodontitis
In the non-ligation groups, TRAP-positive cells and RANKL-positive cells were rare. Few TRAP-positive cells were detected along the alveolar bone surface and RANKL-positive cells were scattered within periodontal tissues (Figure 4A and D). There were no significant differences in the numbers of such positive cells between the two non-ligation groups (P > 0.05). In the ligation groups, increased TRAP-positive cells could be identified in the bone resorption areas and a large number of RANKL-positive cells were located close to the bone and distributed in the inflammatory cell-infiltrated connective tissues. The numbers of TRAP-positive cells and RANKL-positive cells in the CD19Cre group were significantly higher than that in the WT group (7.50 ± 1.07 vs 4.38 ± 0.18, 55.88 ± 2.85 vs 42.38 ± 2.35, respectively, P < 0.01) (Figure 4E and H).
Similarly, in the non-ligation groups, few IL-1β-positive cells and TNF-α-positive cells were observed and the numbers of positive cells were comparable between the two non-ligation groups. The amounts of IL-1β positive cell and TNF-α positive cell significantly increased in the inflammatory connective tissue in the ligation groups, while they were detected more in the CD19Cre group than in the WT group (17.38 ± 1.13 vs 12.75 ± 0.59, P < 0.05; 128.4 ± 13.15 vs 80.5 ± 12.4, P < 0.01; respectively) (Figure 4B, C, F, and G).
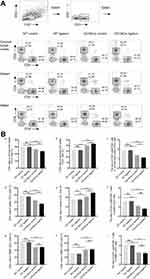
Decreased CD4+/CD8+ T-Cell Ratio Was Observed in the Cervical LN and Spleen and Among PBMC from CD19Cre Mice with Ligature-Induced Periodontitis
The percentage of CD3+ cells was increased in the cervical LN of the CD19Cre strain independent of whether the mice were ligated, whereas there were no significant changes of that in the spleen or among PBMC. In the cervical LN and spleen and among PBMC, the frequency of CD4+ T cells was lower and the frequency of CD8+ T cells higher in CD19Cre mice than in WT mice, which resulted in a lower CD4+/CD8+ T cell ratio in CD19Cre mice than that found in WT mice (P < 0.05). In WT mice, ligature-induced periodontitis did not change the frequencies of CD4+ T cells or CD8+ T cells in all the three types of samples. By contrast, in CD19Cre mice, ligation led to a significant reduction in the frequency of CD4+T cells and a significant increase in the frequency of CD8+T cells in the cervical LN but not in the spleen or among PBMC (Figure 5). Percentages of CD19+ B cells is reduced significantly in the cervical LN, spleen and among PBMC of the CD19Cre mice (less than 0.03%) compared with that in WT mice (Figure S1).
Discussion
The role of B cells in experimental periodontitis has been reported in several studies, although there were some contradictions. Klausen et al established a B cell-deficient rat model by anti-μ treatment administered by i.p. injections. They indicated that anti‐μ-treated rats displayed significantly less periodontal bone support than normal rats after inoculation with Actinomyces viscosus, Bacteroides gingivalis, and a strain of oral spirochetes, suggesting that B cells may play a protective role in the induction of periodontitis. However, this was a temporary B lymphocyte‐deficient model. At the termination of the anti‐μ treatment, a significant reduction was observed in B cell numbers in the spleen and inguinal LN of the anti‐μ-treated rats, while 3 months later, no difference was found between the anti‐μ-treated and control rats.23 While the B cell numbers and functions recovered with time, the degree thereof was indeterminable, which might affect the development of experimental periodontitis. In contrast, another study demonstrated that μMT mice were protected from P. gingivalis-induced alveolar bone loss.24 However, it should be noted that non-inoculated μMT mice already displayed obvious bone loss in the study, which was greater than that in the inoculated WT control.24 This restricted the comparability in the bone resorption between the μMT and WT mice. Moreover, local inoculation of human putative periodontal pathogen was selected to induce experimental periodontitis in the two aforementioned studies. Although this method could be employed effectively in exploring the role of human periodontitis-specific pathogens in the pathogenesis of periodontitis, it may be limited in several aspects, including: (1) the putative periodontal pathogen in humans and in mice are not identical; (2) the severity of periodontitis induced by different bacteria is different; (3) this model lacks the early stage of periodontitis (transformation of the microbiota); (4) bacteria-induced infection lacks chronicity and persistence, which is inconsistent with periodontitis characterized by chronic and persistent infection; (5) bacteria inoculated in the oral cavity may enter and affect the microbiota of the gastrointestinal tract to cause changes in the systemic immune status.30–32
In this study, we assessed the role of B cells in ligature-induced periodontitis using CD19Cre mice, a frequently used B cell-deficient transgenic mouse strain. CD19Cre mice have a deficiency in the B-1 subset together with a concomitant reduction in serum IgM. Their ability to respond to T-cell-dependent antigens was severely impaired, and they failed to form splenic germinal centers.33–35 Instead of bacteria smearing, as applied in previous studies, ligation was chosen to induce periodontitis in the present study. Placement of a ligature leads to the imbalance of the periodontal ecosystem, followed by dysbacteriosis and microulceration of the sulcular epithelium, which, in turn, facilitate the invasion of periodontal pathogens into connective tissue.30 This method is convenient and suitable for observing the disease progression of periodontitis for an extended time period. In the present study, ligature-induced periodontitis manifested significant and persistent inflammation for 4 weeks (till the end of the study). Furthermore, micro-CT scanning was applied in the present study for bone level assessment, which could overcome the limitations of the linear data provided by traditional 2D radiographic images and histomorphometry.28 Micro-CT is also superior in recognizing the subtle variations in bone loss (0.043 mm between the two periodontitis groups in the present study, and approximately 0.02 mm in a previous study24). Our results showed that the alveolar bone was stable in CD19Cre mice at baseline, and B-cell deficiency exacerbated alveolar bone loss in ligature-induced periodontitis mice (Figure 1).
To investigate the effects of B cells on adaptive immunity in periodontitis, Baker et al examined the alveolar bone loss of IgD-deficient mice with P. gingivalis-induced periodontitis and proved that IgD deficiency could protect mice from bone resorption.36 Because the binding of B cell membrane IgD to IgD receptors on CD4+ T cells facilitates B cell antigen presentation to CD4+ T cells, leading to coactivation of both B and T cells of the immune system,37,38 the results implied a destructive role of B cell-mediated adaptive immunity in periodontitis. In contrast, in our study, exacerbation of alveolar bone loss was observed in CD19Cre mice with periodontitis. Considering the fact that CD19 deficiency results in both compromised B cell-mediated innate and adaptive immune responses, it is speculated that B cell-mediated innate immune responses may play immune protective functions in the occurrence of experimental periodontitis.
It is widely accepted that the RANK/RANKL/OPG axis plays important roles in the regulation of the bone loss and remodeling. Evidence from clinical and preclinical studies of periodontitis indicate a high RANKL/OPG ratio as the primary determinant of osteolytic activity, whereas a low RANKL/OPG ratio is frequently observed in inactive lesions.39 Collectively, in this context, RANKL and OPG mRNA and protein expression were significantly higher, while RANKL/OPG ratio was lower after ligature placement. Similarly, histology showed upregulated RANKL level in mice with periodontitis. These findings closely resemble the observations made by previous studies.40–42 An upregulated mRNA level and protein levels of RANKL and downregulated levels of OPG were detected in CD19Cre ligation mice than in WT ligation mice. IHC also showed that B cell-deficiency enhanced RANKL-immunopositive staining in periodontal lesions. These changes led to higher RANKL/OPG ratio, subsequent increased osteoclast number and greater bone loss in CD19Cre ligation mice, which might be associated with the upregulated proinflammatory cytokines in the periodontal lesions.
IL-1β and TNF-α contribute substantially to the pathological periodontal alveolar bone resorption and collagen tissue destructions by affecting the activities of leukocytes, osteoclasts and collagenolytic enzyme MMPs.43–45 Consistent with previous studies,40,42 both IL-1β and TNF-α expressions were significantly higher after ligation. B cell-deficiency further upregulated the mRNA and protein expressions of IL-1β and TNF-α in gingiva, as well as immunopositive cells in periodontal lesions. Osteoclastogenesis and bone resorption could be promoted through up-regulations of RANK and RANKL as a consequence.
Proinflammatory cytokines secreted by Th1 and Th17 cells directly modulate RANKL/OPG expression, together with providing pro-osteoclastogenic support. Conversely, the cooperative action between Th2 and regulatory T (Treg) cells subsets creates an anti-inflammatory and preparative milieu associated with lesion stability.39,40 In our study, higher INF-γ, IL-12, and IL‐17 levels and a lower IL‐4 mRNA levels in the gingiva of CD19Cre periodontitis mice indicated the cytokine expression profiles of Th1 and Th17 responses. Through CD40-CD40L interaction, the antigen-activated B cells preferentially induce IL-4 synthesis, which favors the differentiation of naive CD4+ T cells into Th2 effector cells.46 Thus, B-cell deficiency in CD19Cre mice could impair the Th2 response.
One possible reason for the elevated proinflammatory cytokines and enhanced Th1 and Th17 responses might be the deficiency of protective B cell-subgroups in CD19Cre mice. Innate response activator (IRA)-B cells could recognize and clear bacterial infection in innate immunity depending on pattern recognition receptors and GM-CSF. Specific block of IRA-B cell activity impairs bacterial clearance and elicits a cytokine storm.47 Recently, regulatory B cells have been found to inhibit the differentiation of inflammatory Th1, Th17 and NKT cells and suppress immune-mediated inflammatory responses and through the production of different cytokines such as IL-10 and IL-35, TGF-β and through the cell contact-dependent suppressive mechanisms.9,16,48
Additionally, B-cell deficiency did not increase the RANKL level at baseline, even though the expression of proinflammatory cytokines in gingiva was upregulated slightly. This could be explained by the natural low‐affinity IgM and IgA antibodies in CD19Cre mice,33,34 which could prevent commensal flora from causing tissue destruction. Therefore, the CD19Cre mouse appears to be a suitable strain for investigating the contribution of B cells in experimental periodontitis.
Consistent with previous reports, CD19Cre mice displayed a decreased CD4+/CD8+ T cell ratio in the cervical LN and spleen and among the PBMC in the present study (Figure 5).24,36 This could be explained by the lack of B cell antigen-presentation and co-stimulation of CD4+ T cells. Compared to other antigen‐presenting cells (eg, Langerhans cells, macrophages, and dendritic cells), antigen presentation by B cells is specific and effective. They can recognize T cells in secondary lymphoid organs shortly after antigen entrance. B cell receptor (BCR)-mediated endocytosis allows them to concentrate small amounts of specific antigen.49
Conclusions
The present study demonstrated that B-cell deficiency exacerbated inflammation and alveolar bone loss in ligature-induced periodontitis in mice, indicating an overall protective role for B cells in the initiation of periodontitis. Our results will provide a better understanding of the immune pathogenesis mechanism of periodontitis and a foundation for future investigations of B cell-targeted therapies in periodontitis.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81470740).
Disclosure
All authors declare that there is no conflict of interest in this study.
References
1. Kinane DF. Causation and pathogenesis of periodontal disease. Periodontol. 2001;25(1):8–20. doi:10.1034/j.1600-0757.2001.22250102.x
2. Berglundh T, Donati M. Aspects of adaptive host response in periodontitis. J Clin Periodontol. 2005;32(Suppl 6):87–107. doi:10.1111/j.1600-051X.2005.00820.x
3. Cyster JG, Allen CDC. B cell responses: cell interaction dynamics and decisions. Cell. 2019;177(3):524–540. doi:10.1016/j.cell.2019.03.016
4. Coat J, Demoersman J, Beuzit S, et al. Anti-B lymphocyte immunotherapy is associated with improvement of periodontal status in subjects with rheumatoid arthritis. J Clin Periodontol. 2015;42(9):817–823. doi:10.1111/jcpe.12433
5. Kawai T, Matsuyama T, Hosokawa Y, et al. B and T lymphocytes are the primary sources of RANKL in the bone resorptive lesion of periodontal disease. Am J Pathol. 2006;169(3):987–998. doi:10.2353/ajpath.2006.060180
6. Jarry CR, Martinez EF, Peruzzo DC, et al. Expression of SOFAT by T- and B-lineage cells may contribute to bone loss. Mol Med Rep. 2016;13(5):4252–4258. doi:10.3892/mmr.2016.5045
7. Figueredo CM, Lira-Junior R, Love RM. T and B cells in periodontal disease: new functions in A complex scenario. Int J Mol Sci. 2019;20(16):3949. doi:10.3390/ijms20163949
8. Jansen K, Cevhertas L, Ma S, Satitsuksanoa P, Akdis M, van de Veen W. Regulatory B cells, A to Z. Allergy. 2021;76(9):2699–2715. doi:10.1111/all.14763
9. Chekol Abebe E, Asmamaw Dejenie T, Mengie Ayele T, Dagnew Baye N, Agegnehu Teshome A, Tilahun Muche Z. The role of regulatory B cells in health and diseases: a systematic review. J Inflamm Res. 2021;14:75–84. doi:10.2147/jir.S286426
10. Michaud D, Steward CR, Mirlekar B, Pylayeva-Gupta Y. Regulatory B cells in cancer. Immunol Rev. 2021;299(1):74–92. doi:10.1111/imr.12939
11. Beckett J, Hester J, Issa F, Shankar S. Regulatory B cells in transplantation: roadmaps to clinic. Transpl Int. 2020;33(11):1353–1368. doi:10.1111/tri.13706
12. Menon M, Hussell T, Ali Shuwa H. Regulatory B cells in respiratory health and diseases. Immunol Rev. 2021;299(1):61–73. doi:10.1111/imr.12941
13. Dasgupta S, Dasgupta S, Bandyopadhyay M. Regulatory B cells in infection, inflammation, and autoimmunity. Cell Immunol. 2020;352:104076. doi:10.1016/j.cellimm.2020.104076
14. Wang Y, Yu X, Lin J, et al. B10 cells alleviate periodontal bone loss in experimental periodontitis. Infect Immun. 2017;85(9). doi:10.1128/iai.00335-17
15. Yu P, Hu Y, Liu Z, et al. Local induction of b cell interleukin-10 competency alleviates inflammation and bone loss in ligature-induced experimental periodontitis in mice. Infect Immun. 2017;85(1). doi:10.1128/iai.00645-16
16. Gu Y, Han X. Toll-like receptor signaling and immune regulatory lymphocytes in periodontal disease. Int J Mol Sci. 2020;21(9):9. doi:10.3390/ijms21093329
17. Cao G, Zhang X, Song Y, et al. Local promotion of B10 function alleviates experimental periodontitis bone loss through antagonizing RANKL-expressing neutrophils. J Periodontol. 2021;92(6):907–920. doi:10.1002/jper.20-0074
18. Shi T, Jin Y, Miao Y, Wang Y, Zhou Y, Lin X. IL-10 secreting B cells regulate periodontal immune response during periodontitis. Odontology. 2020;108(3):350–357. doi:10.1007/s10266-019-00470-2
19. Han Y, Yu C, Yu Y, Bi L. CD25+ B cells produced IL-35 and alleviated local inflammation during experimental periodontitis. Oral Dis. 2021. doi:10.1111/odi.13939
20. Watanabe R, Fujimoto M, Ishiura N, et al. CD19 expression in B cells is important for suppression of contact hypersensitivity. Am J Pathol. 2007;171(2):560–570. doi:10.2353/ajpath.2007.061279
21. Mizoguchi A, Mizoguchi E, Takedatsu H, Blumberg RS, Bhan AK. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity. 2002;16(2):219–230. doi:10.1016/S1074-7613(02)00274-1
22. Mauri C, Gray D, Mushtaq N, Londei M. Prevention of arthritis by interleukin 10-producing B cells. J Exp Med. 2003;197(4):489–501. doi:10.1084/jem.20021293
23. Klausen B, Hougen HP, Fiehn N-E. Increased periodontal bone loss in temporarily B lymphocyte-deficient rats. J Periodontal Res. 1989;24(6):384–390. doi:10.1111/j.1600-0765.1989.tb00887.x
24. Oliver-Bell J, Butcher JP, Malcolm J, et al. Periodontitis in the absence of B cells and specific anti-bacterial antibody. Mol Oral Microbiol. 2015;30(2):160–169. doi:10.1111/omi.12082
25. Abe T, Hajishengallis G. Optimization of the ligature-induced periodontitis model in mice. J Immunol Methods. 2013;394(1–2):49–54. doi:10.1016/j.jim.2013.05.002
26. Mizraji G, Segev H, Wilensky A, Hovav A-H. Isolation, processing and analysis of murine gingival cells. J Vis Exp. 2013;(77):e50388. doi:10.3791/50388
27. Virto L, Haugen HJ, Fernández-Mateos P, et al. Melatonin expression in periodontitis and obesity: an experimental in-vivo investigation. J Periodontal Res. 2018;53(5):825–831. doi:10.1111/jre.12571
28. Park CH, Abramson ZR, Taba M
29. Liu YF, Wu LA, Wang J, Wen LY, Wang XJ. Micro-computerized tomography analysis of alveolar bone loss in ligature- and nicotine-induced experimental periodontitis in rats. J Periodontal Res. 2010;45(6):714–719. doi:10.1111/j.1600-0765.2010.01290.x
30. Graves DT, Kang J, Andriankaja O, Wada K, Rossa C
31. Guggenheim B, Gaegauf-Zollinger R, Hefti A, Burckhardt JJ. The effect of cyclosporin A on periodontal disease in rats monoassociated with Actinomyces viscosus Ny 1. J Periodontal Res. 1981;16(1):26–38. doi:10.1111/j.1600-0765.1981.tb00947.x
32. Baker PJ, Dixon M, Roopenian DC. Genetic control of susceptibility to Porphyromonas gingivalis-induced alveolar bone loss in mice. Infect Immun. 2000;68(10):5864–5868. doi:10.1128/iai.68.10.5864-5868.2000
33. Rickert RC, Roes J, Rajewsky K. B lymphocyte-specific, Cre-mediated mutagenesis in mice. Nucleic Acids Res. 1997;25(6):1317–1318. doi:10.1093/nar/25.6.1317
34. Rickert RC, Rajewsky K, Roes J. Impairment of T-cell-dependent B-cell responses and B-l cell development in CD19-deficient mice. Nature. 1995;376(6538):352–355. doi:10.1038/376352a0
35. Wang K, Wei G, Liu D. CD19: a biomarker for B cell development, lymphoma diagnosis and therapy. Exp Hematol Oncol. 2012;1(1):36. doi:10.1186/2162-3619-1-36
36. Baker PJ, Boutaugh NR, Tiffany M, Roopenian DC, Cell IgD B. Deletion prevents alveolar bone loss following murine oral infection. Interdiscip Perspect Infect Dis. 2009;2009:864359. doi:10.1155/2009/864359
37. Roes J, Rajewsky K. Immunoglobulin D (IgD)-deficient mice reveal an auxiliary receptor function for IgD in antigen-mediated recruitment of B cells. J Exp Med. 1993;177(1):45–55. doi:10.1084/jem.177.1.45
38. Lakshmi Tamma SM, Wu Y, Toporovsky I, Lima V, Coico RF. IgD receptor-mediated signal transduction in T cells. Cell Immunol. 2001;207(2):110–117. doi:10.1006/cimm.2000.1747
39. Cavalla F, Letra A, Silva RM, Garlet GP. Determinants of periodontal/periapical lesion stability and progression. J Dent Res. 2021;100(1):29–36. doi:10.1177/0022034520952341
40. Bi C-S, Sun L-J, Qu H-L, Chen F, Tian B-M, Chen F-M. The relationship between T-helper cell polarization and the RANKL/OPG ratio in gingival tissues from chronic periodontitis patients. Clin Exp Dent Res. 2019;5(4):377–388. doi:10.1002/cre2.192
41. Cafferata EA, Terraza-Aguirre C, Barrera R, et al. Interleukin-35 inhibits alveolar bone resorption by modulating the Th17/Treg imbalance during periodontitis. J Clin Periodontol. 2020;47(6):676–688. doi:10.1111/jcpe.13282
42. de Molon RS, Park CH, Jin Q, Sugai J, Cirelli JA. Characterization of ligature-induced experimental periodontitis. Microsc Res Tech. 2018;81(12):1412–1421. doi:10.1002/jemt.23101
43. Graves DT, Li J, Cochran DL. Inflammation and uncoupling as mechanisms of periodontal bone loss. J Dent Res. 2011;90(2):143–153. doi:10.1177/0022034510385236
44. Graves DT, Cochran D. The contribution of interleukin-1 and tumor necrosis factor to periodontal tissue destruction. J Periodontol. 2003;74(3):391–401. doi:10.1902/jop.2003.74.3.391
45. Cekici A, Kantarci A, Hasturk H, Van Dyke TE. Inflammatory and immune pathways in the pathogenesis of periodontal disease. Periodontology. 2014;64(1):57–80. doi:10.1111/prd.12002
46. Macaulay AE, DeKruyff RH, Goodnow CC, Umetsu DT. Antigen-specific B cells preferentially induce CD4+ T cells to produce IL-4. J Immunol. 1997;158(9):4171–4179.
47. Rauch PJ, Chudnovskiy A, Robbins CS, et al. Innate response activator B cells protect against microbial sepsis. Science. 2012;335(6068):597–601. doi:10.1126/science.1215173
48. Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. 2008;28(5):639–650. doi:10.1016/j.immuni.2008.03.017
49. Rodríguez-Pinto D. B cells as antigen presenting cells. Cell Immunol. 2005;238(2):67–75. doi:10.1016/j.cellimm.2006.02.005
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.