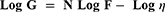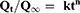Back to Journals » International Journal of Nanomedicine » Volume 17
Augmented in vitro and in vivo Profiles of Brimonidine Tartrate Using Gelatinized-Core Liposomes
Authors Abdel Azim EA, Elkheshen SA , Hathout RM , Fouly MA , El Hoffy NM
Received 10 April 2022
Accepted for publication 7 June 2022
Published 25 June 2022 Volume 2022:17 Pages 2753—2776
DOI https://doi.org/10.2147/IJN.S370192
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Anderson Oliveira Lobo
Engy A Abdel Azim,1 Seham A Elkheshen,2,* Rania M Hathout,3,* Marwa A Fouly,4 Nada M El Hoffy1
1Department of Pharmaceutics and Pharmaceutical Technology, Faculty of Pharmacy, Future University in Egypt, New Cairo, Cairo, 11835, Egypt; 2Department of Pharmaceutics and Industrial Pharmacy, Faculty of Pharmacy, Cairo University, Cairo, 11562, Egypt; 3Department of Pharmaceutics and Industrial Pharmacy, Faculty of Pharmacy, Ain Shams University, Cairo, Egypt; 4Research Institute of Ophthalmology, Giza, Cairo, 12557, Egypt
*These authors contributed equally to this work
Correspondence: Rania M Hathout, Department of Pharmaceutics and Industrial Pharmacy, Faculty of Pharmacy, Ain Shams University, African Union Organization Street, P.O. Box: 11566, Abbassia, Cairo, Egypt, Email [email protected]; [email protected]
Abstract:
Background: The low entrapment efficiency of the hydrophilic drugs such as brimonidine tartrate (BRT) in liposomes represents a challenge that requires interventions. Gelatinized core liposomes (GCLs) were fabricated to increase the drug entrapment, corneal penetration, and physical stability of the investigated molecule.
Research Design and Methods: GCLs encapsulating BRT were prepared and optimized utilizing D-optimal design (DOD). The effect of plasticizer incorporation on the physicochemical characteristics and on the in vivo performance was studied. The optimized formulations were investigated for pH, rheological properties, morphological characteristics, in vitro release profiles, biological performance, safety profile. The effects of storage and gamma sterilization were also investigated.
Results: The results revealed the great success of the prepared formulations to achieve high entrapment efficiency reaching 98% after a maturation period of 10 days. The addition of glycerol as plasticizer significantly minimized the particle size and shortened the maturation period to 7 days. The selected formulations were stable for 3 months after gamma sterilization. The formulations showed significant lowering of intra-ocular pressure (IOP) in glaucomatous rabbits with sustainment of the pharmacological effect for 24 hours compared to drug solution.
Conclusions: Enhanced in vitro and in vivo profiles of brimonidine tartrate loaded gelatinized-core-liposomes were obtained.
Keywords: gelatin, brimonidine tartrate, D-optimal design, glaucoma, gelatinized core liposomes, ocular drug delivery, plasticizer, intra-ocular pressure, BRT, IOP, DOD
Introduction
Normally, there is many obstacles facing drug-delivery to the ocular tissues due to the pioneer defense mechanisms of the eye anatomical structure and physiological performance.1 The blood aqueous barrier, corneal epithelium, retinal pigment epithelium, sclera and blood capillary endothelial cells, represent the eye’s static barriers, whereas the tear drainage, choroidal blood and lymphatic circulations2 and conjunctival blood and lymph clearance denote the dynamic ocular barriers. As a result, less than 5% of the injected dose normally reaches the eye interiors [12]. Recently, many advancements in the field of drug delivery systems finds its way for application in the ocular therapy to overcome these obstacles in order to improve patients’ compliance since the conventional pharmaceutical forms namely; solutions and suspensions, have numerous impediments,3 particularly in disorders like glaucoma.4
Vesicular systems are innovative means of delivering drugs in a controlled manner in order to enhance their bioavailability and prolong their therapeutic effects. Working to improve the drawbacks of liposomes as a poor vesicular carrier for hydrophilic molecules was the main goal for many researchers.5,6 In recent years, gelatinized core liposomes (GCL) offered a valuable modification for effectively delivering drugs of hydrophilic nature with better vesicular stability and higher entrapment efficiency compared to the conventional forms. Gelatin, being hydrophilic in nature with a thixotropic quality, can entrap efficiently the hydrophilic drugs and minimize their leakage in addition to extending their release profile.7 Moreover, gelatin is considered a cheap molecule compared to other synthetic analogues. This could encourage its transformation to the large scale production.8
Brimonidine tartrate (BRT) is a selective alpha 2-agonist9 exhibiting a unique ocular hypotensive effect which has a dual action mechanism due to its ability to decrease the aqueous humour production as well as increase the uveoscleral outflow.10 Its selectivity towards alpha-2 adrenergic receptors and its neuroprotective activity on retinal ganglion cells position it as an important therapeutic agent for the treatment of the open-angle glaucoma.11 Unlike beta adrenergic blockers such as timolol maleate, it is not contraindicated in patients with cardiopulmonary disease.10
The aim of the current work was to boost the use of BRT, being a water-soluble drug, in the topical treatment of glaucoma by enhancing its entrapment efficiency and corneal penetration through encapsulating the drug in gelatinized core liposomes. The GCLs were prepared by the thin-film hydration technique and the effect of the independent formulation factors on the dependent ones were studied using D-optimal design (DOD). The effect of incorporation of glycerin as a plasticizer was studied on both the maturation period of the prepared vesicles as well as on the particle size. After applying the desirability function, three selected formulae were further investigated for in vitro drug release, morphological examination using transmission electron microscopy, possible interactions using differential scanning calorimetry (DSC) and FTIR, rheological behavior and stability over a storage period of 3 months. The biological performance of the selected formulations was studied using glaucomatous Albino rabbits’ model where the intra-ocular pressure (IOP) reduction of the rabbits’ eyes was evaluated applying simple randomized parallel design. The safety profile was assessed using histopathological examinations. Moreover, Draize test was performed to evaluate the irritancy effect of the tested formulations.
Materials and Methods
Materials
BRT was a gift from Holdipharma (Cairo, Egypt). L-α Phosphatidylcholine from dried egg yolk, Type X-E ≥40% (enzymatic), gelatin A bloom gel strength-300g from porcine skin, cholesterol from sheep wool, phosphate buffered saline tablets and the dialysis membrane (12,000–14,000 molecular weight cut-off) were purchased from Sigma-Aldrich (Saint Louis, USA). Ethanol and Glycerol were purchased from El-Nasr Pharmaceutical Co. (Cairo, Egypt). Proparacaine HCl, betamethasone suspension, glacial acetic acid, ethyl alcohol (95% v/v) and neutral buffered formalin (10% v/v) were purchased from Holdipharma (Cairo, Egypt).
Methodology
Preparation of the Gelatinized Core Liposomes (GCL) Applying the D-Optimal Design
Experimental Design
The DOD was utilized for devising the formulae to study the effect of different formulation variables on the properties of the obtained gelatinized core liposomes as well as for optimization procedure. Three independent formulation variables were evaluated, namely: 1) the amount of gelatin (Gel) in mg per formula; 2) the volume of organic solvent in mL (Org) used for dissolving phospholipids; and 3) The percentage of phosphatidylcholine (PC%) per the total lipids weight (200 mg; of phosphatidylcholine (PC%) + cholesterol (CHL). The corresponding levels (3 levels for each variable) were selected based on previous researches12,13) .Twelve formulations were devised by the Design© Expert v. 9 software (Stat-Ease Inc., Minneapolis, MN) applying the DOD after applying the point exchange technique.13 Table 1 presents the composition of the prepared formulations with and without the incorporation of 1% glycerol as a plasticizer together with its role in adjusting the osmotic pressure across the liposomal walls.14
 |
Table 1 The Effect of the Independent Factors on the Characteristics of the GCLs |
Preparation of Gelatinized Core Liposomes
Thin film hydration method was adopted for the preparation of the GCLs12 compounding different amounts of phospholipid/cholesterol, gelatin and organic solvents according to the previously devised set of formulations. Briefly, the total amount of lipids, equivalent to 200 mg, was dissolved in an accurate amount of ethanol. The dispersion was introduced into a rotary evaporating flask, thermostated at 40℃. The flask rotated at 150 rpm for 30 minutes until a thin film was obtained on the walls of the flask. The flask was kept in the rotary evaporator for another half an hour to ensure the complete removal of the organic solvent. Meanwhile, gelatin was dispersed in 10 mL of an isotonic phosphate buffer of the drug (containing 10 mg drug) and kept at 40℃. The prepared aqueous solution was utilized to rehydrate the obtained thin film by addition portion wise with tilting the flask. At the end, the liposomal dispersion was kept for 1 hour in the rotary evaporator’s water bath at 40℃. Finally, glycerol was added to the GLG 1–12 set of liposomal dispersion, to adjust the osmotic pressure across the phospholipid liposomal membrane. All formulations were kept at 4℃ for 7 to 10 days until complete maturation of the developed vesicles after preliminary examination of the required period for reaching constant particle size, PDI and Zeta potential.
Characterization of the Prepared Gelatinized Core Liposomes
Determination of Particle Size, PDI and Zeta Potential
The particle size and PDI of the prepared vesicles were determined applying the dynamic light scattering (DLS) technique using Malvern-ZEN® 3600 ZetaSizer (UK). Measurements were conducted in triplicates at 25℃. The PDI was determined by the same apparatus as a dimensionless number indicating the width and uniformity of the size distribution, where PDI less than 0.4 indicates a homogenous monodisperse population.15
Zeta potential was determined using the laser-Doppler velocimetry technique using ZetaSizer. Samples were prepared by dilution of 0.1 mL of the prepared formulation with 50 mL deionized water followed by shaking for 1 minute16 prior to measurement. Results were the average of three determinations.
Determination of the Entrapment Efficiency (EE%) in the Prepared Gelatinized Core Liposomes
The EE% of BRT was calculated by measuring the concentration of the un-entrapped drug in the supernatant after centrifuging the vesicular dispersion and the separation of the precipitated vesicular pellets.12,17 Briefly, 1 mL of the prepared Gel was centrifugated at 4℃ in a cooling high speed centrifuge at 19,000 g for 1 hour. This was followed by decantation of the supernatant and determining the amount of the free drug spectrophotometrically at 249 nm. The percent entrapment efficiency was calculated applying the following equation:
where Wt is the total amount of the drug in the formulation and Wf is the amount of the free drug in the supernatant.12
Selecting Formulations for Further Investigations Using a Statistical Desirability Function
The desirability function of the Design Expert® software was utilized to select the formulations for further investigations. The targeted criteria were to both maximize and minimize the particle size (one-at-a-time) in order to compare the effect of the particle size in the micrometer range versus the nanometer range on the in vivo performance of the prepared formulations. Furthermore, the formulae were also selected to satisfy some other criteria including the incorporation of both high and low concentration of gelatin being a viscosity inducer and hence expected to affect the ocular retention time and hence the ocular availability. Moreover, the effect of the presence and absence of glycerol in the formulations on their biological performance (ocular availability of the drug and irritancy of the formula) was also investigated.
In vitro Characterization of the Selected Formulations
The selected formulations meeting the target characteristics were subjected to further investigations in terms of their rheological properties, morphological characteristics, possible interactions between their components using DSC and FTIR, in vitro drug release and kinetics and stability towards sterilization using Gamma irradiations as well as sterility testing.
pH Determination of the Selected Gelatinized Core Liposomes
The pH meter (model 3510, Jenway, UK) was calibrated before usage utilizing standard pH 4, 7 and 9.2 buffer solutions.18 The electrode was immersed in the sample solution (10 mL) without dilution until a steady reading was reached and recorded.
Determination of the Rheological Behavior of the Selected Gelatinized Core Liposomes
The viscosity of the selected formulations was measured at 25 ℃ ± 0.2 using cone and plate computer interfaced rheometer fitted with the spindle CS-40 (Brookfield Model: DV3T, USA).19 Simply 1 mL of the formulations was placed on the tray of a plate and cone apparatus with 20 mm diameter/4° angle with a fixed shear rate (sec−1). The running temperature was 25℃ ± 0.2, the cone rotation speed ranged between 10 rpm and 1000 rpm on the up curves and down with the same speed rate. The rheological behavior of each formula was evaluated according to Farrow’s equation by plotting the values of the shear stress versus shear rate.:
Morphological Examination of the Selected Gelatinized Core Liposomes
The morphological characteristics of the selected formulations were examined using transmission electron microscope (TEM) (H-7500, Hitachi, Japan) operating at an accelerating voltage of 80 kV.20,21 Carefully, a drop of each of the vesicular dispersion was placed on a carbon-coated copper grid and left for 2 minutes to adhere to the carbon substrate. A drop of 2% w/v solution of phosphotungstic acid was spread onto the carbon grids and samples were left to dry in the air. The obtained film of stained vesicles was examined at a magnification of 50× to 200,000×.
Differential Scanning Calorimetry Studies
DSC (DSC-50, Shimadzu, Japan) was carried out for the dehydrated vesicles; freeze dried (Christ ALPHA 2–4 LD plus, Germany). DSC thermograms for the individual components namely, phosphatidyl choline, cholesterol, gelatin, the drug as well as their physical mixture and the freeze-dried selected formulations were performed. Briefly, an accurately weighed amount of the sample was placed into an aluminum pan and analyzed against an empty aluminum pan as reference. The process was operated at a heating rate of 10℃/min and over a temperature range from 20 to 300℃ under inert nitrogen flow of 25 mL/min.
Fourier Transform Infrared Spectroscopy (FTIR)
The FTIR spectra were recorded for BRT, phosphatidyl choline, cholesterol, gelatin, their physical mixtures, and the selected formulae using FTIR spectrophotometer (Model 22, Bruker, UK) applying the KBr disk technique. The FTIR measurements were performed in the scanning range of 4000–40℃ m−1 at ambient temperature.
Drug Release and Kinetic Model Studies
In vitro drug release studies of BRT from the selected formulations and their corresponding vesicle-free analogues containing the same concentrations of both the drug and gelatin were carried out using the dialysis bag method, where a cellulose membrane dialysis bag (6 cm long, 2.2 cm wide, molecular weight cut off of 12–14,000 Daltons) was used as the donor compartment22 after soaking in a buffer of pH 7.4 for 24 hours. Exactly, 1 mL BRT GCL was injected into the dialysis bag, which was fastened from both sides and folded and fastened once again to have both ends together. The assembly was immersed in 50 mL isotonic phosphate buffer of pH 7.4 adjusted at 35 ℃± 0.5 ℃, to simulate the normal eye temperature,23 in an incubator shaker (IKA, KS4000IC, Staufen, Germany) working at a rate of 100 stroke per minute. At pre-determined time intervals, samples of 3 mL from the release medium were withdrawn and replaced with equal volume of fresh receptor medium. Samples were analyzed for the drug content spectrophotometrically at a pre-determined wavelength of 249nm.24
The percentage of the cumulative drug release was calculated using a previously developed standard curve of the drug concentration versus absorbance at the same wave length. The mean percentage of the cumulative drug release was plotted against time and consequently the release data were kinetically analyzed using different kinetic models to determine the mechanism of drug release from the selected formulations. The release parameters were calculated to compare between the investigated formulations. They involved: 1) The release half-life (T50); the time required for the release of half the initial drug dose from the prepared formulation; and 2) Mean dissolution time (MDT); the arithmetic mean value of the release profiles which describes the drug release rate and reflects the retarding ability of the formulation for the drug release.25
The kinetics of BRT release from GCL was determined using linear regression analysis in order to find the best fit of the release data to equations representing: zero, 1st and Higuchi diffusion models and results were confirmed by applying Korsmeyer-Peppas equation as follows26–28
where, Qt/Q∞ is the fraction of drug released at time t, k is a constant incorporating structural and geometric characteristics of the controlled release device and n is the release exponent and is indicative of the mechanism of drug release.29 The kinetic parameters (k and n) were calculated from the plot of log (Qt/Q∞) versus log t where, (Qt/Q∞) is less than 0.6 as this model is valid only for early stages of drug release (≤60%).29
Effect of Storage on the Physicochemical Characteristics of the Selected Gelatinized Core Liposomes
The physical stability of the selected formulations GLG1, GLG4 and GL12 were monitored over a period of 3-months storage under refrigeration at a temperature of 2–8℃. The PS, PDI, Zeta potential and EE% were measured after the specified period and compared statistically to the initial values of the freshly prepared formulations. All data are presented as the mean and standard deviations of three separate determinations.
Sterilization Study of the Selected Gelatinized Core Liposomes and Sterility Testing
Sterilization by Gamma Irradiations and Re-Evaluation
The selected formulations received a radiation dose of 10 Kgy for the sterilization purposes30 using cobalt-60 gamma chamber 4000-A (National Centre for Radiation Research and technology (NCRRT) in the Egyptian Atomic Energy Authority, Cairo, Egypt). Re-evaluation of the particle size, PDI, Zeta potential and EE% of the sterilized formulations was performed applying the same procedures as described previously and compared statistically to the initial values of the freshly prepared formulations.
Sterility Test
Following sterilization by gamma irradiation, the sterility testing was performed implementing the test tube method followed by the pour plate method. For the test tube method, two media were used, namely; fluid thioglycollate media (FTM) which supports bacterial growth and trypticase soy broth (TSB) media which supports the growth of fungi. Exactly 1 mL from each selected formulation was transferred under aseptic conditions to a previously sterilized test tube containing 5 mL of either of the previously mentioned media then were tightly closed. All tubes were incubated (TITANOX s.r.j. Fabbrica Artticoli Medico Sanitari, Italy) for 14 days at 37℃ or at 25℃ for FTM and TSB, respectively.31 For comparison, negative and positive controls were prepared for both thioglycollate media and TSB media. The positive control was inoculated with Staphylococcus aureus for FTM and Candida albicans for TSB to assure that the media contain the essential requirements and can promote the growth of the microorganisms. On the other hand, the negative controls were the media with only the nutrients incubated under the same conditions to make sure that they maintained sterility during the preparation procedure.32,33 After 14 days of incubation, 1 mL from each tube was transferred to brain heart agar media plate and trypticase soya agar media plate and incubated for 24 h at 37℃ and for 72 h at 25℃, respectively for the above mentioned media to detect the presence of any bacterial or fungal growth.34
In vivo Evaluation of the Selected Formulations
Selected formulations, namely, GLG1, GLG4 and GL12, were evaluated regarding their biological performance regarding their IOP lowering capacity in glaucomatous Albino rabbits’ eyes as well as their irritancy and pathological effect in comparison with the drug solution in isotonic buffer.
Protocol
The protocol of the animal studies was approved by the research ethics committee for experimental and clinical studies of the faculty of Pharmacy at Future University in Egypt; Serial No.: REC-FPSPI-11/79 on 25/7/2018 and in accordance with use of Laboratory Animals of the National Institutes of Health (NIH Publications No. 80–23, revised 2011).
Evaluation of the Ocular Pressure Lowering Capacity of the Selected Formulations
Animals
Sixteen male New Zealand rabbits (mean body weight of 3± 0.25 Kg) were randomly distributed into four groups; four animals per each. Rabbits were kept in individual cages in controlled room under standardized conditions of humidity, temperature- and light cycles of 12-hrs of light and 12-hrs of dark. Rabbits were fed with a standard rabbit diet accompanied by free access to water. The rabbits’ eyes were examined for ocular abnormalities on external and slit-lamp prior to the beginning of the experiment. At the end of the study, rabbits were sacrificed through an intramuscular injection of a high dose of an anesthetic (ketamine and xylazine). The bodies were frozen at −20 ℃ till incineration in the Research Institute of Ophthalmology in Giza, which was responsible for getting rid of the corps.
Study Design
Selected formulations (GLG1, GLG4, and GL12) were compared to a drug solution in isotonic phosphate buffer with equivalent drug concentration (1 mg/mL). The study was performed using a simple randomized parallel group design where four rabbits were randomly assigned to each of the four treatment groups (A, B, C and D). Group A received GLG1 formulation, Group B received GLG4 formulation, Group C received GL12 formulation and Group D received the drug solution.
The Intra-Ocular Pressure Measurements
The procedure to establish an animal model with a raised IOP using steroids in suspension was adopted as described by Melena et al 1998.35 The rabbits were injected weekly for two weeks with a sub-conjunctival dose of 0.7 mL per each of the right and left eyes with a mixture of betamethasone sodium phosphate and betamethasone acetate (3 mg/mL) each. This combination provides a readily available sodium phosphate and a slow released acetate fraction of betamethasone. In details, 1–2 drops of 5% proparacaine HCl was instilled to each eye to induce local anesthesia. After few minutes, betamethasone suspension was injected in the sub-conjunctiva of the rabbits’ eyes. A mild pressure was applied on the eye for a short period of time. To avoid diurnal variation of IOP, the measurements were started at the same time of the day (9:00 am) throughout the observational period of two weeks. The first measurement was taken immediately before injecting the betamethasone suspension then was recorded twice a week after injection for two weeks where four measurements of the IOP were taken during this period. Animals exhibiting fluctuations >5mm Hg in their IOP and/or not reaching the required starting IOP value (37.2 mmHg) were excluded from the study with multi-measurements (n = 5). The rabbits were held in specially designed wooden boxes during the measurements.
Two weeks after glaucoma induction; one drop (0.1 mL) of the formulations (equivalent to 1 mgBRT/mL) was installed in the right eye of each animal, followed by fluorescein in order to stain the cornea. Afterwards, measuring the IOP was performed using a standardized Schiotz® tonometer (Model AEI-23, Ajanta, India) as described by Shields in 198736 in both eyes at 1, 2, 4, 6, 8, 12 and 24 hours. The pressure in the left eye (the untreated one) was considered as a base line for the treated right eye at each time interval.
Eye Irritancy Test (Modified Draize Test)
The aim of the current test was to determine whether the investigated formulations (GLG1, GLG4 and GL12 versus drug solution) would pose any kind of irritation to the rabbits’ eyes as an indicator of similar effect on the humans’ eyes. For this test, 8 male New Zealand healthy rabbits of age 4–5-month and weight of 2 ± 0.5 kg were used. The normal eye tissues were examined prior to the beginning of the experiment to exclude any animal showing even minor irritation signs. Rabbits were hosted individually in separate cages to guard against accidental injuries with temperature and humidity not exciding 21℃ and 60%, respectively. They were fed on conventional laboratory diets with free access to drinking water. Exactly, 0.1 mL of each of the selected formulations was instilled into the lower conjunctival cul-de-sac of the right eye. The left eye was kept as a control for comparison. The eyelids were held together for several seconds after dose application followed by allowing free blinking. Pre- and post-insertion examination was performed by external observation under proper illumination. The evaluations were performed at 1, 24, 48, 72 h (if reactions occur the eyes should be observed for 21 days to make sure that the reactions are reversible). The ocular changes were graded by a scoring system that includes rating any alterations to the cornea, iris and conjunctiva as a simplified scoring system of that described by Draize et al.37–39
Histopathological Study
To complete the assessment of the adverse effect of the selected GCLs on the tissues of the rabbits’ eyes, the histopathological effect of the three formulations namely; GLG1, GLG4 and GL12 in reference to the drug solution was investigated. The corneal, retinal, filtration apparatus and choroidal tissues of the right treated eyes of the rabbits were examined for the histopathological changes after the administration of the selected formulations as well as the drug solution as one drop every 12 hours for 7 days using microscopical examination. Tissue specimens from the left untreated eyes of the rabbits were examined for normal histological structure for comparison.
Briefly, following slaughter, the rabbits’ eyes were instantly immersed after trimming in Davidson’s solution. Following enucleating and trimming, eyes were kept submerged by means of a gauze. The globe remained in the solution for two days while the eyes were then taken out of the solution and placed in 10% formalin. The specimens were trimmed off, rinsed and dehydrated in alcohol of increasing concentrations. The dehydrated specimens were then washed in xylene, inserted into paraffin slabs and segmented at 4–6 µm thick. The obtained tissue sections were de-paraffinized using xylol and stained utilizing hematoxylin and eosin (H&E) followed by using the electric light microscope for histopathological examination.38
Statistical Analysis
All data were presented as mean ± standard deviation. One-way ANOVA was performed using GraphPad Prism 7.0 ® software (GraphPad Software, San-Diego, CA) to assess the effects of the storage for three months and the sterilization by gamma irradiation on the vesicular characteristics (particle size, PDI, Zeta potential and encapsulation efficiency) as well as for comparing the pharmacodynamic parameters of the selected formulations followed by the least significant difference (LSD) test. Design-Expert® software (version 9; Stat-Ease, Inc., Minneapolis, MN) was adapted for analysing the results of the DOD experiments to investigation the effect of formulation variables on the particle size, PDI, Zeta potential and encapsulation efficiency, followed by ANOVA to test the significance and separate the effects of the independent variables. The statistical significance level was set at p-value ≤ 0.05 in all experiments.
Results
Preparation and Optimization of Gelatinized Core Liposomes Applying D-Optimal Design
The Preparation of the Gelatinized Core Liposomes
The prepared GCLs exhibited a dispersion of milky white appearance that undergo typical sol-gel-sol transformation upon changing the temperature between refrigeration and room temperature and/or agitation which is a logical consequence to the presence of free unentrapped gelatin.
The GCLs with or without the addition of glycerol required an average of 7 to 10 days, to reach maturation in terms of particle size (Figure S1A and B), PDI (Figure S1C and D) and Zeta potential (Figure S1E and F) while the final stable particle size, PDI and Zeta potentials are presented in Table 1.
The time required for maturation may be attributed to the presence of gelatin-A, which acquires a positive charge at pH 7.4 that increases its affinity to the liposomes’ phospholipids being negatively charged. This affects the mutual interaction between the oppositely charged components. This fact also affects the incorporation of gelatin during the assembly of the bilayers of the newly developed vesicles both inside their aqueous core and on their surfaces, which might require an equilibration period for complete formation of the vesicular structure. In other words, the presence of gelatin may have retarded the final assembly of the vesicles, thus prolonged the maturation of the vesicles.
As a result of the unstable PS that matures over 7 to 10 days, the PDI values also presented a fluctuated pattern throughout the maturation period until it reached stable state after maturation. It is clear that the instability of the values of the PDI was in direct agreement with the instability of the vesicles size during the formation and maturation period where molecules of the components of the gelatinized-core vesicles were in dynamic movement exchanging between the dispersion medium and the vesicular structure; both their core and wall. Only when the vesicles reached their mature status the PDI reached its minimum values with the stability of the particle size.
It was clear that the net charges on the surface of the vesicles were a function of the components of the vesicles which have different charges namely; gelatin possessing a positive charge, phosphatidylcholine possessing a negative charge and the drug may pose a possibility of developing positive poles on the ternary amine group specially under the influence of glycerol, being a polyol, and can act as a polarizing agent.
Response Surface Analysis of the Characteristics of the Gelatinized Core Liposomes
Effect of Formulation Variables on Particle Size, Polydispersity Index, and Zeta Potential of the Mature Gelatinized Core Liposomes
Response surface methodology (RSM) using the DOD was adopted to study the effect of the independent variables on the particle size, PDI and Zeta potential of the prepared GCLs. Model regression analysis for the mature vesicles were developed and the significance of the effect of independent variables and their interactions over the responses was tested applying ANOVA.
The model parameters for the influence of formulations variables on the particle size for the glycerol-free formulations (GLs); GL1:GL12 and glycerol containing ones; GLG1:GLG12 are presented in Table 2. The response surfaces of the particle size for both sets were constructed as shown in Figure 1A and B). Furthermore, investigation of the particle size models with respect to a required power transformation were confirmed though constructing the Box Cox plot for both the glycerol free as well as the glycerol containing formulae as presented in Figure 1C and D).
 |
Table 2 Model Summary Statistics for the Influence of Formulations Variables on the Particle Size of the GCLs |
Results revealed the significance of a quadratic models adopted for the influence of formulation factors on the particle size of the obtained GLs formulae (p = 0.0057) as well as GLGs formulae (p = 0.0022). Models were significant and highly fitting the results with close values of both R2 and adjusted R2 of 0.9987 and 0.9930 for GLs formulae, respectively and 1.000 and 1.000 for the GLGs formulae, respectively. Furthermore, high correlation between the experimental and predicted results were observed for the generated D-optimal models as confirmed by their high predicted R2 confirming the capability of the models to forecast the un-carried experiments within the design area.13 In addition, the adequate precision values of the two models, which indicate the signal-to-noise ratio, were 50.107 and 1145.508 for GLs and GLGs, respectively. Usually, values above 4 assures sensitivity and adequacy of the models to navigate the experimental space.13,40
The Box–Cox transformation searches for an appropriate exponent (lambda) in order to determines whether the response needs to be measured on a different scale or not to improve the skewed variables and create a model equation that best fit the data.41,42 The selected model for the GLs was effective with no need for further data transformation, where the current lambda (1.00) laid within the 95% confidence interval of the best lambda value (0.93). On the other hand, the generated model for the glycerol-containing formulations needed further transformation, where the current lambda was superimposed with the best lambda (lambda = 0.2) as shown in Figure 1C and D) and Table 2.
The results of the analysis of variance (ANOVA) for the significance of different factors, factors’ interactions as well as their quadratic level on the particle size of the glycerol free formulae showed that all the independent factors namely; gelatin mass (A), volume of organic solvent (B) and phosphatidyl choline concentration (C), had significant (p = 0.0015, p = 0.0361 and p = 0.0059, respectively) effects on the particle size of the obtained GCLs with the most significant effect being due to the mass of the added gelatin. Additionally, the factor combination; AB as well as the quadratic levels of the three factors; A2, B2 and C2 significantly (p = 0.0216, p = 0.0067, p = 0.0069 and p = 0.0170, respectively) affected the PS of GLs.
The significant increase in the particle size of the glycerol free formulations upon increasing gelatin concentration at all levels of PC concentration and organic solvent volume (p = 0.0015, Table 2 and Figure 1A may be due to the fact that gelatin A acquires a positive charge at physiological pH which augments its affinity to the phospholipids of the liposome bilayer, being negatively charged, and therefore favor its incorporation both inside the liposomal core and its wall. Nevertheless, the excess amount of gelatin in the dispersion medium can also increase the particle size by bridging the dispersed particles leading to aggregation as observed in GL12 which contains the highest amount of gelatin that contributes to a relatively large particle size of 1308.68 ± 125.33 nm. Similarly, it was reported that increasing the gelatin mass increased the particle size of the GCLs prepared for the delivery of timolol maleate.12
Concerning the effect of the organic solvent, it showed negative coefficient in the response surface equation, indicating that increasing the volume of the organic solvent leads to significant (p = 0.0361) reduction in particle size. This could be explained on the basis of more spread of the PC/cholesterol dispersion during the rotary-evaporation, developing a thinner film which allows the formation of larger number of vesicles with smaller particle size during the hydration process. Furthermore, the decrease in the amount of organic solvent with an increase in the lipid amount may have resulted in less dispersion of the lipids, formation of thicker film, less efficient hydration with the aqueous phase and hence producing vesicles of larger particle size at different levels of the other factors namely; PC% and gelatin amount. On the other hand, at the high level of gelatin, increasing the organic solvent led to a significant increase in particle size as reflected by the significant interaction; AB. The better dispersion of the lipids and the magnified surface area of the produced film as a result of increasing the volume of the organic solvent may have allowed for better interaction between the positively charged gelatin-A and the negatively charged lipids upon hydration leading to the incorporation of larger amounts of gelatin into the aqueous compartment of the liposomes. Consequently, higher volume core was obtained that pressurized the thin walls of the liposomes leading to vesicles with larger particle sizes.
The concentration of phosphatidylcholine with a negative coefficient in the model equation had a significant (p = 0.0059) decreasing effect on the particle size of the obtained vesicles. This can be explained on the basis that PC produces more or less a flexible bilayer wall that allows the spontaneous subdivision of the vesicles yielding smaller liposomes where its counterpart of the lipid content; cholesterol is responsible for rigidifying the liposomal wall and hence stabilizing them. Needless to say, that increasing the PC concentration will be deducted from the amount of cholesterol (total lipid amount = 200 mg).
On the other hand, increasing the cholesterol concentration on behalf of PC may explain the increase in particle size of the vesicles as shown with formulations, GL3, GL7, GL11 and GL12. The presence of higher concentration of CHL rigidifies the vesicles wall preventing their subdivision into smaller size. It is well reported that the amount of the incorporated CHL in the vesicular structure significantly affects numerous characteristics of the membrane including elasticity, stability, permeability to ions, fluidity, aggregation, shape and size.43 It increases the rigidity of the bilayer through increasing the chain order of lipids’ bilayers and decreasing their phase transition temperature as well.44 It was previously reported that the effect of cholesterol on the particle size as well as the encapsulation capacity of liposomes varied with the manufacturing conditions and/or the properties of the incorporated polymer as gelatin.12 These results are also in line with that reported by other researchers.45,46
Furthermore, it was evident that the addition of glycerol to the formulations GLG1 though GLG12 significantly (p = <0.0001) reduced the particle size reaching the nanometer range. This may be due to the incorporation of glycerol in the vesicles’ bilayers, hence increasing its deformability being a small polyol alcohol which in turn facilitates the vesicles’ subdivision ability. It was reported that different components of liposomes can affect the final particle size.47 It was also reported that the final size of the spontaneously formed liposomes after reconstitution in aqueous solution is function of their thermodynamic stability, which is highly affected by the polar group that stabilize them through the formation of H-bonds with the surrounding medium. Water, the natural stabilizing compound of the formed liposomes, can be replaced by the polyol, glycerol in the hydrogen bond formation. This interaction allows glycerol to penetrate the bilayer, relaxing the membrane tensions and lowering the surface free energy consequently support the formation of smaller size liposomes48–50Finally, being a cryoprotectant,51 glycerol would prevent the aggregation and the fusion of the liposomes during formation which contributes to the smaller particle size as well as the shorter maturation period of the glycerol containing formulations.
The ANOVA analysis of the glycerol containing formulations, GLG1:GLG12, showed that all the independent factors, A, B and C were significant with F values of 143500 (p = 0.0017), 173600 (p = 0.0015) and 12663.37 (p = 0.0057), respectively besides their quadratic parameters; A2, B2 and C2 in the model equation as shown in Table 2 and the 3D response surface Figure 1B and for the same reasons as discussed previously.
Furthermore, the combinations of the two factors namely; the gelatin mass and the volume of the organic solvent (AB) interact antagonistically over the vesicular particle size while the organic solvent volume and phosphatidylcholine concentration % (BC) interacted synergistically as reflected by their negative and the positive coefficients in the response surface equation, respectively Table 2. As previously explained, the decrease in the amount of organic solvent accompanied by an increase in the lipid amount lead to less dispersion of the lipids and less surface area of the produced thin film. Consequently, the less efficient hydration with the aqueous phase yields vesicles of larger particle size. This coincides with the increase in gelatin amount that exerts its size increasing effect either by increasing the core volume due to increasing the viscosity or by exerting aggregation effect.
Model-analysis as well as ANOVA revealed insignificance of the predicted models for both the PDI and zeta potential of the plasticizer-modified and non-modified groups of formulations and showed that the overall mean is a better predictor of the responses than the constructed models. Never the less, it was clear that their was some what agreement exist between the PS and the PDI. As the particle size for the mature vesicles increases they also show higher PDI values.
However the model of zeta potential was not significant, it was obvious that all formulae lacking glycerol turned from positively charged vesicles to negatively charged ones after maturation. This might prove the stability of the vesicular structure with the negative charge being imparted by the bilayer of the negatively charged phospholipid, where more rigid wall incorporating most of the gelatin, with its positive charge, inside the aqueous core of the vesicle as confirmed by the larger particle size of those vesicles compared to the GLGs formulations. On the other hand, the glycerol containing formulations remained positively charged after maturation which may be explained on the basis of the adsorption of some of the gelatin molecules that were not incorporated inside the vesicles’ core on their surface due to the sub-deviation of the particles into smaller sizes under the influence of the presence of glycerol as a plasticizer which increased the deformability of the vesicles. Another possible reason is the adsorption of the leaked drug molecule on the vesicles’ surface as reflected by the reduction in the encapsulation efficiency (Section 3.1.2.2) of those type of vesicles. The drug molecule with its ternary amine group have a chance of acquiring a proton from glycerol, being a nucleophile, turning the drug’s ternary amine group into a quaternary amine group and developing a positive charge on the surface of the vesicles Similarly Alonso et al51 studied the Effect of glycerol on the stability and surface properties of liposomes and reported that glycerol affected the interfacial properties and was responsible for some permeation changes. Arceo et al52 also reported the effect of the used glycerol on the liposomal permeation properties.
Effect of Formulation Variables on the EE% of the Prepared Gelatinized Core Liposomes
It is obvious that the presence of gelatin leads to a significant increase in entrapment of the water-soluble drug when compared to literature data for the plain liposomal formulations of water soluble drugs.53 It was reported previously that incorporating gelatin can fully entrap hydrophilic drugs in lysosomes, decrease their leakage out due to increasing the viscosity of the core as well as the dispersion medium around the vesicles’ bilayer,7,12 which is attributed to the hydrophilic nature of gelatin and its viscous gel-like structure at rest and at room temperature.54
However, the presence of the glycerol in GLG1 through GLG12 significantly (p = 0.0001, one-way ANOVA) decreased the entrapment efficiency compared to those formulations lacking glycerol. This may be due to the formation of more flexible walls of the vesicles in the presence of glycerol which increase their deformability and hence leakage of the drug.48 Similarly some studies suggested that the replacement of the interfacial water at liposomal surfaces by sugars or permeant molecules such as glycerol would affect the mechanism of permeability of the lipid bilayers. The aqueous interfaces contribute to the permeability properties both from a thermodynamic as well as from a structural point of view. Structural changes can affect permeation if it takes place.51,52 Furthermore, the presence of glycerol may have led to the dehydration of the bilayer through the low water activity at each side of the membrane as a result of the replacement of the stabilizing water with the polyol.51,55 This, in turn, may result in the partial leakage of the entrapped drug.
The Criteria for Selecting Formulations for Further in vitro and in vivo Evaluation
The desirability function of the Design Expert v.9.0® software together with some logic judgment were utilized to select formulations for further in vitro evaluation and to investigate the effect of their physicochemical characteristics on the in vivo performance. The criteria were to include formulae possessing nano and micron size particles according to the desirability function of the Design Expert v.9.0® software. The particle size is an important criterion in order to investigate its effect on the ocular uptake and hence the IOP lowering ability. Also, certain formulae containing a high and a low level of gelatin which is expected to have effect both on the drug release rate, ocular retention time and hence the in vivo performance of the formulation, were selected. Moreover, the presence and absence of glycerol in the formulation as an isotonicity adjusting agent was investigated to assess its effect on the eye irritancy during the ocular administration. Consequently, formulae GLG1, GLG4 and GL12 were chosen. Figure 1E reveals the particle size zones of selection according to the generated models.
In vitro Characterization of the Selected Gelatinized Core Liposomes
pH Determination of the Selected Gelatinized Core Liposomes
All pH measurements were performed at room temperature; 25℃. The control of the pH of ocular formulations is important for the stability of therapeutic agents, the ocular acceptability of the formulation and the permeation of drugs across the cornea. It is also known that the pH and the buffer capacity directly affect the subsequent discomfort from the formulation after instillation into the eye. Ocular drugs are better formulated to be within a pH range that is non-irritating to the eye to avoid lachrymal secretion which wash out the drug (pH 4.3–8.4).56,57 All the selected formulations; GLG1, GLG4 and GL12 exhibited pH value within the acceptable range for ocular administration; 4.97 ± 0.01, 5.87 ± 0.06 and 6.64 ± 0.02, respectively. No further pH adjustment was required.
Determination of the Rheological Behavior of the Selected Gelatinized Core Liposomes
Topical preparations intended for ocular administration require evaluation for their characteristics during administration specially the viscosity of the eye drops to ensure the evenness of flow and freedom from clogging. As computed from Farrow’s equation, the Farrow’s constant for the selected formulations (GLG1, GLG4 and GL12) was found to be almost equal unity which indicates a flow close to Newtonian behavior with slight thixotropy. This can be further ensured by comparing the viscosity at both maximum and minimum applied shear stresses. The viscosity coefficient (ɳ) of GLG1 at maximum and minimum shear stresses was identical; 1.05 cP, while both GLG4 and GL12 possessed close ɳ of 2.62 cP at the maximum shear stress and 1.3℃P at the minimum shear stress. This Newtonian behavior with viscosity close to that of tears is expected to decrease the discomfort and the damage to ocular epithelia.58
Morphological Examination of the Selected Gelatinized Core Liposomes
The morphological characteristics of the selected formulations (GLG1, GLG4 and GL12) were imaged by transmission electron microscopy after staining using 1% phosphotungstic acid. The obtained images showed a particle size of 133.85 nm, 426.03 nm and > 740 nm for GLG1, GLG4 and GL12, respectively, as shown in Figure 2. These results are in agreement with those obtained from zetasizer measurements. All investigated formulations showed nearly spherical morphology with slight deformations and the internal hydrophilic core appeared darker in color. These deformations confirm the gelation of the core as the gelatin is present below its melting point (Tm) in the gel state.53 The darker hydrophilic core is due to the concentration of the hydrophilic dye, phosphotungstic acid, in the aqueous domain.21,59,60
 |
Figure 2 Transmission electron micrograph (TEM) of the selected formulations, (A) GLG1, (B) GLG4 and (C) GL12. |
Differential Scanning Calorimetry
DSC is particularly useful in the investigation of the crystallinity of the drug within the prepared formulations. Crystalline forms of the drugs are thermodynamically unstable and can undergo crystal growth on storage which affect physical stability and solubility. Amorphization of the drug is thus the main target of many formulation development, which leads to a significant improvement of its bioavailability and stability after incorporation into the formulated vesicles.61,62 Figure 3 represent the thermogram of pure components; BRT, cholesterol, phosphatidylcholine and gelatin, their physical mixture and the lyophilized formulation; GLG1, GLG4 and GL12, respectively.
 |
Figure 3 DSC thermogram of pure components namely; BRT, cholesterol, phosphatidylcholine and gelatin, their physical mixture and the lyophilized formulation; GLG1, GLG4 and GL12. |
The thermogram of the pure drug demonstrated its crystalline structure with a sharp melting endothermic peak at 215.65 ℃. The obtained melting point for BRT was similar to that previously reported.63,64 In addition, another exothermic transition at 269.43℃ was observed representing the thermal decomposition of BRT.63,64 Phosphatidylcholine showed a melting point peak at 207.58℃ which was also similar to that previously reported.65,66 The thermogram of cholesterol demonstrated an endothermic peak at 63.83℃, while gelatin showed a melting point peak at 64.44℃ which was similar to that previously reported.67
It was clear that the peak of BRT was slightly shifted in the thermogram of the physical mixtures of the components of the selected formulations (GLG1, GLG4 and GL12). This can be attributed to the dilution effect of the excipients.21 It was clear that the peak of BRT disappeared in GLG1, GLG4 and GL12 formulation, indicating the molecular dispersion of BRT in amorphous form in the GCLs. Furthermore, upon comparison of DSC thermograms of blank cholesterol, phosphatidylcholine, and gelatin it can be confirmed that an interaction has taken place between phosphatidylcholine and gelatin, which suggests the formation of GCLs as indicated by the disappearance of their peaks in GLG1, GLG4 and GL12. All liposomal formulations exhibited two endothermic peaks corresponding to the pre- and main transitions of GLG1 at 44.54℃ and 198.77℃, GLG4 at 47.72℃ and 197.75℃ and GL12 at 47.8℃ and 53.6℃, respectively, This is in accordance with what was previously reported by Kitayama et al.68
Fourier Transform Infrared Spectroscopy (FTIR)
The interaction of drug with excipients in the selected formulation was studied by FTIR spectroscopy. The FTIR spectra have been depicted in Figure 4. BRT showed absorption bands at 3266.45 cm−1 due to N–H stretching, at 1262.13 cm−1 representing the C–N stretching, at 1595.14 cm−1 due to the carboxylate ions stretching and at 1650.73 cm−1 corresponding to C=O stretching. All of which conforms with previous findings.69
 |
Figure 4 FTIR spectrums of pure BRT, cholesterol, phosphatidylcholine, gelatin, their physical mixture and the selected formulations; GLG1, GLG4 and GL12. |
The FTIR spectrum of pure cholesterol showed characteristics peaks at 3394 cm−1 representing the aliphatic O-H stretching and at 2931 cm−1 due to the C-H asymmetric stretching of the CH3 group as reported in previous findings.70 Phosphatidylcholine spectrum showed characteristic peaks at 2923 cm–1, 1737 cm−1, and 1233 cm−1 as a result of the vibrational stretching of ester C-O, ester carbonyl group C=O and C-H stretching, respectively as reported in previous findings.71 Additionally, the gelatin spectrum showed characteristics peaks at 3423.14 cm−1 corresponding to the N-H stretching vibration, 2924.98 cm−1 due to C-H stretching vibration, 1635.61 cm−1 for the amide I, C=O stretching vibration, 1449.06 cm−1 for the amide II, N-H bending vibration, and at 1236.35 cm−1 for the amide III, N-H bending vibration as reported in previous findings.72,73
Similar peaks were identified in the spectrum of the selected formulation and physical mixture with minor differences in frequencies. Hence, it can be postulated that the drug had no chemical interaction with excipients of the vesicles. These slight shifts may be attributed to the hydrophobic interactions between BRT and liposome constituents. These interactions might be helpful in sustaining the drug release from the liposomes69 however does not affect its chemical integrity. Yet, it was noticed that three types of (C-O) groups were clearly recognized in the phosphatidyl choline spectrum associated with the disappearance of other three (C=O) bands in the gelatin spectrum in GLG1, GLG4 and GLG12 spectra which suggests the formation of new hydrogen bonds.88 These results assure the increased stability of the formulations (tautomeric effect). Moreover, narrowing of the (O-H) peaks and the increase in their intensity in the GCLs spectra assures the claimed interaction between gelatin and phosphatidyl choline.
In vitro Drug Release and Kinetic Model Studies
Figure 5 shows the percentage BRT released from the selected formulations of the GCLs with respect to time. It can be observed that GL12 demonstrated the slowest and the most sustained release profile with 95% released in 24 hours compared to the formulations containing glycerol; GLG1 and GLG4 which release 95% in 8 hours. GL12 showed much lower burst release in 5 minutes (20%) compared to GLG1 and GLG4 (40% for both of them). On the other hand, the corresponding free drug solution formulations containing the same corresponding gelatin concentrations (drug solutions; 1, 2 and 3, each containing 10 mg of the drug and the equivalent amount of gelatin content of GLG1, GLG4 and GL12, respectively) reached 100% release in only 4 hours which ensures that the retardation in release is attributed to the vesicular carrier system. In order to determine the release kinetics, the release data was analyzed according to zero order, 1st order, Higuchi diffusion model and was confirmed by calculating the release exponent (n) following the Korsmeyer-Peppas equation.26,27,74
The best fitting equation was applied to calculate the time for 50% release (t50%) and the MDT. The calculated parameters have indicated a 3-fold increase in the time to 50% release and 3- to 5-fold in the MDT for GL12 compared to the two formulations containing
The retardation of drug release in formulation GL12 is related to both the composition of the formulation as well as the vesicular particle size. The particle size of GL12 is 9- and 4-fold larger than GLG1 and GLG4, respectively. The smaller vesicles offer larger surface area in contact with the release medium with smaller core and hence more diffusion driving force and shorter diffusion pass for the drug to be released from both GLG1 and GLG4 compared to GL12. Furthermore, GL12 have a higher content of gelatin (80 mg) and cholesterol (100 mg) compared to both GLG1 (40 mg gelatin and 50 mg cholesterol) and GLG4 (70 mg gelatin and 25 mg cholesterol) in addition to the presence of glycerol in the two later formulations. The higher content of gelatin may retard the release of the entrapped drug from the gelatinized core being of higher viscosity while the higher concentration of cholesterol in the wall would increase its rigidity and hinder the release of the drug molecules. It was reported previously that the higher content of cholesterol increases the rigidity of the bilayer controlling the fluidity and permeability of the membrane, making it permeable to water but not to ions and protons.43,44,75 The presence of glycerol in GLG1 and GLG4 which act as a plasticizer may have facilitated the escape of the drug from the vesicles. Furthermore, the presence of glycerol may have led to the dehydration of the bilayer on both sides of the membrane as a result of the replacement of the stabilizing water with the polyol; glycerol.51,55 This in turn results in decreasing the vesicle size as well as enhancing the leakage of the entrapped drug to the release medium.50
Effect of Storage on the Physicochemical Characteristics of the Selected Gelatinized Core Liposomes
The physical stability of the selected formulations; GLG1, GLG4 and GL12 were monitored over a period of 3-months of storage under refrigeration at a temperature of 2–8℃. The PS, PDI, zeta potential as well as the EE% were measured after the specified period and compared statistically to the initial values of the freshly prepared formulations. All data are presented as the mean and standard deviations of three measurements as shown in Figure S2A. Upon statistically comparing the parameters before and after storage using one-way ANOVA, it was proven that GLG1 and GL12 formulations showed insignificantly (p>0.05) different parameters (particle size, zeta potential, PDI and entrapment efficiency) which assures the stability of the prepared formulations, while GLG4 showed insignificantly (p>0.05) different particle size, PDI and entrapment efficiency while Zeta potential increased from 14.28 ±1.22 to 42.07 ± 1.07, which was significantly different (p<0.05) but favorable to stability.
Effect of Sterilization
Effect on Physicochemical Properties of the Selected Formulations
The selected formulations received a radiation dose of 10 kGy for the sterilization purposes.30 Re-evaluation of the particle size, PDI, Zeta potential and entrapment efficiency of the sterilized formulations was performed. Upon statistically comparing the parameters using one-way ANOVA for GLG1, GLG4 and GL12, they turned out to be insignificantly (p>0.05) different from those of the untreated formulae except for the zeta potential of GLG1 and GL12 which significantly increased (p<0.05) upon sterilization (Figure S2B). It was reported previously76 that sterilization by gamma irradiation seemed to be an appropriate technique for sterilization of vesicular systems with no effect on drug loading or their physical properties but may increase the zeta potential and consequently augment the vesical stability. This is in accordance with the findings of Napia et al, who proved the significant increase in zeta potential of liposomal formulation upon gamma irradiation sterilization.
Testing Sterility of the Sterilized Formulations
After gamma sterilization, the sterility testing was performed on both the FTM which supports bacterial growth and the TSB media which supports the fungal growth. After two weeks of incubation, neither bacterial nor fungal growth was observed in the tested formulations, which confirms the efficiency of the gamma irradiation process in sterilizing the GCLs formulations. On the other hand, the positive controls showed growth of bacteria and fungi, which ensure the suitability of the selected media for supporting their growth while the negative controls showed no growth, which confirms the sterility of the medium under the processing condition.
Biological Evaluation of the Selected Brimonidine Tartrate Gelatinized Core Liposomes
The Effect on the Intra-Ocular Pressure of Rabbits
IOP was measured after instillation of the selected formulations (GLG1, GLG4 and GL12) into the glaucomatous rabbits’ eyes (all rabbits were treated with a mixture of betamethasone sodium phosphate and betamethasone acetate (3 mg/mL) each until reaching a reading of 5.5 for the 10g plunger load of the standardized Schiotz® tonometer; equivalent to 37.2 mmHg) and the respective results were compared to the drug solution. All the investigated formulations as well as the drug solution in an isotonic buffer had equivalent amounts of BRT/mL of 1 mg/mL. The values of reduction in the IOP are presented in Figure 6. The results revealed that the drug solution caused a sudden decrease in the IOP from the initial IOP of 37.20 mmHg to 29.47 ± 1.88 mmHg in the first hour followed by the gradual reduction in IOP till it reached the lowest level of 19.73 ± 2.70 mmHg after 6 hours and back to above 30 mmHg in 8 hours. On the other hand, GLG1, GLG4 and GL12 caused a smoother IOP lowering profile over 12 hours with fewer fluctuations. The average lowest IOP levels of 17.28 ± 1.34 mmHg, 15.83 ± 1.17 mmHg, and 17.53 ± 1.46 mmHg, for formulations GLG1, GLG4 and GL12, respectively were insignificantly (p>0.05) different from each other where they returned back to 30 mmHg in 24 h. The Area of ocular pressure reduction (the area between the initial ocular pressure of the glaucomatous rabbits’ eyes and the IOP reduction curve) of the selected formulations; GLG1, GLG4, GL12 and the drug solution having the same drug concentration were calculated using the trapezoidal rule to be 143.1 ± 27.60 mmHg, 166.775 ± 8.65 mmHg, 143.9667 ± 33.06 mmHg and 71.46 ± 7.66 mmHg, respectively, for the 10g plunger load of the tonometer.14
 |
Figure 6 IOP lowering over 24 hours for the selected formulations compared to drug solution in isotonic buffer (DS) and control eye (C). |
The superiority of the tested formulations; GLG1, GLG4 and GL12, in comparison to the aqueous solution of the drug was proven by the significantly different (p>0.5) area of IOP reduction after applying one-way ANOVA followed by the least significance difference test using GraphPad Prism® v.5.0 software. Following the 2015 guidelines of the American Academy of Ophthalmology, the primary goal of glaucoma treatment is a 25% decrease in IOP following monotherapy to achieve a slow control of further nerve damage. The success of treatment does not depend only on the efficacy of lowering the IOP, but also on minimizing its fluctuations, which worsen the neural damage.20
The selected formulations demonstrated significantly higher and steady efficacy in reducing the IOP when compared to the drug solution. The variance in efficacy of the drug solution was explained in the literature to be a direct function of the drug drainage upon mechanical blinking as well as tearing which is responsible for the 10-fold loss of the drug concentration within 20 minutes of drug administration.77,78
With respect to the gelatinized core formulations, these structures contain gelatin which has mucoadhesive properties that facilitate interactions of the formulation with the intraocular compartments. Gelatin is known to acquire positive charge on the amine groups at the physiological pH. This positive charge interacts with the negatively charged mucus layer of the cornea increasing the pre-corneal residence time compared to the drug solution. Furthermore, an increase in the residence time of drug in contact with the corneal surface occurs due to the transformation of the formulae into gel which is not easily eroded or cleared by the tears. This finding was reported in similar studies.79,80 Another reason for the improved ocular availability and extended IOP reduction duration would be the enhancement of ocular penetration of the nano encapsulated form of drugs.8,81,82
The debate between the superiority of the nano-sized formulations and the micro-sized ones in ocular drug delivery has been issued for years. Some studies revealed that the nanoparticle formulation reduced the corneal toxicity of eye drops due to the smaller particle size. In addition, the nanoparticle formulations may promote better bioavailability, resulting in reduced drug toxicity as confirmed by Nagai et al83 Furthermore, Lin wu et al84 claimed that the poor corneal penetration and short pre-ocular retention of a clinical hydrophilic drug such as pilocarpine nitrate, is due to the large particle size, so they investigated the potential of liquid crystal nanoparticles (LCNPs) for the ocular delivery of the drug and reported their potential as controlled-release ocular drug delivery system.
On the other hand, other researchers claimed that the micron-sized particles might represent a potential for the application of the long-term delivery of drugs to the eye. Yeh et al76 investigated the preparation of poly (dl-lactide-co-glycolide) microparticles containing 5-fluorouracil (5-FU) applying an oil-in-oil emulsion/solvent extraction technique. They concluded that they were capable of controlling the release of 5-FU over 21 days in a rate of 0.4 µg 5-FU/mg particles/day. Preliminary animal studies indicated that the 5-FU loaded microparticles showed no ocular toxicity and no significant inflammatory response in rabbits during drug delivery over 2 months.
With respect to the current investigation, the biological evaluation of the selected formulations having different particle size range demonstrated that the reduction of IOP was achieved with both the nano-range particle size (GLG1 and GLG4) as well as by the micron-range particle (GL12)85 with no significant difference between them with respect to the peak and duration of IOP reduction. It seems that each particle size range offered a specific advantage,83,84 while the nano-sized particles achieved better penetration,86 the larger particle of the micro-size range posed a better retention in the ocular region due to the particle size compared to the nano-sized ones.
Eye Irritancy Test (Modified Draize Test)
Eye irritancy testing adopting a simplified scoring system as presented in Table 3, which is based on the Draize sensitivity test87 was performed to spot the possible adverse effects of the selected formulations versus the drug solution in isotonic buffer. All formulae as well as the drug solution caused no irritation on the Draize scale with no detected change in the corneal opacity, conjunctival redness or chemosis or increased discharge after the application of the formulations.
 |
Table 3 Simplified Scoring System Based on the Draize’s Scale of Weighted Scores for Grading the Severity of Ocular Lesions |
Histopathological Studies
Microscopic examination of the eye’s tissue included the evaluation of cornea, filtration apparatus, choroid, and retina as presented in Figure 7. All tissues were examined microscopically for all the animals’ groups after instilling the formulations every 12 hours for 7 days. Concerning the cornea, normal histological structure was observed. Regarding the filtration apparatus, the iridocorneal angle showed a normally appearing trabecular meshwork that is responsible for the drainage of aqueous humor and a ciliary body which is a ring-shaped tissue located in the posterior ocular chamber. The ciliary muscle and a double layer of two, partly folded, neuro-epithelia namely: the non-pigmented and the pigmented epithelium can be clearly recognized. The Choroid appeared as blood vessel and connective tissue layer situated between the retina and sclera. The retina showed normally appearing layers. The non-altered histopathological findings of all the examined tissues assured the safety of the prepared GCLs; GLG1, GLG4 and GL12 and their compatibility with the ocular tissue.
Conclusions
Based on the above-mentioned outcomes, the potentiality of formulating BRT in the form of GCLs, with or without the addition of glycerol, for better entrapment of the hydrophilic drug associated with increased in vivo efficacy, safe histological profile and sustainment in the release pattern guarantees a successful glaucoma therapy. However, despite the spontaneous system formation after hydration, yet, it requires a maturation period in the final container for achieving the stable particle size, PDI, and zeta potential which suggests the storage for this period before dispatching if required for commercialization.
Acknowledgments
Authors would like to express their gratitude to the Research Institutions of Ophthalmology in Giza for supporting the conduct of the in vivo part in this research at their animal house under the supervision of Dr. Essam Eliraqy, Dr. Mohamed Ali Ghazaly, MD, Marwa Elfouly and Prof. Anisa S. Mohamed.
Disclosure
The authors declare no conflicts of interest in relation to this work.
References
1. Weng Y, Liu J, Jin S, Guo W, Liang X, Hu Z. Nanotechnology-based strategies for treatment of ocular disease. Acta Pharm Sin B. 2017;7(3):281–291. doi:10.1016/j.apsb.2016.09.001
2. Cholkar K, Dasari SR, Pal D, Mitra AK. Eye: anatomy, physiology and barriers to drug delivery. In: Ocular Transporters and Receptors. Elsevier; 2013:1–36.
3. El Hoffy NM, Azim EAA, Hathout RM, Fouly MA, Elkheshen SA. Glaucoma: management and future perspectives for nanotechnology-based treatment modalities. Eur J Pharm Sci. 2020;158:105648. doi:10.1016/j.ejps.2020.105648
4. Mandal A, Gote V, Pal D, Ogundele A, Mitra AK. Ocular pharmacokinetics of a topical ophthalmic nanomicellar solution of cyclosporine (Cequa®) for dry eye disease. Pharm Res. 2019;36(2):1–21. doi:10.1007/s11095-018-2556-5
5. Prakash P, Gnanaprakasam P, Emmanuel R, Arokiyaraj S, Saravanan M. Green synthesis of silver nanoparticles from leaf extract of Mimusops elengi, Linn. for enhanced antibacterial activity against multi drug resistant clinical isolates. Colloids Surf B Biointerfaces. 2013;108:255–259. doi:10.1016/j.colsurfb.2013.03.017
6. Yamamoto T, Yokoyama M, Opanasopit P, Hayama A, Kawano K, Maitani Y. What are determining factors for stable drug incorporation into polymeric micelle carriers? Consideration on physical and chemical characters of the micelle inner core. J Control Release. 2007;123(1):11–18. doi:10.1016/j.jconrel.2007.07.008
7. Naguib SS, Hathout RM, Mansour S. Optimizing novel penetration enhancing hybridized vesicles for augmenting the in-vivo effect of an anti-glaucoma drug. Drug Deliv. 2017;24(1):99–108. doi:10.1080/10717544.2016.1233588
8. Hathout RM, Omran MK. Gelatin-based particulate systems in ocular drug delivery. Pharm Dev Technol. 2016;21(3):379–386. doi:10.3109/10837450.2014.999786
9. Wheeler L, WoldeMussie E, Lai R. Role of alpha-2 agonists in neuroprotection. Surv Ophthalmol. 2003;48(2):S47–S51. doi:10.1016/S0039-6257(03)00004-3
10. WoldeMussie E, Ruiz G, Wijono M, Wheeler LA. Neuroprotection of retinal ganglion cells by brimonidine in rats with laser-induced chronic ocular hypertension. Invest Ophthalmol Vis Sci. 2001;42(12):2849–2855.
11. Adkins JC, Balfour JA. Brimonidine. Drugs Aging. 1998;12(3):225–241. doi:10.2165/00002512-199812030-00005
12. Hathout RM, Gad HA, Abdel-Hafez SM, et al. Gelatinized core liposomes: a new Trojan horse for the development of a novel timolol maleate glaucoma medication. Int J Pharm. 2019;556:192–199. doi:10.1016/j.ijpharm.2018.12.015
13. Abdel-Hafez SM, Hathout RM, Sammour OA. Towards better modeling of chitosan nanoparticles production: screening different factors and comparing two experimental designs. Int J Biol Macromol. 2014;64:334–340. doi:10.1016/j.ijbiomac.2013.11.041
14. Shokry M, Hathout RM, Mansour S. Exploring gelatin nanoparticles as novel nanocarriers for Timolol Maleate: augmented in-vivo efficacy and safe histological profile. Int J Pharm. 2018;545(1–2):229–239. doi:10.1016/j.ijpharm.2018.04.059
15. Pathan I. Transdermal delivery of ethosomes as a novel vesicular carrier for paroxetine hydrochloride: in vitro evaluation and In vivo study. Marmara Pharm J. 2016;20(1):1–6. doi:10.12991/mpj.201620113534
16. Ramadan AA, Eladawy SA, El-Enin ASMA, Hussein ZM. Development and investigation of timolol maleate niosomal formulations for the treatment of glaucoma. J Pharm Investig. 2020;50(1):59–70. doi:10.1007/s40005-019-00427-1
17. Abdel-Hafez SM, Hathout RM, Sammour OA. Curcumin-loaded ultradeformable nanovesicles as a potential delivery system for breast cancer therapy. Colloids Surf B Biointerfaces. 2018;167:63–72. doi:10.1016/j.colsurfb.2018.03.051
18. Nagargoje S, Phatak A, Bhingare C, Chaudhari S. Formulation and evaluation of ophthalmic delivery of fluconazole from ion activated in situ gelling system. Der Pharmacia Lettre. 2012;4(4):1228–1235.
19. Chen H, Chang X, Weng T, et al. A study of microemulsion systems for transdermal delivery of triptolide. J Control Release. 2004;98(3):427–436. doi:10.1016/j.jconrel.2004.06.001
20. Guinedi AS, Mortada ND, Mansour S, Hathout RM. Preparation and evaluation of reverse-phase evaporation and multilamellar niosomes as ophthalmic carriers of Acetazolamide. Int J Pharm. 2005;306(1–2):71–82. doi:10.1016/j.ijpharm.2005.09.023
21. Hathout RM, Mansour S, Mortada ND, Guinedi AS. Liposomes as an ocular delivery system for Acetazolamide: in vitro and in vivo studies. Aaps Pharmscitech. 2007;8(1):E1–E12. doi:10.1208/pt0801001
22. Aggarwal D, Kaur IP. Improved pharmacodynamics of timolol maleate from a mucoadhesive niosomal ophthalmic drug delivery system. Int J Pharm. 2005;290(1–2):155–159. doi:10.1016/j.ijpharm.2004.10.026
23. Efron N, Young G, Brennan NA. Ocular surface temperature. Curr Eye Res. 1989;8(9):901–906.
24. Annapurna M, Sushmitha M, Sevyatha V. Simultaneous determination of brimonidine tartrate and timolol maleate by first derivative and ratio derivative spectroscopy. J Anal Pharm Res. 2017;4(6):00120.
25. Podczeck F. Comparison of in vitro dissolution profiles by calculating mean dissolution time (MDT) or mean residence time (MRT). Int J Pharm. 1993;97(1–3):93–100. doi:10.1016/0378-5173(93)90129-4
26. Korsmeyer R, Gurny R, Doelker E, Buri P, Peppas N. Mechanisms of potassium chloride release from compressed, hydrophilic, polymeric matrices: effect of entrapped air. J Pharm Sci. 1983;72(10):1189–1191. doi:10.1002/jps.2600721021
27. Korsmeyer RW, Gurny R, Doelker E, Buri P, Peppas NA. Mechanisms of solute release from porous hydrophilic polymers. Int J Pharm. 1983;15(1):25–35. doi:10.1016/0378-5173(83)90064-9
28. Peppas N Analysis of fickian and non-fickian drug release from polymers; 1985.
29. Peppas N. Analysis of fickian and non-fickian drug release from polymers. Pharm Acta Helv. 1985;60(4):110–111.
30. Sakar F, Özer A, Erdogan S, et al. Nano drug delivery systems and gamma radiation sterilization. Pharm Dev Technol. 2017;22(6):775–784. doi:10.3109/10837450.2016.1163393
31. Kaur IP, Aggarwal D, Singh H, Kakkar S. Improved ocular absorption kinetics of timolol maleate loaded into a bioadhesive niosomal delivery system. Graefes Arch Clin Exp Ophthalmol. 2010;248(10):1467–1472. doi:10.1007/s00417-010-1383-0
32. Litamoi J, Palya V, Sylla D. Quality control testing of contagious bovine pleuropneumonia live attenuated vaccine: standard operating procedures: food & agriculture org; 1996.
33. Zolner WJ. Quality-control analytical methods: a guide to quality control testing for the compounding pharmacist. Int J Pharm Compd. 2006;10(4):281.
34. Wiegand I, Hilpert K, Hancock RE. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat Protoc. 2008;3(2):163. doi:10.1038/nprot.2007.521
35. Melena J, Santafé J, Segarra J. The effect of topical diltiazem on the intraocular pressure in betamethasone-induced ocular hypertensive rabbits. J Pharmacol Exp Ther. 1998;284(1):278–282.
36. Shields MB. Textbook of Glaucoma. Williams & Wilkins; 1987.
37. Draize JH, Woodard G, Calvery HO. Methods for the study of irritation and toxicity of substances applied topically to the skin and mucous membranes. J Pharmacol Exp Ther. 1944;82(3):377–390.
38. Sharpe R. The Draize test—motivations for change. Food Chem Toxicol. 1985;23(2):139–143. doi:10.1016/0278-6915(85)90005-5
39. Shamim B. Alternatives to the Draize Eye Test. PharmaTutor. 2014;2(10):45–57.
40. Abozeid SM, Hathout RM, Abou-Aisha K. Silencing of the metastasis-linked gene, AEG-1, using siRNA-loaded cholamine surface-modified gelatin nanoparticles in the breast carcinoma cell line MCF-7. Colloids Surf B Biointerfaces. 2016;145:607–616. doi:10.1016/j.colsurfb.2016.05.066
41. Box GE, Cox DR. An analysis of transformations. J R Stat Soc. 1964;26(2):211–243.
42. Osborne J. Improving your data transformations: applying the Box-Cox transformation. Pract Assess Res Evaluation. 2010;15(1):12.
43. Essa EA. Effect of formulation and processing variables on the particle size of sorbitan monopalmitate niosomes. Asian J Pharm. 2014;4(4):1.
44. Kazi KM, Mandal AS, Biswas N, et al. Niosome: a future of targeted drug delivery systems. J Adv Pharm Technol Res. 2010;1(4):374. doi:10.4103/0110-5558.76435
45. Lelkes PI. Methodological Aspects Dealing with Stability Measurements of Liposomes in vitro Using the Carboxyfluorescein-Assay. Boca Raton, FL: CRC Press; 1984.
46. Ammar H, Haider M, Ibrahim M, El Hoffy N. In vitro and in vivo investigation for optimization of niosomal ability for sustainment and bioavailability enhancement of diltiazem after nasal administration. Drug Deliv. 2017;24(1):414–421. doi:10.1080/10717544.2016.1259371
47. hui-hui Z, qiu-hua L, zhi-jun Y, wei-san P, shu-fang N. Novel ophthalmic timolol meleate liposomal-hydrogel and its improved local glaucomatous therapeutic effect in vivo. Drug Deliv. 2011;18(7):502–510. doi:10.3109/10717544.2011.595839
48. El Kayal M, Nasr M, Mortada N, Elkheshen S. Optimization of the colloidal properties of different vesicular systems aiming to encapsulate (-)- epigallocatechin-3-gallate. Farmacia. 2020;68(1):97–110. doi:10.31925/farmacia.2020.1.14
49. Arnett EM, Harvey N, Johnson E, Johnston DS, Chapman D. No phospholipid monolayer-sugar interactions. Biochemistry. 1986;25(18):5239–5242. doi:10.1021/bi00366a038
50. Cadenhead D, Bean K. Selected lipid monolayers on aqueous-glycerol and aqueous-urea substrates. Biochimica et Biophysica Acta (BBA)-Biomembranes. 1972;290:43–50. doi:10.1016/0005-2736(72)90050-8
51. Alonso-Romanowski S, Biondi A, Disalvo E. Effect of carbohydrates and glycerol on the stability and surface properties of lyophilized liposomes. J Membr Biol. 1989;108(1):1–11. doi:10.1007/BF01870420
52. Arceo E, Marsden P, Bergman RG, Ellman JA, An efficient didehydroxylation method for the biomass-derived polyols glycerol and erythritol. Mechanistic studies of a formic acid-mediated deoxygenation. Chem Comm. 2009;23:3357–3359. doi:10.1039/b907746d
53. Hathout RM, Gad HA, Metwally AA. Gelatinized‐core liposomes: toward a more robust carrier for hydrophilic molecules. J Biomed Mater Res A. 2017;105(11):3086–3092. doi:10.1002/jbm.a.36175
54. Kim NJ, Harris A, Gerber A, et al. Nanotechnology and glaucoma: a review of the potential implications of glaucoma nanomedicine. Br J Ophthalmol. 2014;98(4):427–431. doi:10.1136/bjophthalmol-2013-304028
55. Gruner SM. Intrinsic curvature hypothesis for biomembrane lipid composition: a role for nonbilayer lipids. Proc Natl Acad Sci. 1985;82(11):3665–3669. doi:10.1073/pnas.82.11.3665
56. Chiou G Topical treatment of ocular hypertension, glaucoma, ischemic retinopathy and age-related macular degeneration with ophthalmic formulation of dopamine antagonists: google patents; 2003.
57. Ahmed I, Chaudhuri B. Evaluation of buffer systems in ophthalmic product development. Int J Pharm. 1988;44(1–3):97–105. doi:10.1016/0378-5173(88)90105-6
58. Zhu H, Chauhan A. Effect of viscosity on tear drainage and ocular residence time. Optom Vis Sci. 2008;85(8):E715–E725. doi:10.1097/OPX.0b013e3181824dc4
59. Mura S, Manconi M, Sinico C, Valenti D, Fadda AM. Penetration enhancer-containing vesicles (PEVs) as carriers for cutaneous delivery of minoxidil. Int J Pharm. 2009;380(1–2):72–79. doi:10.1016/j.ijpharm.2009.06.040
60. Shen Y, Jin E, Zhang B, et al. Prodrugs forming high drug loading multifunctional nanocapsules for intracellular cancer drug delivery. J Am Chem Soc. 2010;132(12):4259–4265. doi:10.1021/ja909475m
61. Pattnaik S, Swain K, Mallick S, Lin Z. Effect of casting solvent on crystallinity of ondansetron in transdermal films. Int J Pharm. 2011;406(1–2):106–110. doi:10.1016/j.ijpharm.2011.01.009
62. Coleman NJ, Craig DQ. Modulated temperature differential scanning calorimetry: a novel approach to pharmaceutical thermal analysis. Int J Pharm. 1996;135(1–2):13–29. doi:10.1016/0378-5173(95)04463-9
63. De Souza JF, Maia KN, Patrício PSDO, et al. Ocular inserts based on chitosan and brimonidine tartrate: development, characterization and biocompatibility. J Drug Deliv Sci Technol. 2016;32:21–30. doi:10.1016/j.jddst.2016.01.008
64. Singh HK, Shinde AU. Development and evaluation of novel polymeric nanoparticles of brimonidine tartrate. Curr Drug Deliv. 2010;7(3):244–251. doi:10.2174/156720110791561008
65. Chapman D, Collin D. Differential thermal analysis of phospholipids. Nature. 1965;206(4980):189. doi:10.1038/206189a0
66. Demetzos C. Differential scanning calorimetry (DSC): a tool to study the thermal behavior of lipid bilayers and liposomal stability. J Liposome Res. 2008;18(3):159–173. doi:10.1080/08982100802310261
67. Mukherjee I, Rosolen M. Thermal transitions of gelatin evaluated using DSC sample pans of various seal integrities. J Therm Anal Calorim. 2013;114(3):1161–1166. doi:10.1007/s10973-013-3166-4
68. Kitayama H, Takechi Y, Tamai N, Matsuki H, Yomota C, Saito H. Thermotropic phase behavior of hydrogenated soybean phosphatidylcholine–cholesterol binary liposome membrane. Chem Pharm Bull (Tokyo). 2014;62(1):58–63. doi:10.1248/cpb.c13-00587
69. Maiti S, Paul S, Mondol R, Ray S, Sa B. Nanovesicular formulation of brimonidine tartrate for the management of glaucoma: in vitro and in vivo evaluation. Aaps Pharmscitech. 2011;12(2):755–763. doi:10.1208/s12249-011-9643-9
70. Fathalla D, Soliman G, Fouad E. Development and in vitro/in vivo evaluation of liposomal gels for the sustained ocular delivery of latanoprost. J Clin Exp Ophthalmol. 2015;6(390):2.
71. Sikkandar S, Jayakumar S, Gunasekaran S, Renugadevi T, Alwar B. Study on the analysis of human gallstones using Fourier transform infrared spectroscopic technique. Int J ChemTech Res. 2011;3(1):149–154.
72. Lai J-Y, Li Y-T. Influence of cross-linker concentration on the functionality of carbodiimide cross-linked gelatin membranes for retinal sheet carriers. J Biomater Sci Polym Ed. 2011;22(1–3):277–295. doi:10.1163/092050609X12603600753204
73. Lai J-Y, Hsieh A-C. A gelatin-g-poly (N-isopropylacrylamide) biodegradable in situ gelling delivery system for the intracameral administration of pilocarpine. Biomaterials. 2012;33(7):2372–2387. doi:10.1016/j.biomaterials.2011.11.085
74. Ritger PL, Peppas NA. A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J Control Release. 1987;5(1):37–42. doi:10.1016/0168-3659(87)90035-6
75. Campbell M. Lipids and membranes. In: Campbell MK, editor. Biochemistry. New York, NY, USA: Saunders College Publishing; 1995:1423–1430.
76. Yeh M-K, Tung S-M, Lu D-W, Chen J-L, Chiang C-H. Formulation factors for preparing ocular biodegradable delivery system of 5-fluorouracil microparticles. J Microencapsul. 2001;18(4):507–519. doi:10.1080/02652040010018100
77. Maurice D. Kinetics of topically applied ophthalmic drugs. In: Ophthalmic Drug Delivery. Springer; 1987:19–26.
78. Balasubramaniam J, Kant S, Pandit JK. In vitro and in vivo evaluation of Gelrite® gellan gum-based ocular delivery system for indomethacin. ACTA PHARMACEUTICA-ZAGREB. 2003;53(4):251–262.
79. Singh J, Chhabra G, Pathak K. Development of Acetazolamide-loaded, pH-triggered polymeric nanoparticulate in situ gel for sustained ocular delivery: in vitro. ex vivo evaluation and pharmacodynamic study. Drug Dev Ind Pharm. 2014;40(9):1223–1232. doi:10.3109/03639045.2013.814061
80. Friess W. Collagen–biomaterial for drug delivery. Eur J Pharm Biopharm. 1998;45(2):113–136. doi:10.1016/s0939-6411(98)00017-4
81. Yousry C, Fahmy RH, Essam T, El-Laithy HM, Elkheshen SA. Nanoparticles as tool for enhanced ophthalmic delivery of vancomycin: a multidistrict-based microbiological study, solid lipid nanoparticles formulation and evaluation. Drug Dev Ind Pharm. 2016;42(11):1752–1762. doi:10.3109/03639045.2016.1171335
82. Yousry C, Elkheshen SA, El-Laithy HM, Essam T, Fahmy RH. Studying the influence of formulation and process variables on vancomycin-loaded polymeric nanoparticles as potential carrier for enhanced ophthalmic delivery. Eur J Pharm Sci. 2017;100:142–154. doi:10.1016/j.ejps.2017.01.013
83. Nagai N, Yoshioka C, Mano Y, et al. A nanoparticle formulation of disulfiram prolongs corneal residence time of the drug and reduces intraocular pressure. Exp Eye Res. 2015;132:115–123. doi:10.1016/j.exer.2015.01.022
84. Li J, Wu L, Wu W, et al. A potential carrier based on liquid crystal nanoparticles for ophthalmic delivery of pilocarpine nitrate. Int J Pharm. 2013;455(1–2):75–84. doi:10.1016/j.ijpharm.2013.07.057
85. Hathout RM. Particulate systems in the enhancement of the antiglaucomatous drug pharmacodynamics: a meta-analysis study. ACS omega. 2019;4(26):21909–21913. doi:10.1021/acsomega.9b02895
86. ElMasry SR, Hathout RM, Abdel-Halim M, Mansour M. In vitro transdermal delivery of sesamol using oleic acid chemically-modified gelatin nanoparticles as a potential breast cancer medication. J Drug Deliv Sci Technol. 2018;48:30–39. doi:10.1016/j.jddst.2018.08.017
87. Draize JH. Methods for the study of irritation and toxicity of substances applied topically to the skin and mucous membranes. J Pharmacol Exp Ther. 1944;82:377–390.
88. Ossama M, Hathout RM, Attia DA, Mortada ND. Enhanced Allicin Cytotoxicity on HEPG-2 Cells Using Glycyrrhetinic Acid Surface-Decorated Gelatin Nanoparticles. ACS Omega. 2019;4(6):11293-11300. doi:10.1021/acsomega.9b01580
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.