Back to Journals » Journal of Hepatocellular Carcinoma » Volume 11
Atezolizumab Plus Bevacizumab Combined with Transarterial Embolization Plus Hepatic Arterial Infusion Chemotherapy for Unresectable Hepatocellular Carcinoma with a Diameter >8 Cm: A Retrospective Study
Authors Cai H, Chen S, Wu Z, Wang F, Tang S, Chen L, Guo W
Received 6 September 2023
Accepted for publication 17 January 2024
Published 26 February 2024 Volume 2024:11 Pages 399—409
DOI https://doi.org/10.2147/JHC.S439001
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr David Gerber
Hongjie Cai,1 Song Chen,2 Zhiqiang Wu,1 Fan Wang,1 Shuangyan Tang,1 Ludan Chen,1 Wenbo Guo1
1Department of Interventional Radiology, The First Affiliated Hospital of Sun Yat-Sen University, Guangzhou, 510062, People’s Republic of China; 2Department of Minimally Invasive Interventional Therapy, Sun Yat-Sen University Cancer Center and Sun Yat-Sen University State Key Laboratory of Oncology in South China, and Collaborative Innovation Center for Cancer Medicine, Guangzhou, 510060, People’s Republic of China
Correspondence: Wenbo Guo, Email [email protected]
Purpose: Local in combination with systemic therapy might be an option for patients with advanced unresectable hepatocellular carcinoma (uHCC). This study examined the clinical benefits and adverse events (AEs) of first-line transarterial embolization (TAE) and hepatic arterial infusion chemotherapy (HAIC) combined with atezolizumab (Atezo) and bevacizumab (Bev) in patients with uHCC of a diameter larger than 8 cm.
Patients and methods: This retrospective study included patients with uHCC of a diameter larger than 8 cm who were treated with first-line Atezo-Bev and TAE+HAIC at the First Affiliated Hospital of Sun Yat-Sen University between September 30, 2019, and September 30, 2022. Progression-free survival (PFS), overall survival (OS), tumor response according to mRECIST, and AEs were analyzed. Multivariable Cox analyses were performed to examine the factors associated with PFS.
Results: Thirty patients were included. The objective response rate (ORR) was 74.4% (95% confidence interval [CI], 59.3%-89.5%), and the disease control rate (DCR) was 93.3% (95% CI, 85.4%-98.6%). The median follow-up was 11.4 (inter-quartile range [IQR], 5.5– 17.9) months. The median PFS was 6.8 (95% CI, 2.6– 11.1) months. The 3-, 6-, 9-, and 12-month survival rates were 86.2%, 82.5%, 68.6%, and 60%, respectively. The median OS was not estimated. Extrahepatic metastasis was independently associated with PFS (hazard ratio [HR]=3.468, 95% CI, 1.001– 12.023). The most common AEs were fever (46.7%). Grade 4 AEs occurred one time as hematemesis but no 5 AEs were observed.
Conclusion: Atezo-Bev combined with TAE and HAIC might benefit patients with uHCC of a diameter larger than 8 cm, with manageable AEs.
Keywords: immune checkpoint inhibitors, tyrosine kine inhibitors, local therapy, systemic therapy, large unresectable hepatocellular carcinoma
Introduction
Hepatocellular carcinoma (HCC) is a highly lethal invasive carcinoma arising in the liver,1 with an estimated 905,677 new cases and 830,180 deaths in 2020.2 China has a high prevalence of hepatitis B and C and a consequent high incidence of hepatitis-related HCC.3 Unfortunately, only 30–40% of the patients are amenable to surgery.4 Trials showed that transarterial embolization (TAE) and transarterial chemoembolization (TACE) could achieve benefits in selected patients with unresectable HCC (uHCC).5–7 The Chinese guidelines recommend TACE for China liver cancer staging (CNLC) grade IIb, IIIa, and part of IIIb and hepatic arterial infusion chemotherapy (HAIC) for uHCC.8 TAE and TACE can reduce tumor burden effectively and rapidly by causing an ischemic injury, leading to tumor regression in up to 50% of the patients.9–12 In recent years, HAIC with FOLFOX alone or in combination with tyrosine kine inhibitors (TKIs) has improved survival in patients with advanced HCC in China,13–15 especially in patients with portal vein tumor thrombosis (PVTT)13,16–18 and patients with large uHCC.19
According to the Barcelona Clinic liver cancer (BCLC) stage, atezolizumab (Atezo) plus bevacizumab (Bev) is the preferred first-line treatment of HCC patients with BCLC stage C.20–22 The IMbrave150 trial established the position of Atezo-Bev as the first-line treatment for advanced HCC.20 However, the patients with large uHCC were underrepresented in the IMbrave150 trial, and Atezo-Bev appeared to have limited efficacy in these patients, with a median overall survival (OS) of 7.6 months.20,21
In patients with high-risk uHCC, such as those with larger than 8 cm in diameter,22 the effect of first-line systemic therapy alone is limited. In order to improve survival in patients with advanced uHCC, a combination of locoregional and systemic therapies is recommended.23,24 HAIC can be combined with lenvatinib and immune checkpoint inhibitors (ICIs).25–27 Although triple therapy had a high objective response rate (ORR), 40.5% of the patients still did not respond.28 According to the LAUNCH trial, TACE in combination with lenvatinib in patients with advanced uHCC, showed more promising results than lenvatinib alone.29 Systemic therapy +HAIC is effective, while Atezo-Bev+TACE is still being investigated.30–32 Combining the advantages of HAIC and TACE, there might be a synergistic effect of using TAE on top of Atezo-Bev+HAIC. Indeed, local therapies like TAE and TACE can reduce tumor burden and expose the tumor antigens, enhancing the anticancer effects of ICIs.23,24,33 Therefore, for patients with large uHCC, TAE+HAIC combined with Atezo-Bev might improve the efficacy compared with Atezo-Bev alone, but such a strategy has not been reported before.
Therefore, this retrospective study examined the clinical benefits and the safety of TAE+HAIC combined with Atezo-Bev in patients with uHCC of a diameter larger than 8 cm. The results could provide a novel therapeutic option for patients with a poor prognosis.
Methods
Study Design and Patients
This retrospective study included patients with uHCC of a diameter larger than 8 cm who were treated with Atezo-Bev combined with TAE and HAIC at the First Affiliated Hospital of Sun Yat-Sen University between September 30, 2019, and September 30, 2022. The study was approved by the ethics committee of Sun Yat-Sen University. The requirement for informed consent for the present study was waived by the committee due to the retrospective nature of the study. This study was conducted in accordance with the 1964 Helsinki declaration and its later amendments.
HCC was diagnosed clinically or histopathologically based on the European Association for the Study of the Liver (EASL) HCC clinical practice guidelines and the Chinese guidelines.31,34 The inclusion criteria were: 1) advanced uHCC with a diameter larger than 8 cm confirmed by histopathological examination or imaging; 2) Child-Pugh grade of A or B; 3) Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 or 1; and 4) treated with the Atezo-Bev within 3 weeks after TAE+HAIC. The exclusion criteria were: 1) PVTT without collateral vein formation; 2) non-measurable lesions at baseline; or 3) previously treated with targeted therapy (eg, sorafenib), interventional procedures, or immunotherapy.
Tae + Haic
The catheter was super-selectively sent to the artery supplying the tumor. Iodine oil (5–20 mL) was first perfused through the catheter, and then microspheres or gelatin sponge particles were injected as thoroughly as possible for embolization.35,36 The aim was to reduce tumor burden as much as possible. The procedure aimed to achieve total tumor embolization, but large HCCs often have several feeding arteries and collaterals, and complete embolization is sometimes impossible. Furthermore, complete embolisation of the tumour is not necessary to reduce the tumour burden before HAIC and to avoid excessive liver damage. Finally, the catheter was super-selectively inserted into the blood supply artery of the tumor, and the patient was returned to the ward for the FOLFOX regimen (day 1: oxaliplatin 100 mg/m2, over 2 h, calcium levovinate 200 mg/m2, over 2 h, 5-fluorouracil 400 mg/m2, over 15 min, and 5-fluorouracil 1200 mg/m2, over 22 h; day 2: 5- fluorouracil 1200 mg/m2, over 22 h). After the first interventional surgery, the patient returned to the hospital every 3 weeks for re-examination using upper abdominal enhanced Magnetic Resonance Imaging (MRI) and a-fetoprotein (AFP) measurements. According to the results of the examinations, the decision was made whether to continue interventional surgery. If the lesion were stable, no interventional surgery was given. Repeat interventional therapy was adapted to the situation of the lesion at each follow-up. The total number of interventional operations did not exceed six times.
Atezolizumab + Bevacizumab
According to the patient’s condition, intravenous atezolizumab and bevacizumab were given within 3 weeks after TAE and HAIC. Atezolizumab was given intravenously at the recommended dose of 1200 mg/time, followed by intravenous bevacizumab at 15 mg/kg/time. This regimen was administered every 3 weeks until disease progression or intolerable toxicity.
Data Collection and Outcomes
The baseline data, including age, sex, body mass index (BMI), ECOG PS, hepatitis, AFP, BCLC, tumor size, Child-Pugh grade, number of lesions, PVTT grade, and tumor to liver percentage, as well as treatment information, including number of TAE+HAIC and number of Atezo+Bev, were collected from the patient charts.
The outcomes were the ORR, disease control rate (DCR), PFS, 3-, 6-, 9-, and 12-month survival rates, OS, and adverse events (AEs). ORR was the proportion of patients achieving a complete response (CR) or partial response (PR). DCR was the proportion of patients achieving CR, PR, or stable disease (SD). PFS was defined as the time from the first TAE+HAIC to disease progression or death, whichever occurred first. OS was defined as the time from the first TAE+HAIC to death from any cause. The evaluation of the target lesions was based on the mRECIST. The AEs were evaluated according to the Common Terminology Criteria for Adverse Events (CTCAE) 4.03.
Statistical Analysis
All statistical analyses were performed using SPSS 25.0 (IBM Corp., Armonk, NY, USA). PFS and OS were estimated using the Kaplan-Meier method. A Logrank test was performed to analyze differences in PFS between subgroups. Univariable and multivariable Cox proportional hazard ratio analyses were performed to examine the factors associated with PFS. Variables with P<0.10 in the univariable analyses were included in the multivariable analysis. Two-sided P-values <0.05 were considered statistically significant.
Results
Characteristics of the Patients
Thirty patients were included (Figure 1). At the data of the last follow-up (September 30, 2022), 18 (60%) patients were still receiving treatment, and 12 (40%) were discontinued due to PD or death.
 |
Figure 1 Patient selection flowchart. |
The characteristics of the patients are shown in Table 1. The median age was 55.5 (IQR, 49.0–66.0) years. The median BMI was 22.71 (IQR, 20.47–25.25) kg/m2, and ECOG PS was 0 in 70% of patients and 1 in 30%. BCLC stage B accounted for 33.33%, and stage C for 66.67%. The median maximum tumor diameter was 11.6 (IQR, 9.8–13.2) cm. Tumor to liver volume ratio was ≥50% in 53.33% of patients. The proportions of patients with portal vein invasion were 80%, with 23.3% having Vp III and 30.00% having Vp IV PVTT. Extrahepatic metastasis was present in 36.67% of patients. Child-Pugh grade A was observed in 66.67% of patients and grade B in 33.33%. The predominant cause of liver cancer was hepatitis B, affecting 96.67% of patients. The median AFP levels were 391.8 (IQR, 14.16–9656.99) ng/mL, with 46.67% of patients having AFP levels ≥ 400 ng/mL.
 |
Table 1 Baseline Characteristics of the Patients |
At the time of the last follow-up, the patients had received an average of 3.6±1.9 TAE+HAIC sessions and an average of 7.8±2.4 Atezo+Bev cycles.
Clinical Benefits
The median follow-up was 11.4 (inter-quartile range [IQR], 5.5 to 17.9) months. Among the 30 patients, two (6.7%) had the best efficacy evaluation of CR, and 20 (67.7%) patients had the best efficacy evaluation of PR, with an ORR of 74.4% and a DCR of 93.3% (Table 2). The median PFS (mPFS) was 6.83 (95% CI, 2.59 to 11.07) months (Figure 2A). The median OS was not estimated (NE). The survival rates at 3, 6, 9, and 12 months were 86.2%, 82.5%, 68.6%, and 60.0%, respectively (Figure 2B). Patients without extrahepatic metastases had a longer PFS (mPFS not reached) than those with extrahepatic metastasis (mPFS=4.3 months) (P<0.001) (Figure 3). One patient in the study had a tumor shrinking after treatment and received successful surgical resection.
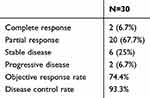 |
Table 2 Treatment Effectiveness |
 |
Figure 2 (A) Kaplan-Meier curve for progression-free survival (PFS). (B) Kaplan-Meier curve for overall survival (OS). |
 |
Figure 3 Kaplan-Meier curves for progression-free survival (PFS) after stratification for the absence/presence of extrahepatic metastasis. |
Factors Associated with PFS
The factors influencing PFS are shown in Table 3. The univariable analyses showed that AFP ≥400 ng/mL (P=0.042), PVTT (P=0.011), and extrahepatic metastasis (P<0.001) were associated with PFS. The multivariable analysis showed that only extrahepatic metastasis was independently associated with PFS (hazard ratio [HR]=3.468, 95% CI, 1.001 to 12.023, P=0.047).
 |
Table 3 Univariable and Multivariable Cox Analyses for PFS |
Adverse Events
The AEs are shown in Table 4. Most patients (93%) had AEs, and most of which were grade 1/2 (73.3%). The most common AEs were fever (46.7%), emesis (46.7%), increased aspartate transaminase (AST) (43.3%), loss of appetite (40.0%), loss of weight (30.0%), diarrhea (30.0%), and albuminuria (23.3%). Grade ≥3 AEs experienced by ≥2 patients were fever (6.7%) and diarrhea (6.7%). Grade 4 AEs occurred one time as hematemesis but no 5 AEs were identified.
 |
Table 4 Safety |
After three TAE+HAIC sessions and 10 Atezo+Bev cycles, one patient had esophageal and gastric venous rupture bleeding during treatment. Transjugular intrahepatic portosystemic shunt was performed after emergency gastroscopy ligation, and Bev was discontinued. All other AEs in the other patients were managed with symptomatic treatment, and did not interrupt the regimen of Atezo-Bev combined with TAE and HAIC.
Discussion
TAE+HAIC combined with Atezo-Bev could be an option for patients with large uHCC. This retrospective study examined the clinical benefits, and AEs of TAE+HAIC combined with Atezo-Bev in patients with uHCC of a diameter larger than 8 cm. The ORR was 74.4%, and the DCR was 93.3%. The median PFS was 6.8 (95% CI, 2.6–11.1) months, with 3-, 6-, 9-, and 12-month survival rates of 86.2%, 82.5%, 68.6%, and 60%, respectively. The median OS was not estimated. Extrahepatic metastasis was independently associated with PFS. The most common AEs were fever (46.7%) and increased aspartate transaminase (AST) (43.3%). Grade 3 or 4 AEs occurred in six patients (20.0%) without any grade 5 AEs. Further prospective clinical research is necessary to validate the benefits and safety of this treatment strategy.
For intermediate and advanced uHCC, especially large tumors with PVTT or extrahepatic metastases, the available guidelines recommend using first-line Atezo-Bev.37–39 Combining the two theoretically has a synergistic anticancer effect,40 but their efficacy in large uHCC is still limited.20,21 In clinical practice, especially in China, where the prevalence of hepatitis and the incidence of HCC are high,3 the proportion of patients with large HCC account for a significant proportion of the cases,22 but these patients are excluded or underrepresented in most clinical trials.21,41,42 Therefore, extra attention should be paid to patients with large HCC. In addition, in the IMbrave150 and FOHAIC-1 trials, the median OS and PFS for patients with large HCC and PVTT (Vp4 or tumor proportion >50%) were not satisfactory,14,20 but the global populations of these two trials were different from the present study, preventing in-depth comparisons. Research is still needed in this group of patients with large uHCC.
TACE is a classic local treatment for intermediate or advanced uHCC, but residual tumors and high recurrence rates after TACE are still a major clinical problem, seriously affecting the long-term survival rate of patients with intermediate or advanced HCC, especially for patients with large HCC.9,12,23,43 The treatment of advanced HCC with FOLFOX HAIC has a significant clinical effect, and has the advantages of low technical difficulty, high tumor shrinkage rate, low liver function damage, and the possible long-term repeated administration.13,16–18 Indeed, HAIC after TACE might deal with the residual tumor cells. Studies showed that local treatments such as TACE and HAIC combined with systemic treatments such as TKIs and ICIs have synergistic antitumor effects.24,44–47 In this study, TAE as a means to reduce tumor burden, combined with the use of HAIC to locally deal with residual tumor cells, followed by Atezo-Bec as systemic treatment, achieved a median PFS of 6.8 months, which was better than the 5.4 months in the intermediate- and high-risk participants in the IMbrave 150 trial,20 suggesting that the clinical efficacy of Atezo-Bev in patients with high-risk tumors might be limited and that local treatment might further improve the efficacy, especially TAE combined with HAIC.
In the present study, the combination of TAE+HAIC with Atezo-Bev for treating patients with uHCC of a diameter larger than 8 cm achieved (among the 30 evaluable patients) an ORR of 74.4% and a DCR of 93.3%. These numbers are higher than in the IMbrave 150 trial,20 in which patients with uHCC were treated with Atezo-Bev, showing an ORR of 27.3% and a DCR of 73.6%. A study of lenvatinib plus pembrolizumab by Finn et al48 showed an ORR of 36.0%. Lee et al40 reported an ORR of 36% with Atezo-Bev. In the study by Chen et al,28 pembrolizumab plus lenvatinib plus HAIC achieved an ORR of 46.4% and a DCR of 90.5%, but TAE/TACE was not used. In a study of ICI plus lenvatinib plus HAIC, Mei et al26 reported an ORR of 40.0% and a DCR of 77.6%. Despite all these studies being performed in patients with uHCC, the study populations differed from the present study, preventing direct comparisons. Nevertheless, an increase in ORR and DCR can be seen from studies of ICI plus antiangiogenic agents20,40 to those of ICI plus antiangiogenic agents plus HAIC26,28 to those of ICI plus antiangiogenic agents plus HAIC and TAE (the present study). Trials directly comparing those modalities should be designed.
In the present study, the median PFS was 6.8 months, with 3-, 6-, 9-, and 12-month survival rates of 86.2%, 82.5%, 68.6%, and 60%, respectively. The median OS could not be estimated due to too few events. Nevertheless, these numbers are higher than in the IMbrave 150 trial,20 which showed a PFS of 6.8 months and a 12-month OS rate of 67.2%. Finn et al48 showed that lenvatinib plus pembrolizumab led to a median PFS of 8.6 months and a median OS of 22 months. Lee et al40 reported an ORR of 36% with Atezo-Bev. Chen et al28 reported a median PFS of 10.9 months and a median OS of 17.7 months in patients treated with pembrolizumab plus lenvatinib plus HAIC but without TAE/TACE. Mei et al26 reported a median PFS and OS of 8.8 and 15.9 months in patients with advanced HCC treated with an ICI plus lenvatinib plus HAIC. As for the ORR and DCR, the different study populations from the present study prevented direct comparisons. Nevertheless, improved survival is observed in studies of ICI plus antiangiogenic agents,20,40 and even better survival is suggested in studies of ICI plus antiangiogenic agents plus HAIC.26,28 Still, the median PFS was apparently shorter in the present study than in previous studies. It could be due to the differences in study populations.
The multivariable analysis showed that only extrahepatic metastasis was independently associated with PFS. Of course, the presence of extrahepatic metastasis is a well-known factor of poor prognosis.37,39,49,50
In this study, AEs of the TAE+HAIC and Atezo-Bev combination were consistent with those known for TAE,9,23,43,51 HAIC,13–15 and Atezo-Bev.20,21,40 Grade 3 or 4 AEs occurred in 6 (20%) patients, grade 4 AEs occurred one time as hematemesis but no 5 AEs were observed. The most commonly associated AEs were abnormal liver function, fever, albuminuria, and hypertension. In the IMbrave 150 trial,20 the incidence of upper gastrointestinal bleeding in the Atezo-Bev group was 7%. In the present study, one patient (3.3%) developed upper gastrointestinal bleeding. In addition, the biggest concern was liver damage. In this study, the incidence of elevated ALT/AST was high, which might be related to the high proportion of patients with HBV infection included in this study and with local treatments such as TAE and HAIC. As previously reported, TAE can severely impair liver function and affect the ultimate outcome.43,51 In this study, abnormalities in liver function could be controlled by effective treatment and return to normal in a short period, neither affecting subsequent treatment nor causing liver failure. Overall, with appropriate monitoring and management, the AEs of this treatment combination appears manageable.
The present study had several limitations. First, the study design was retrospective, subject to inherent bias, and with a small sample size. Second, HAIC can be performed in a standard procedure, and TAE is used as a means of debulking, although with two experienced interventional physicians, it is difficult to achieve consistent assurance of the degree of embolism. Depending on the degree of embolism, the end result might be completely different. In addition, the inclusion of patients in this study was limited to those with treatment-naïve HCC with PVTT and high-risk tumors, so these results should not be generalized to all patients with HCC. Finally, the impact on the OS was not assessed due to the limited observation period. Therefore, larger, multicenter, long-follow-up cohort studies are needed to validate the present results and determine serum or tissue predictive markers of this combination therapy.
Conclusion
TAE+HAIC combined with Atezo-Bev showed good clinical benefits in patients with uHCC of a diameter larger than 8 cm. In addition, this combination therapy appears to be well tolerated. This combination strategy might be an appropriate treatment option for this patient population, and clinical trials should be designed for confirmation.
Acknowledgments
This paper was presented at ASCO 2023 annual meeting and published at Journal of Clinical Oncology (Wenbo Guo, Hongjie Cai, Song Chen, Zhiqiang Wu, Fan Wang, Ludan Chen, Shuangyan Tang, and Wenquan Zhuang Journal of Clinical Oncology 2023 41:16_suppl, e14617-e14617. DOI: 10.1200/JCO.2023.41.16_suppl.e14617 Journal of Clinical Oncology 41, no. 16_suppl (June 01, 2023) e14617-e14617. Published online May 31, 2023.)
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
All authors declare that they have no competing interests in this work.
References
1. Villanueva A. Hepatocellular carcinoma. N Engl J Med. 2019;380(15):1450–1462. doi:10.1056/NEJMra1713263
2. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
3. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi:10.3322/caac.21338
4. Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362(9399):1907–1917. doi:10.1016/S0140-6736(03)14964-1
5. Llovet JM, Real MI, Montana X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359(9319):1734–1739. doi:10.1016/S0140-6736(02)08649-X
6. Lo CM, Ngan H, Tso WK, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35(5):1164–1171. doi:10.1053/jhep.2002.33156
7. Takayasu K, Arii S, Ikai I, et al. Prospective cohort study of transarterial chemoembolization for unresectable hepatocellular carcinoma in 8510 patients. Gastroenterol. 2006;131(2):461–469. doi:10.1053/j.gastro.2006.05.021
8. Zhou J, Sun H, Wang Z, et al. Guidelines for the diagnosis and treatment of hepatocellular carcinoma (2019 edition). Liver Cancer. 2020;9(6):682–720. doi:10.1159/000509424
9. Pleguezuelo M, Marelli L, Misseri M, et al. TACE versus TAE as therapy for hepatocellular carcinoma. Expert Rev Anticancer Ther. 2008;8(10):1623–1641. doi:10.1586/14737140.8.10.1623
10. Saito R, Amemiya H, Hosomura N, et al. Intended preoperative trans-arterial embolization for large hepatocellular carcinoma: a retrospective cohort study. World J Surg Oncol. 2022;20(1):90. doi:10.1186/s12957-022-02563-9
11. Yasuda S, Ito H, Yoshikawa M, et al. Radiotherapy for large hepatocellular carcinoma combined with transcatheter arterial embolization and percutaneous ethanol injection therapy. Int J Oncol. 1999;15(3):467–473.
12. Wang K, Guo WX, Chen MS, et al. Multimodality treatment for hepatocellular carcinoma with portal vein tumor thrombus: a large-scale, multicenter, propensity mathching score analysis. Medicine. 2016;95(11):e3015. doi:10.1097/MD.0000000000003015
13. Lyu N, Kong Y, Mu L, et al. Hepatic arterial infusion of oxaliplatin plus fluorouracil/leucovorin vs. sorafenib for advanced hepatocellular carcinoma. J Hepatol. 2018;69(1):60–69. doi:10.1016/j.jhep.2018.02.008
14. Lyu N, Wang X, Li JB, et al. Arterial chemotherapy of oxaliplatin plus fluorouracil versus sorafenib in advanced hepatocellular carcinoma: a biomolecular exploratory, randomized, Phase III trial (FOHAIC-1). J Clin Oncol. 2022;40(5):468–480. doi:10.1200/JCO.21.01963
15. He M, Li Q, Zou R, et al. Sorafenib plus hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin vs sorafenib alone for hepatocellular carcinoma with portal vein invasion: a randomized clinical trial. JAMA Oncol. 2019;5(7):953–960. doi:10.1001/jamaoncol.2019.0250
16. Ikeda M, Shimizu S, Sato T, et al. Sorafenib plus hepatic arterial infusion chemotherapy with cisplatin versus sorafenib for advanced hepatocellular carcinoma: randomized Phase II trial. Ann Oncol. 2016;27(11):2090–2096. doi:10.1093/annonc/mdw323
17. Chan SL, Chong CC, Chan AW, Poon DM, Chok KS. Management of hepatocellular carcinoma with portal vein tumor thrombosis: review and update at 2016. World J Gastroenterol. 2016;22(32):7289–7300. doi:10.3748/wjg.v22.i32.7289
18. Deng ZJ, Li L, Teng YX, et al. Treatments of hepatocellular carcinoma with portal vein tumor thrombus: current status and controversy. J Clin Transl Hepatol. 2022;10(1):147–158. doi:10.14218/JCTH.2021.00179
19. Sidaway P. FOLFOX-HAIC active in large HCC. Nat Rev Clin Oncol. 2022;19(1):5. doi:10.1038/s41571-021-00577-y
20. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–1905. doi:10.1056/NEJMoa1915745
21. Breder VV, Vogel A, Merle P, et al. Exploratory efficacy and safety results of hepatocellular carcinoma (HCC) patients (pts) with main trunk and/or contralateral portal vein invasion (Vp4) treated with atezolizumab (atezo) + bevacizumab (bev) versus sorafenib (sor) in a global Ph III study. J Clin Oncol. 2021;39(15_suppl):4073. doi:10.1200/JCO.2021.39.15_suppl.4073
22. Abdel-Wahab M, Sultan AM, Fathy OM, et al. Factors affecting recurrence and survival after living donor liver transplantation for hepatocellular carcinoma. Hepatogastroenty. 2013;60(128):1847–1853.
23. Kudo M, Izumi N, Sakamoto M, et al. Survival analysis over 28 years of 173,378 patients with hepatocellular carcinoma in Japan. Liver Cancer. 2016;5(3):190–197. doi:10.1159/000367775
24. Pinato DJ, Murray SM, Forner A, et al. Trans-arterial chemoembolization as a loco-regional inducer of immunogenic cell death in hepatocellular carcinoma: implications for immunotherapy. J Immunother Cancer. 2021;9(9):e003311. doi:10.1136/jitc-2021-003311
25. Wheeler DC, Toto RD, Stefánsson BV, et al. A pre-specified analysis of the DAPA-CKD trial demonstrates the effects of dapagliflozin on major adverse kidney events in patients with IgA nephropathy. Kidney International. 2021;100(1):215–224. doi:10.1016/j.kint.2021.03.033
26. Mei J, Tang YH, Wei W, et al. Hepatic arterial infusion chemotherapy combined with PD-1 inhibitors plus lenvatinib versus PD-1 inhibitors plus lenvatinib for advanced hepatocellular Carcinoma. Front Oncol. 2021; 11(618206):doi:10.3389/fonc.2021.618206
27. Liu BJ, Gao S, Zhu X, et al. Real-world study of hepatic artery infusion chemotherapy combined with anti-PD-1 immunotherapy and tyrosine kinase inhibitors for advanced hepatocellular carcinoma. Immunotherapy. 2021;13(17):1395–1405. doi:10.2217/imt-2021-0192
28. Chen S, Xu B, Wu Z, et al. Pembrolizumab plus lenvatinib with or without hepatic arterial infusion chemotherapy in selected populations of patients with treatment-naive unresectable hepatocellular carcinoma exhibiting PD-L1 staining: a multicenter retrospective study. BMC Cancer. 2021;21(1):1126. doi:10.1186/s12885-021-08858-6
29. Peng Z, Fan W, Zhu B, et al. Lenvatinib combined with transarterial chemoembolization as first-line treatment for advanced hepatocellular carcinoma: a phase III, randomized clinical trial (LAUNCH). J Clin Oncol. 2023;41(1):117–127. doi:10.1200/JCO.22.00392
30. Li B, Qiiu J, Zheng Y, et al. Conversion to resectability using transarterial chemoembolization combined with hepatic arterial infusion chemotherapy for initially unresectable hepatocellular carcinoma. Ann Surg Open. 2021;2(2):e057. doi:10.1097/AS9.0000000000000057
31. Wu Z, Gao J, Zhuang W, Yang J, Guo W. Efficacy and safety of transcatheter arterial chemoembolization plus hepatic arterial infusion chemotherapy in the treatment of advanced hepatocellular carcinoma with portal vein tumor thrombosis in the main trunk. J Cancer Res Ther. 2022;18(2):345–351. doi:10.4103/jcrt.jcrt_1078_21
32. Ben Khaled N, Seidensticker M, Ricke J, et al. Atezolizumab and bevacizumab with transarterial chemoembolization in hepatocellular carcinoma: the DEMAND trial protocol. Future Oncol. 2022;18(12):1423–1435. doi:10.2217/fon-2021-1261
33. Galluzzi L, Buque A, Kepp O, Zitvogel L, Kroemer G. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol. 2017;17(2):97–111. doi:10.1038/nri.2016.107
34. Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563–567. doi:10.1038/nature14011
35. Kamran AU, Liu Y, Li FE, et al. Transcatheter arterial chemoembolization with gelatin sponge microparticles treated for BCLC stage B hepatocellular carcinoma: a single center retrospective study. Medicine. 2015;94(52):e2154. doi:10.1097/MD.0000000000002154
36. Zhao GS, Liu S, Liu Y, et al. Clinical application of gelatin sponge microparticles combined with pirarubicin for hepatic transcatheter arterial chemoembolization in breast cancer liver metastasis treatment: results of a single-center long-term study. World J Surg Oncol. 2021;19(1):249. doi:10.1186/s12957-021-02332-0
37. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines); Hepatobiliary Cancers. Version 5.2022. Fort Washington: National Comprehensive Cancer Network; 2023.
38. Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American association for the study of liver diseases. Hepatology. 2018;68(2):723–750. doi:10.1002/hep.29913
39. Vogel A, Cervantes A, Chau I, et al. Hepatocellular carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl 4):iv238–iv255. doi:10.1093/annonc/mdy308
40. Lee MS, Ryoo BY, Hsu CH, et al. Atezolizumab with or without bevacizumab in unresectable hepatocellular carcinoma (GO30140): an open-label, multicentre, phase 1b study. Lancet Oncol. 2020;21(6):808–820. doi:10.1016/S1470-2045(20)30156-X
41. Tsuchiya K, Kurosaki M, Marusawa H, et al. The efficacy and safety of lenvatinib in patients who did not meet the inclusion criteria of the Phase 3 trial (REFLECT trial) in real-world practice in Japan: a nationwide multicenter study in Japan. J Clin Oncol. 2019;37(15_suppl):e15629. doi:10.1200/JCO.2019.37.15_suppl.e15629
42. Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25–34. doi:10.1016/S1470-2045(08)70285-7
43. Galle PR, Tovoli F, Foerster F, et al. The treatment of intermediate stage tumours beyond TACE: from surgery to systemic therapy. J Hepatol. 2017;67(1):173–183. doi:10.1016/j.jhep.2017.03.007
44. Zhu H, Shan Y, Ge K, et al. Oxaliplatin induces immunogenic cell death in hepatocellular carcinoma cells and synergizes with immune checkpoint blockade therapy. Cell Oncol Dordr. 2020;43(6):1203–1214. doi:10.1007/s13402-020-00552-2
45. Yamamoto Y, Matsui J, Matsushima T, et al. Lenvatinib, an angiogenesis inhibitor targeting VEGFR/FGFR, shows broad antitumor activity in human tumor xenograft models associated with microvessel density and pericyte coverage. Vasc Cell. 2014;6(18):18. doi:10.1186/2045-824X-6-18
46. Deng H, Kan A, Lyu N, et al. Dual vascular endothelial growth factor receptor and fibroblast growth factor receptor inhibition elicits antitumor immunity and enhances programmed cell death-1 checkpoint blockade in hepatocellular carcinoma. Liver Cancer. 2020;9(3):338–357. doi:10.1159/000505695
47. Chung AS, Lee J, Ferrara N. Targeting the tumour vasculature: insights from physiological angiogenesis. Nat Rev Cancer. 2010;10(7):505–514. doi:10.1038/nrc2868
48. Finn RS, Ikeda M, Zhu AX, et al. Phase ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol. 2020;38(26):2960–2970. doi:10.1200/JCO.20.00808
49. Katyal S, Oliver JH, Peterson MS, et al. Extrahepatic metastases of hepatocellular carcinoma. Radiology. 2000;216(3):698–703. doi:10.1148/radiology.216.3.r00se24698
50. Uka K. Clinical features and prognosis of patients with extrahepatic metastases from hepatocellular carcinoma. World J Gastrol. 2007;13(3):414–420. doi:10.3748/wjg.v13.i3.414
51. Kudo M, Han KH, Ye SL, et al. A changing paradigm for the treatment of intermediate-stage hepatocellular carcinoma: Asia-pacific primary liver cancer expert consensus statements. Liver Cancer. 2020;9(3):245–260. doi:10.1159/000507370
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Simultaneous and Sequential Use of Molecular Targeted Agents Plus Immune Checkpoint Inhibitors for Advanced Hepatocellular Carcinoma: A Real-World Practice in China
Li J, Huang L, Ge C, Zhu X, Qiu M, Chen C, Wei S, Yan Y
Journal of Hepatocellular Carcinoma 2023, 10:949-958
Published Date: 20 June 2023
