Back to Journals » Infection and Drug Resistance » Volume 16
Attenuated Cytokine-Induced Memory-Like Natural Killer Cell Responses to Mycobacterium tuberculosis in Tuberculosis Patients
Authors Liang C, Li S , Yuan J, Song Y, Ren W, Wang W, Shang Y , Tang S , Pang Y
Received 28 February 2023
Accepted for publication 15 April 2023
Published 20 April 2023 Volume 2023:16 Pages 2349—2364
DOI https://doi.org/10.2147/IDR.S407742
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Chen Liang,1,2,* Shanshan Li,1,* Jinfeng Yuan,1 Yanhua Song,3 Weicong Ren,1 Wei Wang,1 Yuanyuan Shang,1 Shenjie Tang,2 Yu Pang1
1Department of Bacteriology and Immunology, Beijing Key Laboratory on Drug-Resistant Tuberculosis Research, Beijing Chest Hospital, Capital Medical University /Beijing Tuberculosis and Thoracic Tumor Research Institute, Beijing, 101149, People’s Republic of China; 2Tuberculosis Clinical Medical Center, Beijing Chest Hospital, Capital Medical University /Beijing Tuberculosis and Thoracic Tumor Research Institute, Beijing, 101149, People’s Republic of China; 3Department of Tuberculosis, Beijing Chest Hospital, Capital Medical University/Beijing Tuberculosis and Thoracic Tumor Research Institute, Beijing, 101149, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Shenjie Tang; Yu Pang, Beijing Chest Hospital, Capital Medical University, No. 97, Machang, Tongzhou District, Beijing, 101149, People’s Republic of China, Tel/Fax +86 010 8950 9367 ; +86 010 8950 9359, Email [email protected]; [email protected]
Purpose: This study aimed to investigate the phenotype, proliferation and functional alterations of cytokine-induced memory-like natural killer (CIML NK) cells from healthy subjects and TB patients, and assessed the efficacy of CIML NK cells in response to H37Rv-infected U937 cells in vitro.
Methods: Fresh peripheral blood mononuclear cells (PBMCs) were isolated from healthy people and tuberculosis patients and activated for 16h using low-dose IL-15, or IL-12, IL-15, IL-18 combination or IL-12, IL-15, IL-18 and MTB H37Rv lysates, respectively, followed by low-dose IL-15 maintenance for another 7 days. Then, the PBMCs were co-cultured with K562 and H37Rv-infected U937, and the purified NK cells were co-cultured with H37Rv infected U937. The phenotype, proliferation and response function of CIML NK cells were assessed using flow cytometry. Finally, colony forming units were enumerated to confirm the survival of intracellular MTB.
Results: CIML NK phenotypes from TB patients were similar to healthy controls. CIML NK cells undergo higher rates of proliferation after IL-12/15/18 pre-activation. Moreover, the poor expansion potential of CIML NK cells co-stimulated with MTB lysates. CIML NK cells from healthy individuals showed enhanced IFN-γ functional to H37Rv infected U937 cells, along with significantly enhanced killing of H37Rv. However, the CIML NK cells from TB patients show attenuated IFN-γ production and now enhanced the ability of killing intracellular MTB compared to those from healthy donors after co-cultured with H37Rv infected U937.
Conclusion: CIML NK cells from healthy individuals exist the increased ability of IFN-γ secretion and boosted anti-MTB activity in vitro, which from TB patients show impaired IFN-γ production and no enhanced anti-MTB activity compared to those from healthy donors. Additionally, we observe the poor expansion potential of CIML NK cells co-stimulated with antigens from MTB. These results open up new possibilities for NK cell-based anti-tuberculosis immunotherapeutic strategies.
Keywords: Mycobacterium tuberculosis, cytokines, NK cells, memory-like NK cells, IFN-γ
Introduction
Mycobacterium tuberculosis (MTB), the causative agent of tuberculosis (TB), is one of the world’s most pernicious human pathogens.1 In 2020, 10.0 million people developed active TB and approximate 1.5 million people died from this disease.2,3 The success of this pathogen lies in its ability to persist within humans throughout the life span.4 It is estimated that one-third of the global population is infected with MTB, posing a major contributor to the pool of active TB cases.5 Current vaccines have poor efficacy, and the prolonged treatment with combinations of drugs is required to cure disease due to eradication of nonreplicating bacilli.6 To overcome these problems, there is a need for a better understanding of MTB in the context of its host for the development of new immunotherapeutic strategies.
As the host’s first line of defense against invading pathogens, innate immune responses play an important role in early clearance of tubercle bacilli, and shaping the nature and magnitude of adaptive immune responses.7 In Innate immunity, NK cells play a well-recognized role in actively inducing death of MTB-infected monocytes and shaping acquired cell-mediated immunity.8 A previous clinical study revealed that newly diagnosed TB patients had significantly lower frequencies of NK cell subsets, coinciding with decreased expression of activating receptors, indicating that NK cells may be functionally impaired during TB infection.9 Correspondingly, another recent study provided evidence that the NK cell cytotoxic activity was restored upon successful anti-TB treatment via monitoring the activity of circulating NK cells before and after anti-TB treatment.10,11 Thus, these studies point towards a remarkable contribution of NK cells in immune defenses against MTB.
Traditionally, the activation of NK cells employs non-antigen specific mechanism during infection, but a growing body of evidence demonstrates that NK cells can elicit recall responses against previously experienced antigens.12 The existence of memory-like NK cells was reported in different species, including non-human primates and human.13,14 Specific memory responses of NK cells were initially identified in cytomegalovirus-infected mice and in the development of delayed-type hypersensitivity responses to chemical semi-antigens and viral antigens,15 and several subsequent studies have shown that NK cells can mediate memory-like responses to a variety of pathogens, including herpes simplex virus,16 human immunodeficiency virus,17,18 Ehrlichial pathogens19 and Mycobacterium tuberculosis.20 The adaptive features of NK cells open new possibilities for NK cell-based therapeutic strategies against TB. Specifically, it has been reported that stimulating NK cells with multiple cytokines was able to endow them with memory-like characteristics.21 A series of pre-clinical and clinical trials have demonstrated the considerable therapeutic effects of cytokine-induced memory-like (CIML) NK cells in patients with different malignancies.22 The enhanced anti-tumor responses are majorly attributed to the increased interferon-γ (IFN-γ) production by CIML NK cells. In view of the essential for IFN-γ in resistance to intracellular tubercle bacilli,23 an interesting question is whether the specific subset of NK cells could bring additional benefits for TB patients to accelerate bacterial clearance. We therefore conducted in vitro experimental study to compare the phenotypes, proliferation and functional alterations of peripheral blood mononuclear cell-induced CIML NK cells in healthy subjects and TB patients, and to assess the efficacy of CIML NK cells in response to H37Rv-infected U937 cells.
Materials and Methods
Reagents, Bacteria and Cell Line
The following anti-human antibodies were used: PerCP/Cyanine 5.5-anti-human CD3 (clone: HIT3a), Brilliant Violet 421 anti-human IFN-γ (clone: B27), PE/Cyanine7 anti-human CD107a (H4A3), Brilliant Violet 650 anti-human CD57 (QA17A04), Zombie NIRTM Fixable Viability and CFSE-FITC (all BioLegend); Brilliant Violet 480 anti-human CD16 (clone: 3G8), PE anti-human CD56 (clone: B159) (all BD Biosciences). The following endotoxin-free recombinant human (rh) cytokines were used: rhIL-12 and rhIL-15 (PeproTech), and rhIL-18 (Biolegend). The standard strain MTB H37Rv strain (ATCC: 27294) was preserved in Department of Bacteriology and Immunology, Beijing Chest Hospital, Capital Medical University, taken out of the −80 °C freezer for recovery, and cultivated in Lowenstein-Jensen medium. MTB H37Rv was lysed using FastPrep-24 5G (MP Biomedicals). The lysates were centrifuged and the supernatants were prepared to obtain MTB H37Rv whole cell lysates. K562 (ATCC: CCL-243; K-562 are lymphoblast cells isolated from the bone marrow of a 53-year-old chronic myelogenous leukemia patient) and U937 cells (ATCC: CRL-1593.2) were stored in liquid nitrogen, resuscitated and thawed at 37 °C for use in our studies, and maintained for <2 months at a time in continuous culture in RPMI-1640 medium containing penicillin/streptomycin and 10% FBS (Gibco). The K562 and U937 cell lines were gently provided by Beijing Children’s Hospital affiliated to Capital Medical University.
Samples Collection and Study Design
Peripheral blood was collected from healthy volunteers and TB patients at Beijing Chest Hospital from May 2022 to November 2022. Healthy volunteers were recruited based on the following inclusion criteria: (i) aged 18–70 years; (ii) T-SPOT negative; (iii) without any tuberculosis-related symptoms, and chest imaging examination without active lesions; (iv) no history of TB and other immune-related diseases. The pulmonary TB patients were diagnosed according to National Health Commission of the People’s Republic of China pulmonary tuberculosis diagnosis and treatment guidelines (WS 288–2017, China). TB patients should met all inclusion criteria as follows: (i) aged 18–70 years; (ii) new cases without any anti-TB treatment before; (iii) positive results for MTB test using sputum culture and/or GeneXpert MTB/RIF test; (iv) pulmonary imaging features indicative of TB; (v) informed consent. All people with MTB previous infection, HIV co-infection, diabetes mellitus or other immune-related comorbidity were excluded from our study.
Donor peripheral blood mononuclear cells (PBMCs) were isolated by Lymphocyte Separation Medium (TBD). Fresh PBMCs were plated at 4–5×106 cells/mL in 12-well cell culture plate and preactivated for 16h hours using [rhIL-12 (10 ng/mL) plus rhIL-15 (50 ng/mL) plus rhIL-18 (50 ng/mL)] or [rhIL-12 (10 ng/mL) plus rhIL-15 (50 ng/mL) plus rhIL-18 (50 ng/mL) and MTB H37Rv whole cell lysates (20 μg/mL)] or control conditions (rhIL-15, 1 ng/mL) in RPMI-1640 medium containing penicillin/streptomycin and 10% FBS, respectively (Cooper et al, 2009; Romee et al, 2016).15,18 Following pre-activation, cells were washed to remove cytokines and cultured in RPMI-1640 supplemented with rhIL-15 (1 ng/mL) to support survival for 7 days. Fresh media was replaced every 2–3 days. On day 7, PBMCs were collected for subsequent experiments. NK cells were isolated from PBMCs using negative magnetic bead separation (Miltenyi Biotec, Bergisch Gladbach, Germany), consistently achieving greater than 95% purity for CD3–CD56+ NK cells (Figure S1).
Proliferation Assays
PBMCs from healthy subjects and TB patients were prepared as described above. PBMCs were adjusted to 107/mL in PBS and incubated with CFSE (working concentration: 0.25μM) for 20 minutes at room temperature in the dark. Next, the suspensions were washed using PBS for three times to remove unbound dye and incubated with the Zombie NIR Fixable Viability for 10min. The cells were incubated with PerCP/Cyanine5.5 anti-human CD3 antibodies and PE anti-human CD56 antibodies to distinguish CD3−CD56+NK cells for 30 min at 4 °C protected from light, the single cells were suspended in D-PBS and data were acquired using LSRFortessa flow cytometer (BD Biosciences, San Diego, CA, USA). The PBMCs collected here before treatment with cytokines at this time are the undivided cells for NK cell proliferation. The rest of the CFSE labeled cells were pre-activated referring to the previous paragraph. On the 8th day, the cells were collected for flow cytometry analysis. Percentage of divided cells=number of cells that have undergone proliferation/total number of cells before stimulation×100%.
Functional Assays and Survival of MTB
Fresh PBMCs were activated for 16 h referring to the previous paragraph and harvested after 7 days with rhIL-15 (1 ng/mL) treatment. Then, cells were restimulation with cytokine or co-cultured with K562 or H37Rv infected U937. For restimulation, PBMCs were plated at 5×105 cells/mL in 12-well cell culture plate restimulated for 6h using IL-12/15/18. For targeting K562, PBMCs were incubated with K562 cells (105) for 6h (effector/target ratio of 5:1). For targeting H37Rv infected U937, U937 cells (105 for PBMCs or 5×104 for purified NK cells) were treated with 1μM of Phorbol 12-myristate 13-acetate (Sigma) overnight into macrophage phenotype prior to H37Rv infection. The optical density (OD) value of H37Rv was adjusted to infect U937 cells at MOI=5 for 4 h. Next, cells were washed 3 times with PBS to remove the un-phagocytosed bacteria. Preactivated PBMCs or purified NK cells were incubated with H37Rv infected U937 for 6 h (effector/target ratio of 5:1). GolgiPlug and GolgiStop (brefeldin A and monensin, respectively) protein transport inhibitors (BD Biosciences) were added 4 hours prior to cell collection in all groups. Next, the supernatant cells were washed twice with D-PBS supplemented with 1% of FBS, and stained with Zombie NIR Fixable Viability kit (BioLegend) to exclude dead cells. Then, the cells were stained for cell surface markers (CD3, CD56, CD16, CD57 and CD107a) with the respective fluorochrome-conjugated antibodies for 30 min on ice protected from light. For intracellular IFN-γ staining, cells were fixed and permeabilized with BD Cytofix/Cytoperm Kit following the manufacturer’s protocol. Finally, cells were washed twice and acquired in a BD LSRFortessa (BD Biosciences) cytometer. NK cells were gated as CD3−CD56+(Figure 1B for details on gating strategies) for all analysis. Flow cytometry data were analyzed with FlowJo v10.8.1. Meanwhile, the adherent cells in the lower layer were washed three times with PBS to confirm the survival of H37Rv. 0.05% SDS (in PBS) was added to lyse the cells, followed by 10-fold serial dilutions and cell lysates were plated on 7H10 agar for 4–6 weeks at 37 °C (5% CO2) to enumerate colony forming units (CFUs).
Statistical Analysis
GraphPad Prism Version 9.0 software was used for graphical representation and statistical analysis. Data were represented using Means ± SEM. The Shapiro–Wilk Test was used to test whether the data conformed to a normal distribution. If the data conformed to a normal distribution, Student’s t test was used for statistical analyses between two groups. And Ordinary one-way or two-way ANOVA with Tukey’s multiple comparisons test was used to determine significant differences among multiple groups. If the data do not conform to a normal distribution, Wilcoxon rank sum test was used for statistical analyses between two groups. A Kruskal–Wallis Test was used to determine significant differences among multiple groups. If the results of a Kruskal–Wallis test are statistically significant, then Dunn’s Test was used to determine exactly which groups are different. Values of P<0.05 were considered statistically significant.
Results
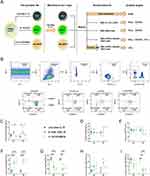
Characteristics of Cytokine-Induced Memory-Like NK Cells in Healthy Donors and Tuberculosis Patients
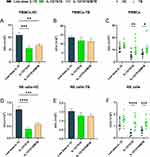
The whole experimental schematic is shown in Figure 1A. Figure 1B was the representative flow cytometry gating strategy, which was set with FMO control for NK cells (Figure S2). In healthy subjects, the percentage of NK cells in the lymphocytes with low-dose IL-15, IL-12/15/18, and IL-12/15/18/MTB induction group were (3.60±0.73) %, (5.83±0.90) and (2.94±0.69) %, respectively (Figure 1C). In TB patients, the percentage of NK cells in the lymphocytes with above three different treatments were (2.98±1.07) %, (6.54±2.12) %, (3.76±1.39) %, respectively (Figure 1C). Compared with control NK group (low-dose IL-15 pretreatment), the percent of NK cells was enhanced after the IL-12/15/18 stimulation in both healthy donors (P<0.05) and TB patients (P<0.01), but there was no significant difference in IL-12/15/18/MTB treatment groups (P>0.05). Further comparison of the proportion of the two major NK subsets found that there was no significant change in the ratio of the CD56dim and CD56bright NK after cytokine induction (Figure 1D and E). However, the proportion of CD16−CD56dim NK cells was considerably increased in IL-12/15/18 and IL-12/15/18/MTB stimulated group (Figure 1F), and the proportion of CD16+CD56dim NK cells was greatly reduced in both stimulated groups (Figure 1G). There was no statistical difference in the change in the proportion of CD16−CD56bright NK cells after cytokine induction (P>0.05, Figure 1H). The percent of CD16+CD56bright NK cells was reduced in IL-12/15/18 and IL-12/15/18/MTB stimulated group in TB (P<0.001, Figure 1I).
Proliferation and Differentiation of Cytokine-Induced Memory-Like NK Cells in Healthy and TB Donors
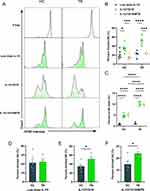
The proliferative effect of NK cells with low dose IL-15, IL-12/15/18 or IL-12/15/18/MTB H37Rv lysate pre-activation on PBMCs from healthy donors and TB patients was investigated by CFSE labeling and dye dilution was evaluated after 7 days. CFSE labeling was measured by flow cytometry for CD3−CD56+ NK cells. Representative CFSE flow cytometry results of CD3−CD56+ NK cells were shown Figure 2A. In healthy subjects, the percentage of divided NK cells in the low-dose IL-15, IL-12/15/18, and the IL-12/15/18/MTB induction group were (21.23±8.32) %, (34.85±8.44) % and (14.83±4.89) %, respectively (Figure 2B). In TB patients, the percentage of divided NK cells in the above three different treatments was (22.18±6.92) %, (51.2±9.26) %, (23.83±2.62) %, respectively (Figure 2B). Compared with control group (low-dose IL-15 pretreatment), the proliferation of NK cells was greatly enhanced after the IL-12/15/18 stimulation in both healthy donors (P<0.05) and TB patients (P<0.0001). However, when PBMCs were co-treated with IL-12/15/18/MTB, the percentage of divided NK cells decreased compared to the IL-12/15/18 group (P<0.001, Figure 2B). When PBMCs were treated with low doses of IL-15, the proportion of NK cell divided was not notably different between healthy individuals and TB patients (P>0.05, Figure 2D). While the proportion of divided NK cells from TB patients was higher than that from healthy people after IL-12/15/18 or IL-12/15/18/MTB induction (P<0.05, Figure 2E and F). Meanwhile, after IL-12/15/18 or IL-12/15/18/MTB induction, the percentage of dead NK cell in TB group was significantly greater than that in healthy group (P<0.0001, Figure 2C). There were no obvious differences noted in the proportion of CD57+ NK cells among these treatments (Figure S3).
Cytokine-Induced Memory-Like NK Cells Exhibited Significantly Enhanced Functional Responses
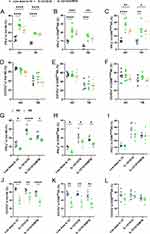
Previous studies have determined that human NK cells which were pre-activated with IL-12, IL-15, and IL-18 exhibit memory-like functional responses to leukemia target cells.24 To evaluate whether NK cells from TB patients affect the IFN-γ release and degranulation levels after cytokine pre-activation, flow cytometry analysis gating on CD3−CD56+NK cells were performed. Representative flow cytometry gating strategy for IFN-γ+ and CD107a+ NK cells as shown Figure 3A and B. And the gating strategy of IFN-γ and CD107a positive quadrant for CD56dim and CD56bright NK cells were shown in Figure S4. Our results found that CIML NK cells exhibited enhanced IFN-γ production with cytokine restimulation (P<0.0001, Figure 3A and C). The levels of IFN-γ secreted by two subsets of NK cells (CD56dim and CD56bright) also showed an enhanced function after cytokine induction (P<0.0001,Figure 3D and E). In healthy subjects, the percentage of IFN-γpositive NK cells in the low-dose IL-15, IL-12/15/18 induction, and the IL-12/15/18/MTB induction group were (2.3±1.04) %, (38.89±10.3) % and (41.46±6.49) % in total NK cells, (1.9±1.00) %, (39.85±10.98) % and (40.27±3.97) % in CD56bright NK, (2.27±0.88) %, (40.93±7.35) % and (41.04±3.88) % in CD56dim NK, respectively. In TB patients, the percentage of IFN-γ positive NK cells in the low-dose IL-15, IL-12/15/18 induction, and the IL-12/15/18/MTB induction group were (2.41±2.52) %, (36.9±6.61) % and (35.48±4.35) % in total NK cells, (3.32±2.46) %, (37.1±8.18) % and (39.55±3.73) % in CD56bright NK, (2.31±2.37) %, (34.73±7.45) % and (32.33±9.05) % in CD56dim NK, respectively. The presence of MTB lysate did not affect IFN-γ secretion by CIML NK cells (Figure 3C–E). The level of total NK cells, CD56dimNK and CD56brightNK degranulation was increased after cytokine preactivated (P<0.01, Figure 3B, F, G and H). In health population, the percentage of CD107a positive NK cells with above three different treatments were (18.2±1.46) %, (38.83±10.27) % and (36.73±5.87) % in total NK, (5.42±2.02) %, (20.87±6.91) % and (22.9±5.06) % in CD56bright NK, (10.10±3.19) %, (25.72±8.96) % and (19.38±5.28) % in CD56dimNK, respectively. In TB patients, the percentage of CD107a positive NK cells with above three different treatments were (20.15±4.93) %, (54.08±10.88) %, (47.80±16.20) % in total NK, (8.95±4.21) %, (26.43±9.83) % and (20.05±4.51) % in CD56bright NK, (7.88±4.25) %, (20.73±4.09) % and (24.68±4.03) % in CD56dimNK, respectively. After cytokine restimulation, IFN-γ release (Figure 3I–K) and CD107a (Figure 3L–N) expression of NK cell were compared between healthy subjects and TB patients, showing no significant differences between the two populations (P > 0.05). These findings demonstrated that IL-12/15/18 and IL-12/15/18/MTB preactivated human NK cells could affect IFN-γ and degranulation levels in both health and TB population.
Cytokine-Induced Memory-Like NK Cells Functional Responses Against K562 Target Cells in HC and TB Population
IFN-γ expression and degranulation levels of NK cells in peripheral blood of TB patients were reduced, and returned to baseline levels after clinical cure.25,26 In order to verify whether the function of NK cells in TB patients could be rescued by cytokine induction, the induced cells were incubated with K652 cells for another 6 hours, and then the levels of IFN-γ and CD107a in NK cells were detected using flow cytometry. In healthy subjects, the percentage of IFN-γ positive NK cells in the low-dose IL-15, IL-12/15/18 induction, and the IL-12/15/18/MTB induction group were (12.78±2.69) %, (28.82±3.31) % and (22.64±3.26) % in total NK cells, (12.83±1.19) %, (36.95±8.04) % and (27.63±2.25) % in CD56bright NK, (9.68±1.18) %, (21.48±5.50) % and (18.87±2.30) % in CD56dim NK, respectively. The percentage of CD107a positive NK cells with above three different treatments were (67.22±1.93) %, (63.83±4.18) % and (58.48±4.52) % in total NK, (54.00±10.29) %, (53.18±9.53) % and (46.20±4.56) % in CD56bright NK, (56.1±3.88) %, (55.37±3.40) % and (52.23±8.38) % in CD56dim NK, respectively. In TB patients, the percentage of IFN-γ positive NK cells in the low-dose IL-15, IL-12/15/18 induction, and the IL-12/15/18/MTB induction group were (7.66±1.19) %, (19.03±0.62) %, (17.76±1.42) % in total NK cells, (11.72±2.33) %, (29.98±9.96) % and (26.8±9.25) % in CD56brightNK, (3.62±1.86) %, (15.07±3.3) % and (12.70±1.78) % in CD56dimNK, respectively. The percentage of CD107a positive NK cells with above three different treatments were (39.98±8.21) %, (48.95±9.39) % and (36.70±6.70) % in total NK, (43.60±9.03) %, (51.84±6.24) % and (41.6±6.81) in CD56brightNK, (32.60±9.92) %, (34.75±11.41) % and (30.78±9.13) % in CD56dimNK, respectively. CIML NK cells exhibited enhanced IFN-γ production in response to K562 leukemia cells both in HC and TB group (P<0.01, Figure 4A–C). However, there was no significant difference in the level of cell degranulation under three treatment condition in either the HC or the TB patient group (P>0.05, Figure 4D–F). The total NK and CD56dim NK cells from TB patients showed conspicuously weaker levels of IFN-γ and CD107a against K562 target cells overall than that from healthy controls (P<0.05, Figure 4G, H, J and K). However, the levels of IFN-γ and CD107a responses of CD56brightNK cells to K562 target cells were not a remarkable discrepancy when compared between healthy individuals and TB patients (P>0.05, Figure 4I and L).
Cytokine-Induced Memory-Like NK Cells Functional Responses Against H37Rv-Infected U937 Cells in HC and TB Population
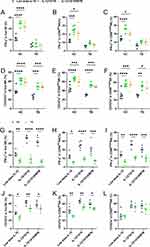
We next verified the efficacy of CIML NK cells in response to H37Rv-infected U937 cells. On day 7 after cytokine induction, the induced PBMCs and purified NK cells were incubated with H37Rv infected U937 cells for 6 h. The levels of IFN-γ and CD107a expressing in NK cells were assayed using flow cytometry in PBMCs. The lower cell layer was lysed and CFUs were counted to evaluate the intracellular bactericidal ability of NK cells. In healthy subjects, the percentage of IFN-γ positive NK cells in the low-dose IL-15, IL-12/15/18 induction, and the IL-12/15/18/MTB induction group were (11.29±2.13) %, (24.52±2.09) % and (22.03±2.98) % in total NK cells, (14.13±1.64) %, (26.25±3.36) % and (19.4±4.07) % in CD56brightNK, (11.8±1.55) %, (25.15±3.88) % and (20.70±6.71) % in CD56dimNK, respectively. In HC group, the levels of IFN-γ positive NK cells were enormously increased with IL-12/15/18 induction after exposure to H37Rv infected U937 (P<0.0001, Figure 5A), and also significantly enhanced in the two major NK cell subtypes CD56dimNK cells (P<0.001, Figure 5B) and CD56brightNK cells (P<0.0001, Figure 5C). However, in TB patients, the percentage of IFN-γ positive NK cells in the low-dose IL-15, IL-12/15/18 induction, and the IL-12/15/18/MTB induction group were (5.24±1.60) %, (6.25±2.62) % and (4.45±1.80) % in total NK cells, (5.87±2.31) %, (10.66±1.62) % and (9.99±2.79) % in CD56bright NK, (5.50±1.05) %, (9.58±1.51) % and (7.00±1.6) % in CD56dim NK, respectively. CIML NK cells derived from PMBCs of TB patients showed little enhanced functional response after co-incubation with H37Rv-infected U937 cells, as evidenced by the absence of a significant increase in the expression of IFN-γ after cell pre-activated by cytokines (P>0.05, Figure 5A).
The expression of CD107a positive cells was also increased after IL-12/15/18 preactivated on the surface of NK cells both in health and TB population (P<0001, Figure 5D). In health people, the percentage of CD107a positive NK cells in the low-dose IL-15, IL-12/15/18 induction, and the IL-12/15/18/MTB induction group were (39.27±7.23) %, (62.50±4.60) % and (54.95±9.30) % in total NK, (31.32±9.75) %, (56.42±5.83) % and (53.94±8.15) % in CD56brightNK, (45.72±8.33) %, (66.33±3.21) % and (59.92±5.64) % in CD56dimNK, respectively. In TB patients, the percentage of CD107a positive NK cells with above three different treatments were (26.63±4.47) %, (48.70±4.75) % and (47.55±6.23) % in total NK, (29.94±2.35) %, (51.98±7.16) % and (46.50±8.35) % in CD56brightNK, (30.48±1.72) %, (53.03±4.34) % and (51.08±6.26) % in CD56dimNK, respectively. Moreover, the IFN-γfunctional response of CIML NK cells from TB patients to H37Rv was weaker than that of healthy people (P<0.0001, Figure 5G–I). Although NK cells in the TB patient group showed increased expression of CD107a after IL-12/15/18 induction, their degranulation levels were significantly weaker compared to healthy subjects (P<0.05, Figure 5J and K). However, there was no significant difference in CD107a expression in CD56brightNK cells when comparing healthy individuals and TB patients (P>0.05, Figure 5L).
Furthermore, our results showed that after cytokine-preactivated PBMCs and purified NK cell co-incubation with H37Rv-infected U937 cells, the CFU counts of healthy human-derived PBMCs and purified NK cells were significantly lower compared to control NK cells (P<0.01, Figure 6A and D). Cytokine-preactivated PBMCs and purified NK cells from TB patients incubated with H37Rv-infected U937 did not contribute to obvious CFU number change under the three treatment conditions (P>0.05, Figure 6B and E). The above results showed that intracellular MTB killing ability of TB patient-derived PBMCs and purified NK cells which were preactivated using IL-12/15/18 or IL-12/15/18/MTB were not markedly enhanced compared with low-dose IL-15 treatment, and were also conspicuously weaker compared to the simultaneously treated PBMCs and purified NK from HC group (P< 0.01, Figure 6C and F).
Discussion
The development of new therapeutic strategies for tuberculosis treatment is urgently needed to facilitate its global eradication.27 An alternative approach is provided by host-directed therapies, which interfere with host cell factors required for survival or replication of tubercle bacilli.28 Motivated to make inroads into the urgent needs within host-directed therapy for TB patients, we investigated whether pre-activation of human PBMCs with IL-12, IL-15, and IL-18 produced enhanced protective immune responses against MTB. First, we analyzed the changes in the CIML NK phenotype. In our results, the expression of CD16 on NK cell surface showed a downward trend after the pre-activation of IL-12/15/18 and IL-12/15/18/MTB. The results were in according to previous report that cytokine exposure resulted in decreased expression of CD16 on the NK cell surface,29 which correlated with increased IFN-γ expression.30 CD16+ NK cells from patients with tuberculous pleuritis have increased susceptibility to apoptosis, which leads to reduced frequency of NK cells.31–33 Therefore, the decrease in the proportion of CD16+NK cells and shedding of CD16 after IL-12/15/18 and IL-12/15/18/MTB pre-activation may be a regulatory mechanism by which NK cell activity is controlled to prevent auto-aggressiveness or activation-induced cell death. The mechanism of surface expression and function of CD16 on NK cells is regulated by disintegrin and metalloproteinase 17 (ADAM17).30 However, the signaling pathway behind the changes of CD16 expression on NK cell after cytokines treatment need to be further investigated. In addition, CD57 is the marker of NK cell terminal maturation, which is only expressed in CD56dim. In this study, there was no statistical difference in the expression of CD57 before and after cytokine induction. Existing studies suggest that CIML NK may not enhance its own function by inducing cell differentiation and maturation.20,34 CIML NK plays a role in long-term persistence and memory through epigenetic (CpG demethylation) and cellular metabolic changes (glucose transporter and transferrin receptor).20,35,36 The expression of CD57 is weak in response to cytokine signals and IFN-γproduced by antigen stimulation is also low.37–39 The CD57+ phenotypes of tuberculosis patients in different states of the disease are also divergent.40–42 This may be the reason for the huge heterogeneity in CD57+ phenotype among TB group in our study.
Furthermore, our results provide a first insight into the boosted anti-TB activity of human CIML NK cells via facilitating the secretion of IFN-γ, but not enhancing cytotoxicity. Similar results were also reported by a recent study when CIML NK cells were restimulated in vitro with a variety of hepatocellular carcinoma cell lines.43 These data highlight the essential role of IFN-γ for controlling MTB growth in patients, which is supported by a previous therapeutic trial demonstrating positive results of safety and efficacy for IFN-γ in accelerating eradication of MTB infection.44,45 In comparison with conventional administration methods, the CIML NK cells have several potential advantages in host-directed therapies. On the one hand, these CIML NK cells persist for a long time and are highly functional, which make them a good alternative for immunotherapy. In other words, they are better able to serve as IFN-γ reservoir to modulate the protective immune responses against MTB. On the other hand, previous experimental studies confirm the high specificity of CIML NK cells and do not demonstrate unwilling effector function towards autologous cells.24 Thus, it has potential for maintaining immune homeostasis and controlling tissue damage that occurs during host-directed therapies. Collectively, the CIML NK based immunotherapy holds great promise for the treatment of TB patients, especially drug-resistant TB patients who respond poorly to existing anti-TB drug therapy.
Another interesting finding of our study was that the CIML NK cells induced by PBMCs of patients with tuberculosis could not enhance the elimination of MTB, and its functional response was significantly weaker than that of healthy people, which may suggest that some of immunosuppressive effect on NK cells associated with TB infection can be overcome with CIML NK cells from health donors rather than TB patients. In previous studies on the mechanisms of NK cells, IL-12 and IL-18 induce NK-cell activity and bias immunity towards a Th1 cell response characterized by profound IFN-γ production, which is considered critical for protection against MTB.46–48 And NK subsets of pulmonary TB patients had significantly compromised NKp30 and NKp46 expression and decreased IFN-γ production upon triggering. This is in agreement with reduced NKp46 mRNA expression in TB patients with ineffective immunity to MTB compared with health donors.49 Although the exact mechanism of defective response of NK cells remains unclear, at present, evidence indicates that MTB may hijack the host gene expression to inhibit the immuno-metabolic reprogramming of NK cells.50,51 Importantly, this immunosuppressed status could not be reversed by cytokine-mediated activation. Further study is urgently required to elucidate the molecular mechanism involved in impaired IFN-γ production capability of NK cells in TB patients.
Despite exhibiting no difference in IFN-γ production between IL-12/15/18 and IL-12/15/18/MTB pre-activated PBMCs, we observed the poor expansion potential of CIML NK cells co-stimulated with antigens from MTB. The amount of NK cells was also found to be remarkedly decreased after 7 day-stimulation with MTB antigens.42 Our results also showed that MTB lysates impaired the proliferative capacity of NK cells, most likely due to virulence factors released after the destruction of bacterial cells.52 This effect is reminiscent of a previous study demonstrating that cytomegalovirus infection has the potential to shape the NK cell repertoire, thus resulting in the selective expansion or inhibition of specific NK cell subsets.12 These reports suggest that the mycobacterial components could hijack NK cell signal transduction and involve in proliferation procedure. On the other, we also found an interesting result that the proportion of CIML NK cell proliferation was higher in TB patients than in healthy subjects. NK cells in TB patients show greater ability to divide after cytokine preactivation, which is accompanied by higher levels of death. Lots of previous studies have demonstrated that there were exhaustion of immune cells during MTB infection.53–55 NK cell exhaustion in chronic diseases appears to have many conserved features, including upregulation of inhibitory receptors and excess proliferation due to cytokine-induced activation.56 In our study, TB patients were previously exposed to MTB infection compared to healthy individuals, and immune cells in TB patients had been activated by the pathogen. Thus, NK cells from TB patients might be able to respond and proliferate rapidly in response to cytokine induction.57–59 Meanwhile, the functional profiles of NK cells are modified in TB endemic settings.48 These may explain why the proliferation of NK cells in TB patients is accompanied by more cell death and weakened functional response. The exact mechanism needs to be further verified in the future.
We also acknowledged several limitations to the present study. First, sample might be too small to detect some marked difference, which needs to be further expanded for verification. Second, despite showing in vitro efficacy against MTB-infected U937 cells, application of our findings to an animal model is deserved, to validate whether the superiority of CIML NK-based immunotherapy is also evident in vivo. Finally, given that the trend of degranulation of CIML NK subsets was comparable between health control and TB patients, it seems reasonable to speculate on the presence of impaired IFN-γ biosynthesis via inhibiting signal transduction in CIML NK cells. Unfortunately, the underlying mechanisms of suppressed IFN-γ production of CIML NK cells from TB patients are not fully understood. Nevertheless, our data provides novel insights into the employment of CIML NK cells in cellular immunotherapy for tuberculosis.
Conclusion
To conclude, our results demonstrated that CIML NK cells from healthy individuals exist the increased ability of IFN-γ secretion and boosted anti-MTB activity in vitro. The CIML NK cells from TB patients show impaired IFN-γ production and no enhanced anti-MTB activity compared to those from healthy donors. Additionally, we observe the poor expansion potential of CIML NK cells co-stimulated with antigens from MTB. Further animal experiments are of great importance to validate whether the superiority of CIML NK-based immunotherapy is also evident against MTB infection in vivo.
Data Sharing Statement
The data used to support the findings of this study are included within the article.
Ethics Approval
This study was approved by the Ethics Committee of Beijing Chest Hospital, Capital Medical University (Ethics approval number: YJS-2021-029), and informed consent was obtained from all individual participants included in the study. The manuscript does not contain any individual person’s identifying details, images, or videos.
Funding
This work was supported by grants from the Beijing Key Clinical Speciality Project (20201214), and the National Natural Science Foundation of China (82072243), and R&D Program of Beijing Municipal Education Commission (KM202210025006).
Disclosure
The authors declare no competing interests.
References
1. World Health Organization. Global Tuberculosis Report 2022. Geneva: World Health Organization; 2022.
2. Shi YH, Zhu SW, Mao XZ, et al. Transcriptome profiling, molecular biological, and physiological studies reveal a major role for ethylene in cotton fiber cell elongation. Plant Cell. 2006;18(3):651–664. doi:10.1105/tpc.105.040303
3. Harding E. WHO global progress report on tuberculosis elimination. Lancet Respir Med. 2020;8(1):19. doi:10.1016/S2213-2600(19)30418-7
4. Russell DG, VanderVen BC, Lee W, et al. Mycobacterium tuberculosis wears what it eats. Cell Host Microbe. 2010;8(1):68–76. doi:10.1016/j.chom.2010.06.002
5. Shah M, Dorman SE. Latent tuberculosis infection. N Engl J Med. 2021;385(24):2271–2280. doi:10.1056/NEJMcp2108501
6. Gengenbacher M, Kaufmann SH. Mycobacterium tuberculosis: success through dormancy. FEMS Microbiol Rev. 2012;36(3):514–532. doi:10.1111/j.1574-6976.2012.00331.x
7. Liu CH, Liu H, Ge B. Innate immunity in tuberculosis: host defense vs pathogen evasion. Cell Mol Immunol. 2017;14(12):963–975. doi:10.1038/cmi.2017.88
8. Maroof A, Beattie L, Zubairi S, Svensson M, Stager S, Kaye PM. Posttranscriptional regulation of II10 gene expression allows natural killer cells to express immunoregulatory function. Immunity. 2008;29(2):295–305. doi:10.1016/j.immuni.2008.06.012
9. Bozzano F, Costa P, Passalacqua G, et al. Functionally relevant decreases in activatory receptor expression on NK cells are associated with pulmonary tuberculosis in vivo and persist after successful treatment. Int Immunol. 2009;21(7):779–791. doi:10.1093/intimm/dxp046
10. Ratcliffe LT, Lukey PT, MacKenzie CR, Ress SR. Reduced NK activity correlates with active disease in HIV- patients with multidrug-resistant pulmonary tuberculosis. Clin Exp Immunol. 1994;97(3):373–379. doi:10.1111/j.1365-2249.1994.tb06097.x
11. Esin S, Batoni G. Natural killer cells: a coherent model for their functional role in Mycobacterium tuberculosis infection. J Innate Immun. 2015;7(1):11–24. doi:10.1159/000363321
12. Lodoen MB, Lanier LL. Natural killer cells as an initial defense against pathogens. Curr Opin Immunol. 2006;18(4):391–398. doi:10.1016/j.coi.2006.05.002
13. Erkes DA, Selvan SR. Hapten-induced contact hypersensitivity, autoimmune reactions, and tumor regression: plausibility of mediating antitumor immunity. J Immunol Res. 2014;2014:175265. doi:10.1155/2014/175265
14. Terren I, Orrantia A, Astarloa-Pando G, Amarilla-Irusta A, Zenarruzabeitia O, Borrego F. Cytokine-induced memory-like NK cells: from the basics to clinical applications. Front Immunol. 2022;13:884648. doi:10.3389/fimmu.2022.884648
15. Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457(7229):557–561. doi:10.1038/nature07665
16. Abdul-Careem MF, Lee AJ, Pek EA, et al. Genital HSV-2 infection induces short-term NK cell memory. PLoS One. 2012;7(3):e32821. doi:10.1371/journal.pone.0032821
17. Paust S, Gill HS, Wang BZ, et al. Critical role for the chemokine receptor CXCR6 in NK cell-mediated antigen-specific memory of haptens and viruses. Nat Immunol. 2010;11(12):1127–1135. doi:10.1038/ni.1953
18. Reeves RK, Li H, Jost S, et al. Antigen-specific NK cell memory in rhesus macaques. Nat Immunol. 2015;16(9):927–932. doi:10.1038/ni.3227
19. Habib S, El Andaloussi A, Hisham A, Ismail N, Cell-Mediated NK. Regulation of protective memory responses against intracellular ehrlichial pathogens. PLoS One. 2016;11(4):e0153223. doi:10.1371/journal.pone.0153223
20. Choreno Parra JA, Martinez Zuniga N, Jimenez Zamudio LA, Jimenez Alvarez LA, Salinas Lara C, Zuniga J. Memory of natural killer cells: a new chance against mycobacterium tuberculosis? Front Immunol. 2017;8:967. doi:10.3389/fimmu.2017.00967
21. Cooper MA, Elliott JM, Keyel PA, Yang L, Carrero JA, Yokoyama WM. Cytokine-induced memory-like natural killer cells. Proc Natl Acad Sci U S A. 2009;106(6):1915–1919. doi:10.1073/pnas.0813192106
22. Gang M, Wong P, Berrien-Elliott MM, Fehniger TA. Memory-like natural killer cells for cancer immunotherapy. Semin Hematol. 2020;57(4):185–193. doi:10.1053/j.seminhematol.2020.11.003
23. Braverman J, Sogi KM, Benjamin D, Nomura DK, Stanley SA. HIF-1alpha is an essential mediator of IFN-gamma-dependent immunity to mycobacterium tuberculosis. J Immunol. 2016;197(4):1287–1297. doi:10.4049/jimmunol.1600266
24. Romee R, Rosario M, Berrien-Elliott MM, et al. Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia. Sci Transl Med. 2016;8(357):357ra123. doi:10.1126/scitranslmed.aaf2341
25. Roy Chowdhury R, Vallania F, Yang Q, et al. A multi-cohort study of the immune factors associated with M. tuberculosis infection outcomes. Nature. 2018;560(7720):644–648. doi:10.1038/s41586-018-0439-x
26. Abebe F. Immunological basis of early clearance of Mycobacterium tuberculosis infection: the role of natural killer cells. Clin Exp Immunol. 2021;204(1):32–40. doi:10.1111/cei.13565
27. Kolloli A, Subbian S. Host-directed therapeutic strategies for tuberculosis. Front Med. 2017;4:171. doi:10.3389/fmed.2017.00171
28. Wallis RS, O’Garra A, Sher A, Wack A. Host-directed immunotherapy of viral and bacterial infections: past, present and future. Nat Rev Immunol. 2023;23(2):121–133. doi:10.1038/s41577-022-00734-z
29. Grzywacz B, Kataria N, Verneris MR. CD56(dim)CD16(+) NK cells downregulate CD16 following target cell induced activation of matrix metalloproteinases. Leukemia. 2007;21(2):356–359. doi:10.1038/sj.leu.2404499
30. Romee R, Foley B, Lenvik T, et al. NK cell CD16 surface expression and function is regulated by a disintegrin and metalloprotease-17 (ADAM17). Blood. 2013;121(18):3599–3608. doi:10.1182/blood-2012-04-425397
31. Schierloh P, Yokobori N, Aleman M, et al. Increased susceptibility to apoptosis of CD56dimCD16+ NK cells induces the enrichment of IFN-gamma-producing CD56bright cells in tuberculous pleurisy. J Immunol. 2005;175(10):6852–6860. doi:10.4049/jimmunol.175.10.6852
32. Rao D, Venkataswamy MM, Vasanthapuram R, Satishchandra P, Desai A. Alterations in natural killer and dendritic cell subsets in individuals with HIV-associated neurotuberculosis. J Med Virol. 2018;90(5):899–906. doi:10.1002/jmv.25042
33. Esaulova E, Das S, Singh DK, et al. The immune landscape in tuberculosis reveals populations linked to disease and latency. Cell Host Microbe. 2021;29(2):165–178 e8. doi:10.1016/j.chom.2020.11.013
34. Sherwood ER, Burelbach KR, McBride MA, et al. Innate immune memory and the host response to infection. J Immunol. 2022;208(4):785–792. doi:10.4049/jimmunol.2101058
35. Terren I, Orrantia A, Mosteiro A, Vitalle J, Zenarruzabeitia O, Borrego F. Metabolic changes of Interleukin-12/15/18-stimulated human NK cells. Sci Rep. 2021;11(1):6472. doi:10.1038/s41598-021-85960-6
36. Tarannum M, Romee R. Cytokine-induced memory-like natural killer cells for cancer immunotherapy. Stem Cell Res Ther. 2021;12(1):592. doi:10.1186/s13287-021-02655-5
37. Lopez-Verges S, Milush JM, Pandey S, et al. CD57 defines a functionally distinct population of mature NK cells in the human CD56dimCD16+ NK-cell subset. Blood. 2010;116(19):3865–3874. doi:10.1182/blood-2010-04-282301
38. Hazeldine J, Lord JM. The impact of ageing on natural killer cell function and potential consequences for health in older adults. Ageing Res Rev. 2013;12(4):1069–1078. doi:10.1016/j.arr.2013.04.003
39. Nielsen CM, White MJ, Goodier MR, Riley EM. Functional Significance of CD57 expression on human NK cells and relevance to disease. Front Immunol. 2013;4:422. doi:10.3389/fimmu.2013.00422
40. Garand M, Goodier M, Owolabi O, Donkor S, Kampmann B, Sutherland JS. Functional and phenotypic changes of natural killer cells in whole blood during mycobacterium tuberculosis infection and disease. Front Immunol. 2018;9:257. doi:10.3389/fimmu.2018.00257
41. Di Vito C, Mikulak J, Mavilio D. On the way to become a natural killer cell. Front Immunol. 2019;10:1812. doi:10.3389/fimmu.2019.01812
42. Albayrak N, Dirix V, Aerts L, et al. Differential expression of maturation and activation markers on NK cells in patients with active and latent tuberculosis. J Leukoc Biol. 2022;111(5):1031–1042. doi:10.1002/JLB.4A1020-641RR
43. Zhuang L, Fulton RJ, Rettman P, et al. Activity of IL-12/15/18 primed natural killer cells against hepatocellular carcinoma. Hepatol Int. 2019;13(1):75–83. doi:10.1007/s12072-018-9909-3
44. Condos R, Rom WN, Schluger NW. Treatment of multidrug-resistant pulmonary tuberculosis with interferon-gamma via aerosol. Lancet. 1997;349(9064):1513–1515. doi:10.1016/S0140-6736(96)12273-X
45. Raju B, Hoshino Y, Kuwabara K, et al. Aerosolized gamma interferon (IFN-gamma) induces expression of the genes encoding the IFN-gamma-inducible 10-kilodalton protein but not inducible nitric oxide synthase in the lung during tuberculosis. Infect Immun. 2004;72(3):1275–1283. doi:10.1128/IAI.72.3.1275-1283.2004
46. Ulrichs T, Kaufmann SH. New insights into the function of granulomas in human tuberculosis. J Pathol. 2006;208(2):261–269. doi:10.1002/path.1906
47. Schneider BE, Korbel D, Hagens K, et al. A role for IL-18 in protective immunity against Mycobacterium tuberculosis. Eur J Immunol. 2010;40(2):396–405. doi:10.1002/eji.200939583
48. Harris LD, Khayumbi J, Ongalo J, et al. Distinct human NK cell phenotypes and functional responses to mycobacterium tuberculosis in adults from TB endemic and non-endemic regions. Front Cell Infect Microbiol. 2020;10:120. doi:10.3389/fcimb.2020.00120
49. Vankayalapati R, Barnes PF. Innate and adaptive immune responses to human Mycobacterium tuberculosis infection. Tuberculosis. 2009;89(Suppl 1):S77–80. doi:10.1016/S1472-9792(09)70018-6
50. Howard NC, Khader SA. Immunometabolism during Mycobacterium tuberculosis Infection. Trends Microbiol. 2020;28(10):832–850. doi:10.1016/j.tim.2020.04.010
51. Rao Y, Gai X, Le Y, et al. Enhanced Proinflammatory Cytokine Production and Immunometabolic Impairment of NK Cells Exposed to Mycobacterium tuberculosis and Cigarette Smoke. Front Cell Infect Microbiol. 2021;11:799276. doi:10.3389/fcimb.2021.799276
52. Kust SA, Streltsova MA, Panteleev AV, et al. HLA-DR-Positive NK cells expand in response to mycobacterium tuberculosis antigens and mediate mycobacteria-induced T cell activation. Front Immunol. 2021;12:662128. doi:10.3389/fimmu.2021.662128
53. Jayaraman P, Jacques MK, Zhu C, et al. TIM3 mediates T cell exhaustion during mycobacterium tuberculosis infection. PLoS Pathog. 2016;12(3):e1005490. doi:10.1371/journal.ppat.1005490
54. Khan N, Vidyarthi A, Amir M, Mushtaq K, Agrewala JN. T-cell exhaustion in tuberculosis: pitfalls and prospects. Crit Rev Microbiol. 2017;43(2):133–141. doi:10.1080/1040841X.2016.1185603
55. Wondmagegn T, Damtie D, Genetu M, Biadgo B, Lemma M, Negash M. T lymphocyte subpopulations and intestinal helminthes profile among tuberculosis patients co-infected with HIV before and after anti tubercular treatment at university of Gondar hospital, Northwest Ethiopia. BMC Infect Dis. 2020;20(1):109. doi:10.1186/s12879-020-4845-y
56. Schafer JL, Muller-Trutwin MC, Reeves RK. NK cell exhaustion: bad news for chronic disease? Oncotarget. 2015;6(26):21797–21798. doi:10.18632/oncotarget.5490
57. Netea MG, Quintin J, van der Meer JW. Trained immunity: a memory for innate host defense. Cell Host Microbe. 2011;9(5):355–361. doi:10.1016/j.chom.2011.04.006
58. Khader SA, Divangahi M, Hanekom W, et al. Targeting innate immunity for tuberculosis vaccination. J Clin Invest. 2019;129(9):3482–3491. doi:10.1172/JCI128877
59. Uppendahl LD, Felices M, Bendzick L, et al. Cytokine-induced memory-like natural killer cells have enhanced function, proliferation, and in vivo expansion against ovarian cancer cells. Gynecol Oncol. 2019;153(1):149–157. doi:10.1016/j.ygyno.2019.01.006
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.