Back to Journals » Journal of Inflammation Research » Volume 15
Augmentation of NAD+ by Dunnione Ameliorates Imiquimod-Induced Psoriasis-Like Dermatitis in Mice
Authors Lee SH, Kim HJ, Oh GS, Lee SB, Khadka D , Cao W, Choe SK, Shim H , Kim CD, Kwak TH, So HS
Received 27 April 2022
Accepted for publication 7 August 2022
Published 13 August 2022 Volume 2022:15 Pages 4623—4636
DOI https://doi.org/10.2147/JIR.S372543
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Zili You
Seung Hoon Lee,1,* Hyung-Jin Kim,1,* Gi-Su Oh,1 Su-Bin Lee,1 Dipendra Khadka,1 Wal Cao,1 Seong-Kyu Choe,1 Hyeok Shim,2 Chang-Deok Kim,3 Tae Hwan Kwak,4 Hong-Seob So1,4
1Department of Microbiology, Wonkwang University School of Medicine, Iksan, Jeonbuk, 54538, Republic of Korea; 2Department of Hemato-Oncology, Wonkwang University School of Medicine, Iksan, Jeonbuk, 54538, Republic of Korea; 3Department of Dermatology, College of Medicine, Chungnam National University, Daejeon, 34134, Republic of Korea; 4R&D Center, NADIANBIO Ltd, Iksan, Jeonbuk, 54538, Republic of Korea
*These authors contributed equally to this work
Correspondence: Hong-Seob So, Department of Microbiology, Wonkwang University School of Medicine, 460 Iksan-Daero, Iksan, Jeonbuk, 54538, Republic of Korea, Tel +82-63-850-6950, Fax +82-63-855-6777, Email [email protected]
Background: Dunnione has anti-inflammatory properties arising from its ability to alter the ratio of NAD+/NADH through NAD(P)H quinone oxidoreductase 1 (NQO1) enzymatic action, followed by subsequent inhibition of NF-κB and inflammatory cytokines. Psoriasis is a chronic, inflammatory skin disorder in which the IL-23/Th17 axis plays an important role in inflammation. However, it is unclear whether modulation of NAD+ levels affects psoriasis, such as skin inflammation. Therefore, in this study, we investigated the effect of NAD+/NADH ratio modulation on imiquimod (IMQ)-induced, psoriasis-like skin inflammation in mice.
Methods: Psoriasis-like skin inflammation was generated by daily topical application of IMQ cream. The severity of dermatitis was assessed using the Psoriasis Area Severity Index (PASI) and histochemistry. Expression of inflammatory cytokines was detected by enzyme-linked immunosorbent assay and quantitative PCR. Acetylation of NF-κB p65 and STAT3 was determined by Western blotting.
Results: Dunnione improved IMQ-induced epidermal hyperplasia and inflammation, consistent with decreased levels of inflammatory cytokines (IL-17, IL-22, and IL-23) in skin lesions. Moreover, we found that an increase in the NAD+/NADH ratio by dunnione restored SIRT1 activity, thereby reduced imiquimod-induced STAT3 acetylation, which modulates the expression of psoriasis-promoting inflammatory cytokines, such as IL-17, IL-22, and IL-23.
Conclusion: Pharmacological modulation of cellular NAD+ levels could be a promising therapeutic approach for psoriasis-like skin disease.
Keywords: psoriasis, NQO1, NAD+, SIRT1, inflammatory cytokine
Introduction
Psoriasis is a chronic, immune-mediated, inflammatory skin disease characterized by hyperproliferative keratinocytes, parakeratosis, hyperkeratosis, and massive infiltration of inflammatory cells.1 Although the pathogenesis of psoriasis is not fully understood, there is growing evidence for the involvement of interleukin (IL)-23 and T helper 17 (Th17) cells and their inflammatory mediators, IL- 17 and IL-22.2 IL-23 plays an important role in promoting the differentiation and development of Th17 cells, which are major producers of cytokines such as IL-17A and IL-22 and play a role in many chronic inflammatory diseases, including psoriasis.3 Th17-secreted cytokines can affect keratinocytes and skin inflammatory cells, causing local inflammation and keratinocyte proliferation.4
Nicotinamide adenine dinucleotide (NAD+), an important regulator of energy metabolism and cellular homeostasis, is a substrate for many NAD+-dependent enzymes including sirtuin (SIRT) and poly(ADPribose) polymerase (PARP).5 Nuclear SIRT1 is an NAD+ -dependent deacetylase that plays important roles in metabolism, aging, stress adaptation, hormone responses, and cell death through the deacetylation of substrates including NF-κB, p53, STAT3, and histones.6,7 SIRT1 also inhibits oxidative stress and inflammation.8 PARP is an abundant ADP-ribosyltransferase that functions as a DNA nick-sensor and contributes to DNA repair, chromatin remodeling, and genomic stability. In particular, PARP1 uses NAD+ to generate large amounts of poly(ADP-ribose), which promotes the recruitment of DNA repair factors. However, excessive activation of PARP1 causes depletion of intracellular NAD+ and ATP levels, eventually leading to cell death.9 Furthermore, PARP1 enhances NF-κB-mediated transcription, which plays a pivotal role in the expression of inflammatory cytokines, chemokines, adhesion molecules, and inflammatory mediators.
NAD(P)H:quinone oxidoreductase 1 (NQO1) is an antioxidant protein with multiple substrates that catalyzes the oxidation of NADH to NAD+. Dunnione, an orange-red pigment obtained from Streptocarpus dunnii Mast, was initially identified as an anti-fungal and anti-tumor agent; more recently, it has been shown to act as a substrate of NQO1, increasing the NAD+/NADH ratio through NQO1-mediated NADH oxidation.10,11 Interestingly, decreased NAD+/NADH ratios lead to ROS production and inflammation.12 We recently demonstrated that increasing the cellular NAD+/NADH ratio by NQO1 enzymatic action using dunnione suppressed caerulein-induced acute pancreatitis by decreasing oxidative stress and inflammatory responses.11 However, it remains unclear whether modulation of NAD+ levels impacts psoriasis-like skin inflammation. Imiquimod (IMQ)-induced dermatitis in mice is very similar to human psoriatic lesions in terms of the development of lesions involving the IL-23/IL-17 axis, as well as in terms of phenotypic and histological features. Therefore, in this study, we investigated the role of NAD+ metabolism in IMQ-induced psoriasis and the effect of dunnione treatment on intracellular NAD+ levels on IMQ-induced psoriasis in wild-type (WT), NQO1−/−, and skin-tissue specific SIRT1−/− mice.
Materials and Methods
Reagents
Dunnione was synthesized by Erum Biotechnologies (Suwon, Korea) and micronized as particles to enhance oral bioavailability. Antibodies against NF-κB p65 (L8F6, Mouse, Cat No. 6956), acetyl-NF-κB p65 (D2S3J, Rabbit, Cat No. 12629), STAT3 (79D7, Rabbit, Cat No. 4904), and acetyl-STAT3 (Rabbit, Cat No. 2523) were purchased from Cell Signaling Inc. (Beverly, MA, USA). The antibody against β-actin (C4, Mouse, Cat No. sc-47778) was obtained from Santa Cruz Biotechnology, Inc. (CA, USA).
Animals
Male C57BL/6 mice were obtained from the Central Laboratory Animal Inc. (Seoul, Korea). NQO1 knockout (KO) mice on a C57BL/6 background were provided by Dr. C. H. Lee (Animal Model Center, Korea Research Institute of Bioscience and Biotechnology, Daejeon, Korea). SIRT1 floxed mice on a C57BL/6 background were provided by Dr. H. S. Kim (Ewha Womans University, Seoul, Korea). K14-Cre mice on a C57BL/6 background were provided by Dr. C. D. Kim (Chungnam National University, Daejeon, Korea). The skin-specific SIRT1 KO mice on a C57BL/6 background were generated by crossing mice carrying a SIRT1 exon 4 floxed allele with mice expressing Cre recombinase driven by the K14 promoter. Neither NQO1 KO nor skin-specific SIRT1 KO mice showed any developmental abnormalities. All mice were fed a standard commercial diet and housed at ambient temperatures of 20–22 °C with a relative humidity of 50% ± 5%, under a 12-hour light-dark cycle in a pathogen-free facility. Experiments were performed with 8-wk-old mice weighing 20–25 g, and all mice were age-matched within three days. All animal experiments were approved by the Institutional Animal Care and Use Committee of Wonkwang University (Approval No. WKU21-104) and complied with the Animal Welfare Act.
Genotyping of KO Mice
Genotyping was done by PCR to distinguish between WT and KO mice (Supplementary Figure S1). DNA was extracted using Extract N AMP Tissue extraction kit. PCR was set up using 1X PCR buffer from Sigma using the following primers: 5’-TGTGTACCGTGTGTATGCAA-3’ (NQO1-WT forward), 5’-CTAAGACCTGGAAGCCACAG-3’ (NQO1-WT reverse) resulting 450 bps, 5’-GAAGGGACTGGCTGCTATTG-3’ (NQO1-KO forward), 5’-AATTCACGGGTAGCCAACG-3’ (NQO1-KO reverse) resulting 400 bps, 5’-CATCTAAACTTTGTTGGCTGC-3’ (SIRT1-wild type reverse), 5’-TCCTTGCCACAGTCACTCAC-3’ (SIRT1-common forward), 5’-ACAGTCCCATTC CCATACC-3’ (SIRT1-floxed reverse) resulting 600 bps for WT and 700 bps for KO. Electrophoresis was conducted on 2% agarose gels and visualized with a Bio-Rad ChemiDoc system (Bio-Rad, USA).
Study Design and Assessment of Dermatitis
Mice were randomly divided into four groups: control (n = 5), IMQ alone (n = 5), IMQ plus dunnione (n = 5), and dunnione alone (n = 5). Psoriasis-like skin inflammation was generated by daily topical application of a dose of 62.5 mg IMQ cream (5%; Aldara®; 3M Pharmaceuticals, UK) on hair-free dorsal skin and the right ear for six consecutive days. Control mice received a similar daily dose of vehicle (Vaseline Lanette cream; Fagron). After 2 days, dunnione dissolved in corn oil was orally administered at 80 mg/kg daily for 4 consecutive days. All animals were assessed for severity of erythema (redness), scaling (desquamation), and thickening (induration) of skin in the treated areas on days 0, 2, 4, and 6, using modified Psoriasis Area Severity Index (PASI) to assign a score of 0–4 (0, none; 1, mild; 2, moderate; 3, severe; 4, very severe). The thickness of the skin folds of the back and the right ears were measured with a caliper (accuracy: ± 0.02 mm, Mitsutomo, Japan). The cumulative score (erythema plus scaling plus thickening) served as a measure of the severity of inflammation (scale 0–12) at 0, 2, 4, and 6 days.
Histology
Skin samples were fixed in 10% neutral buffered formalin solution and embedded in paraffin. Tissue slices were cut from paraffin sections (4 μm thick) and deparaffinized sections were stained with hematoxylin and eosin Y (H&E). Epidermal thickness was measured by averaging five independent field values per section.
Protein Extraction for Measurement of SIRT1 Activity, PARP Activity and Western Blotting
Frozen mouse skin tissue was homogenized in 1 volume of ice-cold NETN buffer (20 mM Tris-HCl pH 8, 100 mM NaCl, 1 mM EDTA, 0.5% NP-40, complete protease inhibitors and phosphatase inhibitors) using a polytron homogenizing probe. Samples were sonicated on ice with a vibra cell sonicator and spun at 10,000 x g for 10 minutes at 4°C. The supernatant was retained and protein concentration was determined for each sample using a Bio-Rad assay kit (Bio-Rad Laboratories, Hercules, CA, USA).
Measurement of SIRT1 Activity
The effects of IMQ and dunnione on SIRT1 activity in skin tissues were determined using a fluorescent SIRT1 assay kit (Cat No. BML-AK555-0001, Enzo Life Sciences International Inc., PA, USA) according to the manufacturer’s instructions. Briefly, skin homogenates were incubated for 10 min at 37°C to allow degradation of any contaminant NAD+. The homogenates (40μg protein/well) were then incubated in SIRT1 assay buffer (25 mM Tris-Cl, pH 8.0, 137 mM NaCl, 2.7 mM KCl, 1 mM MgCl2, and 1 mg/mL BSA) in the presence of Fluor de Lys–SIRT1 substrate, 5 μM TSA and 200 μM NAD+. After incubation at 37 °C for 1 hour, the reaction was terminated by adding a solution containing Fluor de Lys Developer and 2 mM nicotinamide. Deacetylation of the substrate was measured using a CytoFluor series 4000 fluorometer (Perseptive Biosystems Inc., Framingham, MA, USA) with excitation and emission wavelengths set to 360 and 460 nm, respectively.
Measurement of PARP Activity
PARP activity was determined with a Universal Chemiluminescent PARP assay kit (Cat No. 4676–096-K, Trevigen, Gaithersburg, MD, USA) following the manufacturer’s protocol. Total tissue lysates (30 μg/well) were added to wells containing the PARP buffer cocktail and incubated at room temperature for 1 hour. All wells were washed thrice with PBS plus 0.1% Triton X-100 (PBST), followed by incubation with streptavidin-horseradish peroxidase in strep diluent buffer (1:1000 dilution) for 1 hour. After three extensive washes with PBST, chemiluminescence was detected. Background readings were subtracted from sample readings of the samples; and was quantified using a standard curve.
Western Blotting
Twenty micrograms of lysate were then subjected to electrophoresis on 10% SDS-polyacrylamide gels for 1 hour at 100 mA, then transferred to a nitrocellulose membrane. The membrane was incubated in 5% (wt/vol) non-fat skim milk solution in PBS containing 0.05% (v/v) Tween-20 (PBS-T) for 1 hour, washed in PBS-T, then incubated with primary antibody (1:1000) for 1 hour. Next, the membrane was extensively washed with PBS-T and incubated with the appropriate secondary antibody for 1 hour at room temperature. After extensive washes, protein bands on the membrane were exposed to ECL (Enhanced chemiluminescence, SuperSignal Substrate; Pierce, Rockford, IL, USA) and then developed using Amersham hyperfilm and Xenograph developer.
Measurement of NAD+
We used a fluorescent NAD+ detection kit (Cat No. E2ND-100, BioAssay Systems, Hayward, CA, USA), following the manufacturer’s instructions. Briefly, skin tissues were homogenized in 100 μL acidic extraction buffer. Homogenates were heated at 60 °C for 5 minutes and neutralized by the addition of alkaline extraction buffer. After an enzymatic cycling reaction, NAD+ content was measured using a microplate reader.
Measurement of Inflammatory Cytokines
Levels of the cytokines IL-17, IL-22, and IL-23 in skin tissue were determined using a commercial enzyme-linked immunosorbent assay (ELISA) kit (RayBiotech, Norcross, GA, USA) according to the manufacturer’s instructions. The following ELISA kits were used: IL-17 (Cat No. ELM-IL17-1), IL-22 (Cat No. ELM-IL22-1), and IL-23 (Cat No. ELM-IL23p19-1).
Quantitative Real-Time PCR
Total RNA was isolated from cells using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. Three micrograms of RNA were converted to cDNA using the First Strand cDNA Synthesis Superscript kit (Invitrogen) according to the manufacturer’s protocol. Quantitative real-time PCR was performed using SYBR Green Master Mix (Invitrogen). Reactions were performed in triplicate and their specificity was monitored using melting curves after cycling. The following primers were used: IL-17A, 5’-CTG CTG AGC CTG GCG GCT AC-3’, and 5’-CAT TGC GGT GGA GAG TCC AGG G-3’; IL-17F, 5’-ACC CGT GAA ACA GCC ATG GTC AAG-3’, and 5’-CCC ATG GGG AAC TGG AGC GG-3’; IL-22, 5’-CAG CTC CTG TCA CAT CAG CGG T-3’, and 5’- AGG TCC AGT TCC CCA ATC GCC T-3’; IL-23, 5’-TCC TCC AGC CAG AGG ATC ACC C-3’, and 5’- AGA GTT GCT GCT CCG TGG GC-3; GAPDH, 5′-TCC CAC TCT TCC ACC TTC GA-3 and 5′-AGT TGG GAT AGG GCC TCT CTT G-3′. Relative mRNA expression was quantified using the ΔΔCt method, and GAPDH was used as an internal control. All results are expressed as fold changes.
Statistical Analysis
Each experiment was performed at least three times, and all values are presented as means ± standard deviation (S.D.). Statistical multivariate analysis was performed by analysis of variance (ANOVA) and Duncan tests, using SPSS 11 (Chicago, IL, USA) statistical software. Two-way ANOVA and one-way ANOVA were used to determine the significance of the results. Statistics were reviewed by a masters-level biostatistician and statistical significance was set at p < 0.05.
Results
Dunnione Improves the Morphological Features of IMQ-Induced Psoriasis-Like Dermatitis in Mice
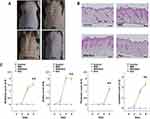
As shown in Figure 1A, psoriasis-like skin inflammation was induced by the topical application of IMQ to dorsal skin and right ear of C57BL/65 WT mice for 6 consecutive days. We administered dunnione on the second day after administering IMQ. Six days later, typical erythema, scaling, and thickening were observed in skin lesions induced by IMQ. However, psoriasis-like features were remarkably prevented in mice treated with dunnione (Figure 1A). The severity of the psoriasis-like skin condition was quantified on days 0, 2, 4, and 6 using the erythema, scaling, and thickening scores of PASI (Figure 1B). There was no significant change in PASI scores in the control and dunnione-treated mice, whereas the scores gradually increased in the IMQ group. Interestingly, mice treated with dunnione + IMQ had significantly lower PASI scores than those treated with IMQ alone. Consistent with the pathological manifestations, skin lesions treated with IMQ showed a thickened prickle cell layer of the epidermis with an increased number of prickle cells, hyperkeratosis, and infiltrating inflammatory cells in the upper dermis compared with control mice. These pathological changes were remarkably alleviated by dunnione, which produced a smoother epidermis, less keratosis, and less epidermal thickening than IMQ alone (Figure 1C). We also measured the thickness of the right ear in the IMQ group and observed a significant increase compared to the control group. However, in the IMQ + dunnione group, ear thickening was significantly reduced relative to that in the IMQ alone group (Figure 2A). In the HE-stained sections of IMQ-treated ear skin, epidermal thickness and subcutaneous tissue were increased compared to controls. Additionally, abnormal keratinocyte differentiation with marked parakeratosis (nuclei in the stratum corneum) was observed in IMQ-treated ear skin. However, dunnione inhibited the IMQ-induced increase in the thickness of both the epidermis and dermal tissue (Figure 2B). These results suggest that the administration of dunnione effectively alleviates IMQ-induced psoriasis-like dermatitis in WT mice.
Dunnione Decreases Expression of Psoriatic Inflammatory Cytokines
To further investigate the anti-inflammatory effects of dunnione, we quantified the expression of genes encoding the psoriasis-associated inflammatory cytokines 17A, IL-17F, IL-22, and IL-23 in skin (Figure 3A) and ear lesions (Figure 3B). As illustrated in Figure 3A and B, cytokine mRNA levels in IMQ-treated mice were significantly higher at day six than those in the normal controls. However, dunnione induced a significant reduction in these cytokine levels compared to those observed with IMQ alone (Figure 3A and B). Similar to the mRNA expression pattern, protein levels of IL-17, IL-22, and IL-23 were increased in the skin lesions in the IMQ group but significantly decreased in the dunnione + IMQ group (Figure 3C). These results demonstrate that the administration of dunnione effectively inhibited the expression of IMQ-induced psoriasis-associated inflammatory cytokines.
Protective Effect of Dunnione Requires NQO1
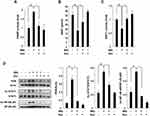
We next investigated whether the protective effect of dunnione on IMQ-induced psoriasis-like dermatitis was mediated by NQO1 using NQO1−/− mice. First, topical administration of IMQ resulted in typical psoriasis-like dermatitis with epidermal hyperplasia and infiltrated inflammatory cells in the skin lesions of NQO1−/− mice. However, unlike the WT mice, dunnione did not alleviate IMQ-induced psoriasis-like dermatitis, as indicated by the PASI score (Figure 4A and B). As shown in Figure 4C, IMQ-induced ear thickening in NQO1−/− mice also was not reduced by dunnione (Figure 4C). We measured the mRNA and protein levels of psoriasis-associated inflammatory cytokines IL-17, IL-22, and IL-23 in NQO1−/− mice. As expected, the levels of psoriatic cytokine mRNAs (Figure 5A) and proteins (Figure 5B) were significantly increased by IMQ; however, the increased levels of these mRNAs and proteins were not attenuated by dunnione in NQO1−/− mice. These results suggest that NQO1 is indispensable for the protective effects of dunnione.
Dunnione Attenuates PARP-1 Hyperactivation by IMQ-Induced Psoriasis-Like Injury and Prevents Decreases in Intracellular NAD+
We previously reported that the beneficial effects of dunnione were mediated by decreases in PARP activity, increases in intracellular NAD+ levels, and increases in SIRT1 activation.10,13 Thus, we measured PARP activity, intracellular NAD+ levels, and SIRT1 activity in our psoriatic mouse model. As shown in Figure 6A and D, both PARP activity and PAR formation were significantly increased in the IMQ group, whereas these increases were considerably attenuated in mice treated with dunnione. However, both intracellular NAD+ levels and SIRT1 activity in mouse skin lesions were significantly decreased by IMQ exposure, whereas dunnione treatment significantly attenuated these decreases (Figure 6B and C). Since activation of SIRT1 is known to induce deacetylation of the NF-κB subunit p65 and STAT3,6,10 we measured acetylation of NF-κB p65 and STAT3 by Western blotting. As shown in Figure 6D, both were significantly increased in skin lesions of IMQ-treated mice, but these effects were completely attenuated by dunnione treatment.
The Protective Effect of Dunnione on IMQ-Induced Psoriasis Requires SIRT1
In epidermal skin-specific SIRT1−/− mice, IMQ administration caused severe psoriatic phenotypes, including erythema, scaling, and thickening. However, unlike the effect observed in WT mice, dunnione failed to attenuate these phenotypes, as indicated by PASI scoring (Figure 7). Similarly, as shown in Figure 8, thickening of the ear in IMQ-treated SIRT1−/− mice was not attenuated by dunnione (Figure 8), suggesting that SIRT1 is required for the protective effects of dunnione.
Discussion
Psoriasis is a common chronic immune-mediated inflammatory skin disease with a prevalence of 2–3% worldwide, characterized by uncontrolled keratinocyte proliferation, recruitment of immune cells to the skin, and release of pro-inflammatory cytokines.1 Psoriasis is associated with multiple comorbidities, including joint destruction, cardiovascular disease, stroke, hypertension, metabolic syndrome, and chronic kidney disease. It is responsible for increasing medical costs every year.14
IMQ is a patient-applied immune response modifier and a TLR7/8 agonist prescribed to treat genital warts and Bowen’s disease (squamous cell carcinoma in situ). Topical IMQ can induce and exacerbate psoriasis. Our IMQ-induced dermatitis model shares many features with psoriasis, including activation of the IL-23/Th17/IL-17 axis,15 dependence on IL-22 to develop lesions, induction of STAT3 phosphorylation in keratinocytes, and oxidative stress and NF-κB activation.3 Although the underlying mechanism of psoriasis remains unclear, previous studies have shown extensive concurrence and cross-correlation of the above pathways and inflammatory cytokines.
In this study, we examined the effects of NAD+ modulation on IMQ-induced psoriasis-like dermatitis in mice. Our results suggest that the reduction in SIRT activity in this dermatitis may be associated with decreased intracellular NAD+ levels due to the hyperactivation of PARP1; moreover, it may play an important role in the development of psoriasis. Furthermore, reduced SIRT1 activity attenuated deacetylation of downstream targets that are activated by acetylation, and exacerbated IMQ-induced psoriasis through inflammatory responses with elevated levels of the inflammatory mediators IL-17A, IL-17F, IL-22, IL-23, as well as keratinocyte over-proliferation. However, dunnione significantly prevented keratinocyte proliferation; inflammatory responses; and gross, histological, cellular, and molecular changes in our mouse model. Dunnione also restored intracellular NAD+ levels and SIRT1 activity, decreased PARP activity, and increased the deacetylation of NF-κB and STAT3.
NAD+ acts as a metabolic cofactor and rate-limiting co-substrate for multiple enzymes involved in energy metabolism and cellular homeostasis; disturbances in cellular NAD+ levels are a feature of many pathological disorders, including diabetes, aging, hypertension, and arterial restenosis. Modulation of exogenous NAD+ shows therapeutic benefits against multiple diseases, including axonal degeneration, cerebral ischemia, and cardiac hypertrophy. Notably, the intracellular NAD+/NADH ratio can be easily modulated by NQO1, which catalyzes the two-electron reduction of natural substrates using NADH as an electron donor.16 Recent studies have shown that an increase in the NAD+/NADH ratio by β-lapachone or dunnione, both of which are NQO1 substrates, positively impacts the features of multiple metabolic syndromes,17 alcoholic ulcers,18 acute pancreatitis,11 cisplatin-mediated ototoxicity10 or kidney injury,12 and adriamycin-induced cardiac dysfunction,19 raising the possibility that this mechanism may represent a potential therapeutic strategy for treating multiple diseases. In this study, we examined the protective role of dunnione in IMQ-treated NQO1−/− mice. In the absence of NQO1, dunnione failed to prevent IMQ-induced psoriasis-like dermatitis. These data suggest that NQO1 is required for the protective effect of dunnione on IMQ-induced psoriasis-like dermatitis and can be a potential therapeutic strategy for treating multiple diseases.
As previously mentioned, NAD+ is a cofactor for multiple enzymes including PARP and SIRT1. One of the primary events in the development of psoriatic lesions is the accumulation of oxidative damage by the phagocytic reaction of neutrophils.20 Oxidative damage under pathological conditions activates PARP, an NAD+-dependent DNA repair enzyme. PARP is a major NAD+ consumer in the process of transferring an ADP-ribose moiety to PARP itself or to other acceptor proteins to build poly(ADPribose) polymers.21 Thus, appropriate PARP activation is required for DNA damage repair for normal cell function. However, PARP hyperactivation through sustained and severe oxidative DNA damage can deplete NAD+, impairing NAD+-dependent processes such as SIRT1 activity, and eventually leading to cell death and related pathological conditions.22 In our study, PARP hyperactivation was observed in IMQ-treated skin lesions, which led to a decline in intracellular NAD+ levels and SIRT1 activity. Conversely, dunnione restored intracellular NAD+ levels and SIRT1 activity through PARP inactivation. The exact roles of NAD+ and PARP1 in psoriasis are unclear and controversial; Martinez-Morcillo et al insisted that NAMPT-derived NAD+ causes PARP1 to promote skin inflammation through PARP1 hyperactivation.23 However, Kiss et al demonstrated that PARP1 depletion enhanced the severity of inflammation in an IMQ-induced model of psoriasis.24 We also demonstrated that increasing NAD+ by NQO1 enzymatic action, using dunnione as a substrate, markedly improved IMQ-induced skin lesions and associated inflammatory responses. There are possible explanations for these apparently contradictory results. There are several approaches to increase NAD+ levels in vitro and in vivo through the activation of de novo and salvage pathways for NAD+ biosynthesis. For example, NAD+ can be synthesized by a de novo pathway using L-tryptophan as a precursor or by a salvage pathway using NAMPT/NMNAT from nicotinamide and NMN as intermediate precursors. In any pathway, making NAD+ requires energy from ATP.25 It has been reported that oxidative stress, including ROS, plays critical roles in psoriasis and causes many pathological alterations in mitochondrial gene, function, and energy production. Under these circumstances, NAD+ production by the salvage pathway using NAMPT and subsequent excessive use by PARP1 will eventually lead to further depletion of ATP, ultimately leading to cell death and tissue damage. However, NAD+ can be increased naturally without energy use by direct oxidation of NADH to NAD+ through the enzymatic activity of NQO1, and by transferring the obtained electrons to dunnione, a substrate of NQO1. Compared with NAMPT, which generates NAD+ by using excess energy from an intermediate, there is no significant difference in terms of final NAD+ generation, but we suggest that it can make a significant difference in terms of effects on cells or tissues. Therefore, it is thought that increased NAD+ without excessive loss of ATP can activate SIRT1 and improve the skin symptoms of psoriasis. In this study, using skin-specific SIRT1−/− mice, we demonstrated that SIRT1 is critical for the protective effect of dunnione. Notably, the significant amelioration of skin inflammation by dunnione was completely restrained by knockout of the SIRT1 gene.
SIRT1, an NAD+-dependent enzyme, uses NAD+ as a substrate to deacetylate its downstream targets such as NF-κB p65 and STAT3, which regulate inflammatory responses. NF-κB is a crucial factor in immune-inflammatory responses implicated in multiple skin disorders, including psoriasis. Several anti-psoriatic drugs act in part by inhibiting NF-κB activation.26,27 In addition, upregulation of NF-κB activation in the psoriatic epidermis of patients with psoriasis has been demonstrated.28 Activation of the NF-κB pathway leads to the transcription of numerous genes, including those encoding cytokines, chemokines, and growth factors, involved in the initiation of the inflammatory response.26 Interestingly, SIRT1 has been shown to suppress NF-κB activation through deacetylation of NF-κB, resulting in the reduction of inflammatory responses.29 Remarkably, SIRT1 physically interacts with nuclear translocated NF-κB p65 and deacetylates NF-κB p65 at residue Lys-310, inhibiting the transcriptional activity of NF-κB.6 In this study, we also observed elevated NF-κB p65 acetylation in IMQ-treated skin lesions, which was facilitated by reduced SIRT1 activity induced by decreased intracellular NAD+ levels. However, the increase in the cellular NAD+ levels by NQO1 by dunnione resulted in downregulation of NF-κB p65 acetylation in the skin lesions, as well as production of inflammatory cytokines through increased SIRT1 activity. STAT3 is also a latent cytoplasmic transcription factor that plays a pathological role by altering the proliferation and differentiation of keratinocytes and inducing the expression of inflammatory molecules. STAT3 regulates the expression of genes controlling survival, proliferation, and angiogenesis through collaboration with other transcription factors, including NF-κB.30 In addition, STAT3 is a key signaling mediator of cytokines such as IL-17, IL-23, and IL-22, which are significantly increased in the psoriatic epidermis.31 Excessive activation of STAT3 has been observed in skin lesions of patients with psoriasis and overexpression of STAT3 in keratinocytes produced a psoriatic phenotype in transgenic mice.32 Notably, STAT3 activation is proportional to acetylation of residue Lys685, since post-translational modification is required for phosphorylation of Tyr705. SIRT1 mediates deacetylation of the STAT3 Lys685 site and negatively regulates STAT3 phosphorylation at Tyr705.33 Recently, it has been demonstrated that IL-22-induced proliferation and inflammation of keratinocytes is negatively regulated by SIRT1 through deacetylation of STAT3.6 In this study, we also found increased STAT3 acetylation in psoriatic lesions. However, restoration of intracellular NAD+ content by treatment with dunnione resulted in the downregulation of STAT3 acetylation through increased SIRT1 activity.
Conclusion
In conclusion, we demonstrated that augmentation of NAD+ levels by NQO1 enzymatic activation using dunnione as a substrate attenuates IMQ-induced psoriasis-like skin inflammation in mice through modulation of the PARP-1, SIRT1, STAT3, and NF-κB signaling pathways and potential inflammatory cytokines (summarized in Figure 8C). Therefore, these results suggest that modulation of cellular NAD+ levels by pharmacological agents such as dunnione is an attractive potential therapeutic strategy for patients with psoriasis and other skin diseases.
Disclosure
Hong-Seob So is an employee of NADIANBIO Ltd. The author reports no other potential conflicts of interest in this work.
References
1. Bochenska K, Smolinska E, Moskot M, Jakobkiewicz-Banecka J, Gabig-Ciminska M. Models in the Research Process of Psoriasis. Int J Mol Sci. 2017;18(12):2514.
2. Kim J, Krueger JG. The immunopathogenesis of psoriasis. Dermatol Clin. 2015;33(1):13–23.
3. Liu T, Li S, Ying S, et al. The IL-23/IL-17 Pathway in Inflammatory Skin Diseases: from Bench to Bedside. Front Immunol. 2020;11:594735.
4. Furue M, Furue K, Tsuji G, Nakahara T. Interleukin-17A and Keratinocytes in Psoriasis. Int J Mol Sci. 2020;21(4):1275.
5. Zapata-Perez R, Wanders RJA, van Karnebeek CDM, Houtkooper RH. NAD(+) homeostasis in human health and disease. EMBO Mol Med. 2021;13(7):e13943.
6. Zhou Y, Zhang F, Ding J. As a Modulator, Multitasking Roles of SIRT1 in Respiratory Diseases. Immune Netw. 2022;22(3):e21.
7. Kim HJ, Oh GS, Shen A, et al. Augmentation of NAD(+) by NQO1 attenuates cisplatin-mediated hearing impairment. Cell Death Dis. 2014;5:e1292.
8. Singh V, Ubaid S. Role of Silent Information Regulator 1 (SIRT1) in Regulating Oxidative Stress and Inflammation. Inflammation. 2020;43(5):1589–1598.
9. Ke Y, Wang C, Zhang J, et al. The Role of PARPs in Inflammation-and Metabolic-Related Diseases: molecular Mechanisms and Beyond. Cells. 2019;8(9):1047.
10. Kim HJ, Pandit A, Oh GS, et al. Dunnione ameliorates cisplatin ototoxicity through modulation of NAD(+) metabolism. Hear Res. 2016;333:235–246.
11. Shen A, Kim HJ, Oh GS, et al. NAD(+) augmentation ameliorates acute pancreatitis through regulation of inflammasome signalling. Sci Rep. 2017;7(1):3006.
12. Oh GS, Kim HJ, Choi JH, et al. Pharmacological activation of NQO1 increases NAD(+) levels and attenuates cisplatin-mediated acute kidney injury in mice. Kidney Int. 2014;85(3):547–560.
13. Pandit A, Kim HJ, Oh GS, et al. Dunnione ameliorates cisplatin-induced small intestinal damage by modulating NAD(+) metabolism. Biochem Biophys Res Commun. 2015;467(4):697–703.
14. Hawkes JE, Yan BY, Chan TC, Krueger JG. Discovery of the IL-23/IL-17 Signaling Pathway and the Treatment of Psoriasis. J Immunol. 2018;201(6):1605–1613.
15. van der Fits L, Mourits S, Voerman JS, et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J Immunol. 2009;182(9):5836–5845.
16. Katsyuba E, Romani M, Hofer D, Auwerx J. NAD(+) homeostasis in health and disease. Nat Metab. 2020;2(1):9–31.
17. Hwang JH, Kim DW, Jo EJ, et al. Pharmacological stimulation of NADH oxidation ameliorates obesity and related phenotypes in mice. Diabetes. 2009;58(4):965–974.
18. Jo IG, Park D, Kyung J, et al. Inhibitory effects of a beta-dunnione compound MB12662 on gastric secretion and ulcers. Lab Anim Res. 2013;29(3):178–181.
19. Khadka D, Kim HJ, Oh GS, et al. Augmentation of NAD(+) levels by enzymatic action of NAD(P)H quinone oxidoreductase 1 attenuates Adriamycin-induced cardiac dysfunction in mice. J Mol Cell Cardiol. 2018;124:45–57.
20. Chiang CC, Cheng WJ, Korinek M, Lin CY, Hwang TL. Neutrophils in Psoriasis. Front Immunol. 2019;10:2376.
21. Hurtado-Bages S, Knobloch G, Ladurner AG, Buschbeck M. The taming of PARP1 and its impact on NAD(+) metabolism. Mol Metab. 2020;38:100950.
22. Murata MM, Kong X, Moncada E, et al. NAD+ consumption by PARP1 in response to DNA damage triggers metabolic shift critical for damaged cell survival. Mol Biol Cell. 2019;30(20):2584–2597.
23. Martinez-Morcillo FJ, Canton-Sandoval J, Martinez-Navarro FJ, et al. NAMPT-derived NAD+ fuels PARP1 to promote skin inflammation through parthanatos cell death. PLoS Biol. 2021;19(11):e3001455.
24. Kiss B, Szanto M, Hegedus C, et al. Poly(ADP-ribose) polymerase-1 depletion enhances the severity of inflammation in an imiquimod-induced model of psoriasis. Exp Dermatol. 2020;29(1):79–85.
25. Hassa PO, Haenni SS, Elser M, Hottiger MO. Nuclear ADP-ribosylation reactions in mammalian cells: where are we today and where are we going? Microbiol Mol Biol Rev. 2006;70(3):789–829.
26. Sun W, Gao Y, Yu X, et al. ‘Psoriasis 1’ reduces psoriasislike skin inflammation by inhibiting the VDRmediated nuclear NFkappaB and STAT signaling pathways. Mol Med Rep. 2018;18(3):2733–2743.
27. Tang L, Li T, Zhang B, et al. Punicalagin Alleviates Psoriasis by Inhibiting NF-kappaB-Mediated IL-1beta Transcription and Caspase-1-Regulated IL-1beta Secretion. Front Pharmacol. 2022;13:817526.
28. Moorchung N, Kulaar JS, Chatterjee M, Vasudevan B, Tripathi T, Dutta V. Role of NF-kappaB in the pathogenesis of psoriasis elucidated by its staining in skin biopsy specimens. Int J Dermatol. 2014;53(5):570–574.
29. de Gregorio E, Colell A, Morales A, Mari M. Relevance of SIRT1-NF-kappaB Axis as Therapeutic Target to Ameliorate Inflammation in Liver Disease. Int J Mol Sci. 2020;21(11):3858.
30. Wang A, Wei J, Lu C, et al. Genistein suppresses psoriasis-related inflammation through a STAT3-NF-kappaB-dependent mechanism in keratinocytes. Int Immunopharmacol. 2019;69:270–278.
31. Bugaut H, Aractingi S. Major Role of the IL17/23 Axis in Psoriasis Supports the Development of New Targeted Therapies. Front Immunol. 2021;12:621956.
32. Nakajima K, Kataoka S, Sato K, et al. Stat3 activation in epidermal keratinocytes induces Langerhans cell activation to form an essential circuit for psoriasis via IL-23 production. J Dermatol Sci. 2019;93(2):82–91.
33. Xu F, Xu J, Xiong X, Deng Y. Salidroside inhibits MAPK, NF-kappaB, and STAT3 pathways in psoriasis-associated oxidative stress via SIRT1 activation. Redox Rep. 2019;24(1):70–74.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.