Back to Journals » Journal of Hepatocellular Carcinoma » Volume 10
Simultaneous and Sequential Use of Molecular Targeted Agents Plus Immune Checkpoint Inhibitors for Advanced Hepatocellular Carcinoma: A Real-World Practice in China
Authors Li J, Huang L, Ge C, Zhu X, Qiu M, Chen C, Wei S, Yan Y
Received 5 April 2023
Accepted for publication 7 June 2023
Published 20 June 2023 Volume 2023:10 Pages 949—958
DOI https://doi.org/10.2147/JHC.S415941
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr David Gerber
Jing Li,1,2,* Liang Huang,1,* Chao Ge,3,* Xingwu Zhu,1 Maixuan Qiu,1 Chaopan Chen,3 Shaohua Wei,2 Yiqun Yan1
1Department of Hepatic Surgery, Eastern Hepatobiliary Surgery Hospital, Shanghai, People’s Republic of China; 2Department of General Surgery, The Second Affiliated Hospital of Soochow University, Suzhou, People’s Republic of China; 3Department of General Surgery, Ningbo Development Zone Hospital, Ningbo, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yiqun Yan, Department of Hepatic Surgery, Shanghai Eastern Hepatobiliary Surgery Hospital, No. 700 North Moyu Road, Shanghai, 201805, People’s Republic of China, Email [email protected] Shaohua Wei, Department of General Surgery, The Second Affiliated Hospital of Soochow University, No. 1055 Shanxiang Road, Suzhou, 215004, People’s Republic of China, Email [email protected]
Purpose: Molecular targeted agents (MTAs) plus immune checkpoint inhibitors (ICIs) treatment for advanced hepatocellular carcinoma (HCC) has shown an exciting prospect. This study aimed to report the efficacy of the Simultaneous and Sequential use of them in a real-world practice.
Patients and Methods: From April 2019 to December 2020, patients with advanced HCC in three Chinese medical centers receiving MTAs and ICIs as their initial systemic therapy were enrolled. Participants were classified into the Simultaneous group (treated with them simultaneously) and the Sequential group (treated with MTAs initially and added ICIs after tumor progression). Toxicity, tumor response, survival outcomes and prognostic factors were investigated.
Results: One hundred and ten consecutive patients participated in the study (64 in the Simultaneous group and 46 in the Sequential group). A total of 93 (84.5%) patients experienced treatment-related adverse events (AEs), of which 55 (85.9%) in the Simultaneous group and 38 (82.6%) in the Sequential group (P=0.19). Grade 3/4 AEs were observed in 9 (8.2%) patients. Patients in the Simultaneous group achieved a higher objective response rate than those in the Sequential group (25.0% vs 4.3%, p=0.04). The median overall survival (OS) of the entire cohort was 14.8 [95% confidence interval (CI): 4.6– 25.5] months and the OS rates at 6 and 12 months were 80.6% and 60.9%, respectively. Patients in the Simultaneous group achieved better survival outcomes than those in the Sequential group, but without statistically significant differences. Child-Pugh 6 scores (HR: 2.97, 95% CI: 1.33– 6.61, P=0.008), tumor number ≤ 3 (HR: 0.18, 95% CI: 0.04– 0.78, P=0.022), extrahepatic metastasis (HR: 3.05, 95% CI: 1.35– 6.87, P=0.007) were independent prognostic factors for survival.
Conclusion: The combined treatment of MTAs and ICIs shows good tumor response and survival outcomes with acceptable toxicity for advanced HCC in the real-world practice, in particular when they are applied simultaneously.
Keywords: hepatocellular carcinoma, systemic therapy, molecular targeted agents, immune checkpoint inhibitors
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common malignancy in men and the seventh most common malignancy in women.1,2 Although the incidence and prevalence in western world countries is lower compared to East Asia and sub-Saharan Africa, HCC represents a major medical and socioeconomic problem worldwide, being one of the leading causes of cancer-related deaths.2,3 Only about 15% of patients with early stage HCC are suitable for curative therapy (resection, ablation, or liver transplantation).4 For those with advanced HCC in the form of macrovascular invasion or extrahepatic metastasis and preserved liver function, systemic therapy is the standard of care.5 While the overall survival of patients with HCC has been improved in the past few years, probably due to stage migration, the survival of patients with advanced cancer has remained unchanged.6 However, with the application of molecular targeted agents (MTAs) and recent immune checkpoint inhibitors (ICIs), systemic treatment outcomes for patients with advanced HCC have greatly improved.7
Given the promising data from the IMbrave150 trial, the combination of atezolizumab and bevacizumab now represents the new first-line systemic therapy for advanced HCC.8,9 Although sorafenib and lenvatinib monotherapy is still the first-line systemic treatment option for advanced HCC, in the real-world practice, MTAs or ICIs are rarely used as monotherapy for advanced HCC nowadays. A more recent systemic review made a comparative assessment of early death (defined as death within the first 3 months of treatment) risk upon ICIs alone or in combination with other agents across solid malignancies, and the results indicated that early death upon first-line ICIs is a clinically relevant phenomenon across solid malignancies and preventable through the addition of other treatments to ICIs.10 Recently, various combinations of MTAs and ICIs to treat advanced HCC have shown exciting prospects, probably becoming another new first-line option in clinical practice.11 However, the addition use of ICIs for patients with advanced HCC who develop tumor progression after MTAs monotherapy is still under debate.
In China, the costs of MTAs are much lower than that of ICIs, and part of them can be paid by the medical insurances for the treatment of unresectable HCC. Therefore, in the real-world practice, some patients can afford MTAs plus ICIs as their initial systemic therapy while others only take MTAs due to financial limitations. Majority of the latter patients, however, will accept the sequential application of ICIs after tumor progression. Since January 2021, most of MTAs and ICIs have been included in the list of medical insurance payment for the treatment of unresectable HCC, and almost all patients could receive MTAs plus ICIs as their initial systemic treatment. Therefore, in the special period (April 2019 to December 2020), we have the opportunity to assess and compare the efficacy of MTAs plus ICIs for advanced HCC simultaneously (first-line use) versus sequentially (second-line use) at the same time. This study aimed to report the clinical outcomes, as well as to investigate the impact of possible prognostic factors on survival.
Materials and Methods
Patients
All the patients receiving systemic therapy with MTAs plus ICIs for unresectable HCC in three medical centers (Shanghai Eastern Hepatobiliary Surgery Hospital, The Second Affiliated Hospital of Soochow University and Ningbo Development Zone Hospital) between April 2019 and December 2020 were retrospectively reviewed. HCC was diagnosed based on the combined findings of clinical examinations including serum level of alpha-fetoprotein (AFP) and contrast-enhanced computed tomography (CT) /magnetic resonance imaging (MRI). Extrahepatic metastasis was investigated and confirmed by the positron emission tomography-computed tomography (PET-CT) if clinically indicated. Patients who received MTAs and ICIs as their initial systemic treatment after diagnosis were enrolled in this study. Further inclusion criteria were age 18 years or older, Child-Pugh A liver function, and an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 or 1. Concurrent locoregional treatments such as transarterial chemoembolization (TACE), hepatic artery infusion chemotherapy (HAIC) or radiotherapy represented no exclusion criteria. Participants were classified into two groups according to the treatment time of MTAs and ICIs. Simultaneous group included patients treated with MTAs plus ICIs simultaneously, while Sequential group included patients treated with MTAs initially and followed with ICIs after tumor progression.
Demographic characteristics and clinical data were retrospectively collected and analysed.
This study was approved by the Ethics Committees of the Shanghai Eastern Hepatobiliary Surgery Hospital, Second Affiliated Hospital of Soochow University and Ningbo Development Zone Hospital, and it followed the standards of the Declaration of Helsinki. Informed consent was obtained from all patients.
Treatment and Response Evaluation
Patients began to receive systemic treatment when the diagnosis of unresetable HCC was confirmed. Practically, patients in the Simultaneous group received MTAs plus ICIs treatment, while those in the Sequential group received MTAs monotherapy. There were two kinds of MTAs including sorafenib (Sorafenib Tosylate Tablets, Bayer HealthCare Pharmaceuticals) and lenvatinib (Lenvatinib Mesilate Capsules, Merck Sharp & Dohme), and two kinds of ICIs (programmed death-1 (PD-1) inhibitors) including pembrolizumab (Pembrolizumab Injection, Merck Sharp & Dohme) and sintilimab (Sintilimab Injection, Innovent Biologics, Suzhou, China) provided for patients. Which one to be used depended on the choice of each patient. The sorafenib dose was 400mg twice a day. The lenvatinib dose depended on the patients’ weight: those who weighed <60kg were administered 8 mg daily, while those who weighed ≥ 60kg were initially administered 12 mg daily. Patients were given the standard dose of 200 mg of intravenous pembrolizumab or sintilimab every 3 weeks. Especially, patients in the Sequential group would continue the use of MTAs (initial MTA or the other MTA) when ICIs were applicated.
Treatment response was evaluated by contrast-enhanced dynamic CT or MRI at baseline and at 6–12 weeks after start of the MTAs plus ICIs therapy in the Simultaneous group, while evaluated at the time of ICIs application and at 6–12 weeks thereafter in the Sequential group. Usually, the evaluation was conducted after 4 cycles of ICIs treatment unless there was suspect super progression. The tumor response was determined according to the Response Evaluation Criteria in Solid Tumors Criteria 1.1 (RECIST 1.1). To assess the tumor response, the largest diameter of the tumor was measured and compared with the base value recorded, and categorized as follows: complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD).12 Objective response rate (ORR) of tumor was defined as the proportion of patients achieving CR and PR, while disease control rate (DCR) of tumor was defined as the proportion of patients achieving CR, PR and SD.
Treatment-related toxicity was graded using the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0.13 Dose reduction or treatment interruption was done in patients developing adverse events (AEs) grade 3 or 4, or AEs considered uncontrollable despite symptomatic management.
Follow-Up
All patients were asked to receive routine follow-ups after initial therapy. Physical examination followed by laboratory tests of liver function and tumor markers were performed every month. A hepatic CT/MRI scan was conducted every 3 months or earlier when clinical indicated. PET-CT examination was performed in patients with suspected distant metastasis. Thyroid function tests, heart function tests, chest CT scans and other specific examinations were performed to detect specific ICI-related AEs where clinically indicated.
Statistical Analysis
The analysis was performed using the SPSS 20 software program (Chicago, IL). Continuous data were expressed as mean ± standard deviation (SD) and compared using the independent sample t-test. Categorical variables were expressed as numbers (percentage) and compared using the χ2 test with Yates correction, or with the Fisher exact test, as appropriate.
The efficacy endpoint was overall survival (OS) which was calculated from the date of treatment commencement to the last available follow-up or death. Treatment commencement was defined as MTAs plus ICIs concurrent therapy in the Simultaneous group and MTAs monotherapy in the Sequential group. Progression-free survival was not investigated because it was asymmetrical between the two groups. Survival rates were analysed using the Kaplan-Meier method, and group differences were compared using the two-sided Log rank test. Cox proportional hazards regression model was used to analyse possible prognostic factors for survival. Statistical significance was set at P<0.05.
Results
Patient Characteristics
A total of 207 cases in the three medical centers were reviewed, of which 110 were enrolled in the study. There were 64 patients (58.2%) in the Simultaneous group and 46 (41.8%) in the Sequential group. Sixty-eight patients (61.8%) received concurrent locoregional treatments including TACE (52 patients), HAIC (10 patients) and radiotherapy (6 patients). Table 1 demonstrates the demographic characteristics and clinical data at the time of diagnosis, which indicates that there are no statistical differences of the treatment related characteristics between the two groups. This is in accordance with the fact that selection of the patients into the Sequential group was based primarily on financial limitations rather than disease-related factors.
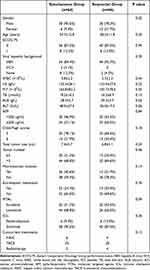 |
Table 1 Demographic and Clinical Characteristics in the Two Groups |
Toxicity
Table 2 summarizes the treatment-related AEs and incidences. A total of 93 (84.5%) patients experienced treatment-related AEs, of which 55 (85.9%) in the Simultaneous group and 38 (82.6%) in the Sequential group (P=0.19). Grade 3/4 AEs were observed in 9 (8.2%) patients (6 in the Simultaneous group and 3 in the Sequential group, P=0.24), all of them discontinued the antitumoral treatments and received specific AEs directed management. No patient died of treatment-related AEs.
 |
Table 2 Treatment Related Adverse Events (CTCAE V. 5.0) |
Treatment Response
There were 4 (3.6%), 14 (12.7%), 46 (41.8%), and 46 (41.8%) patients achieved CR, PR, SD, and PD, respectively. The overall ORR and DCR for the entire cohort were 16.4% and 58.2%, respectively. Patients in the Simultaneous group achieved a significantly higher ORR compared to those in the Sequential group (25.0% vs 4.3%, P=0.04). Table 3 summarizes the treatment response 3 months after MTAs plus ICIs therapy.
 |
Table 3 Treatment Responses and Survival Outcomes in the Two Groups |
At the last follow-up on 31 December 2022, 16 patients (25.0%) in the Simultaneous group and 14 patients (30.4%) in the Sequential group died. Twenty-six patients (40.6%) in the Simultaneous group and 17 patients (40.0%) in the Sequential group were still under systemic therapy. Twenty-two patients undergone locoregional treatment of the tumors during the follow-up period, including 10 with TACE (6 in the Simultaneous group and 4 in the Sequential group); 6 with surgical resection for primary hepatic tumors (2 in the Simultaneous group and 4 in the Sequential group); 4 with radiotherapy for portal vein tumor thrombus (all in the Simultaneous group); and 2 enrolled into a clinical trial (both in the Sequential group).
Survival Outcomes and Prognostic Factors
The median follow-up period was 20 months (range 2–35 months). The median OS of the entire cohort was 14.8 [95% confidence interval (CI): 4.6–25.5] months and the OS rates at 6 and 12 months were 80.6% and 60.9%, respectively. The survival outcomes of the two groups are summarized in Table 3. Patients in the Simultaneous group achieved better survival outcomes compared to those in the Sequential group but without statistically significant differences (Figure 1).
 |
Figure 1 Kaplan-Meier estimates of overall survival in the two groups of patients treated with molecular targeted agents plus immune checkpoint inhibitors. |
On univariate analysis, 5 factors including patient’s age, Child-Pugh scores, tumor number, extrahepatic metastasis and MTAs were related to survival. On multivariate analysis, Child-Pugh 6 scores (HR: 2.97, 95% CI: 1.33–6.61, P=0.008), tumor number ≤3 (HR: 0.18, 95% CI: 0.04–0.78, P=0.022) and extrahepatic metastasis (HR: 3.05, 95% CI: 1.35–6.87, P=0.007) were independent prognostic factors for survival (Figure 2).
 |
Figure 2 Uni- and multivariate analysis of possible factors associated with survival of patients treated with molecular targeted agents plus immune checkpoint inhibitors. |
Discussion
Along with the introduction of immunotherapy in HCC, the systemic treatment strategy for advanced HCC has been revolutionized. With the combination of different agents, higher response rate and longer overall survival may be achieved.14 Thus, many clinical trials are underway to evaluate the efficacy and safety of various combinations of systemic treatments in patients with advanced HCC. Of which the combination of MTAs and ICIs has emerged as a new strategy to treat advanced HCC. The concept of combination therapies is to increase the efficacy of ICIs by further stimulating the immune response, meaning to “make cold tumors hot”. Indeed, also ICIs and MTAs could have synergistic effects, dual blockade of PD-(L)1 and vascular endothelial growth factor (VEGF) has the potential to increase antitumoral activity through joint mechanisms.15,16
Although failed to reach the primary endpoints, the LEAP002 study reported a median OS of 21.2 months in patients with advanced HCC treated by lenvatinib plus pembrolizumab.17 Furthermore, patients in the control arm, which received treatment of lenvatinib and placebo, achieved a median OS of 19 months. In the present study, the Simultaneous group reflexed the first-line use of MTAs plus ICIs for advanced HCC in the real-world practice. The median OS of patients in this group was 16.3 months, which was much lower than that in the LEAP002 study. The relatively poor survival outcome may be to a great extent attributed to the use of sorafenib, which was chosen as MTAs by 31.2% patients. However, the OS rates at 6 and 12 months of this group of patients were 83.7% and 73.2%, respectively, which were better than that in the IMbrave150 trial (72.2% and 54.6%).7 Therefore, we suggest that, in the real-world clinical practice, simultaneous application of MTAs plus ICIs, especially lenvatinib plus PD-1, should be preferred for patients with advanced HCC.
The Sequential group reflexed the second-line use of MTAs plus ICIs for advanced HCC with tumor progression after MTAs monotherapy. The results were only to be expected, patients in the Sequential group had poorer clinical outcomes than that in the Simultaneous group regarding tumor response rate and OS. However, the differences between the two groups were not statistically significant. In addition, the median OS of this group of patients was 12.5 months, which is just a little lower than that of 14.6 months in the clinical trial KENOTE-394 (pembrolizumab as a second-line treatment for advanced HCC after sorafenib or systemic chemotherapy).18 Usually, patients will discontinue the use of initial MTAs when tumor progression happens, in consideration of the drug resistance. This study gives us the information that MTAs plus ICIs treatment is still effective when tumor progression happens after MTAs monotherapy, which indicates that combining an ICI with MTA might overcome the primary resistances observed when the MTA is administered alone.
ICIs has brought a novel spectrum of immune related AEs. Combined therapy with MTAs and ICIs may carry the risk of high toxicities in high-risk patients demanding closer surveillance and hepatologic management.14 All the patients undergoing ICIs treatment in our study are asked to receive comprehensive examinations to identify treatment-related AEs during the follow-up period. The most frequent AE in this study was hand-foot skin reaction (21.8%), followed by diarrhea (18.2%), fatigue (18.2%), and hypertension (16.4%). These AEs were also predominant in previous studies regarding sorafenib and lenvatinib monotherapy.19–23 Pneumonia and myasthenia gravis are ICIs-related specific AEs reported in 3.8% to 6.1% of patients.24,25 In the present study, pneumonia and myasthenia gravis occurred in 4 (3.6%) and 2 (1.8%) patients, respectively. Overall, the tolerability of the combined treatment was good to moderate. Grade 3/4 AEs were developed in 8.2% patients, which was similar to the reports of other study groups using MTAs or ICIs monotherapy.19–25 Furthermore, the AE incidences were not significantly different between the two groups in our study. This may indicate that the simultaneous application of MTAs and ICIs does not increase the toxicity.
It was reported that the expression level of PD-1/PD-L1, tumor mutation burden (TMB) and microsatellite instability/mismatch repair deficiency (MSI/dMMR) were associated with prognosis in various cancers treated with ICIs.26–29 However, the identification of reliable predictors of response to ICIs in HCC patients has not been well addressed so far.30,31 In our clinical practice, the molecular markers are not a routine test for patients treated with ICIs. Instead, several acknowledged clinical factors including high level of AFP, poor liver function, multiple tumor nodules, macrovascular invasion and extrahepatic metastasis were investigated in the present study. Liver function of Child-Pugh 6 scores and extrahepatic metastasis predict poor survival, while tumor number ≤3 predicts good prognosis. These results indicate that the clinical stage of the tumors is still the best predictor for survival outcomes in patients treated with MTAs plus ICIs. However, we still think that the identification of predictive biomarkers represents an unmet need in HCC patients receiving ICIs. The HCC medical community is called to further efforts aimed to elucidate the effective role of PD-L1 expression, TMB, MSI, gut microbiota, and other emerging biomarkers.
Concerning the limitation of this study, the small cohort size and retrospective nature of the analysis may not support the implication in a more persuasive way. A selection bias cannot be fully excluded.
Conclusion
The present real-world study investigated the efficacy and toxicity of MTAs plus ICIs treatment for advanced HCC in three Chinese medical centers. The results suggest that treatment with MTAs plus ICIs can achieve good tumor response and OS rates, in particular when they are applied simultaneously. Moreover, the simultaneous application of MTAs and ICIs seems not to increase the risk of toxicity compared to the sequential application.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Singal AG, Lampertico P, Nahon P. Epidemiology and surveillance for hepatocellular carcinoma: new trends. J Hepatol. 2020;72(2):250–261. doi:10.1016/j.jhep.2019.08.025
2. McGlynn KA, Petrick JL, El-Serag HB. Epidemiology of hepatocellular carcinoma. Hepatology. 2021;73(Suppl 1):4–13. doi:10.1002/hep.31288
3. Arnold M, Abnet CC, Neale RE, et al. Global burden of 5 major types of gastrointestinal cancer. Gastroenterology. 2020;159(1):335–349. e15. doi:10.1053/j.gastro.2020.02.068
4. Tejeda-Maldonado J, Garca-Jurez I, Aguirre-Valadez J, et al. Diagnosis and treatment of hepatocellular carcinoma: an update. World J Hepatol. 2015;7(3):362–376. doi:10.4254/wjh.v7.i3.362
5. Likhitsup A, Razumilava N, Parikh ND. Treatment for advanced hepatocellular carcinoma: current standard and the future. Clin Liver Dis. 2019;13(1):13–19. doi:10.1002/cld.782
6. Toni ED, Schlesinger-Raab A, Fuchs M, et al. Age independent survival benefit for patients with hepatocellular carcinoma (HCC) without metastases at diagnosis: a population-based study. Gut. 2020;69(1):168–176. doi:10.1136/gutjnl-2018-318193
7. Kelley RK. Atezolizumab plus bevacizumab-A landmark in liver cancer. N Engl J Med. 2020;382(20):1953–1955. doi:10.1056/NEJMe2004851
8. Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76(3):681–693. doi:10.1016/j.jhep.2021.11.018
9. NCCN clinical practice guidelines in oncology hepatobiliary cancer. (2022 Version 2). Available from: www.nccn.org/patients.
10. Viscardi G, Tralongo AC, Massari F, et al. Comparative assessment of early mortality risk upon immune checkpoint inhibitors alone or in combination with other agents across solid malignancies: a systematic review and meta-analysis. Eur J Cancer. 2022;177:175–185. doi:10.1016/j.ejca.2022.09.031
11. Greten TF, Lai CW, Li G, Staveley-O’Carroll KF. Targeted and immune-based therapies for hepatocellular carcinoma. Gastroenterology. 2019;15(2):510–524. doi:10.1053/j.gastro.2018.09.051
12. Kuo HY, Chiang NJ, Chuang CH, et al. Impact of immune checkpoint inhibitors with or without a combination of tyrosine kinase inhibitors on organ-specific efficacy and macrovascular invasion in advanced hepatocellular carcinoma. Oncol Res Treat. 2020;43(5):211–220. doi:10.1159/000505933
13. U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) v5.0; 2017. Available from: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf.
14. Mohr R, Jost-Brinkmann F, Zdirik B, et al. Lessons from immune checkpoint inhibitor trials in hepatocellular carcinoma. Front Immunol;2021. 652172. doi:10.3389/fimmu.2021.652172
15. Kudo M. Combination cancer immunotherapy with molecular targeted agents/anti-CTLA-4 antibody for hepatocellular carcinoma. Liver Cancer. 2019;8(1):1–11. doi:10.1159/000496277
16. Hegde PS, Wallin JJ, Mancao C. Predictive markers of anti-VEGF and emerging role of angiogenesis inhibitors as immunotherapeutics. Semin Cancer Biol. 2018;52(Pt2):117–124. doi:10.1016/j.semcancer.2017.12.002
17. Finn RS, Kudo, M, PerlA, P et al. Primary results from the phase III LEAP-002 study: Lenvatinib plus pembrolizumab versus lenvatinib as first-line (1L) therapy for advanced hepatocellular carcinoma (aHCC). Annals of Oncology. 2022;33(suppl_7):S808–S869. doi:10.1016/annonc/annonc1089
18. Qin S, Chen, Z, Fang, W et al. Pembrolizumab plus best supportive care versus placebo plus best supportive care as second-line therapy in patients in Asia with advanced hepatocellular carcinoma (HCC): Phase 3 KEYNOTE-394 study. Journal of Clinical Oncology. 2022;40(no. 4_suppl):383–383. doi:10.1200/JCO.2022.40.4_suppl.383
19. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. doi:10.1056/NEJMoa0708857
20. Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a Phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25–34. doi:10.1016/S1470-2045(08)70285-7
21. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in Simultaneous treatment of patients with unresectable hepatocellular carcinoma: a randomised Phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–1173. doi:10.1016/S0140-6736(18)30207-1
22. Ikeda M, Okusaka T, Mitsunaga S, et al. Safety and pharmacokinetics of lenvatinib in patients with advanced hepatocellular carcinoma. Clin Cancer Res. 2016;22(6):1385–1394. doi:10.1158/1078-0432.CCR-15-1354
23. Komatsu S, Yano Y, Sofue K, et al. Assessment of lenvatinib treatment for unresectable hepatocellular carcinoma with liver cirrhosis. HPB. 2020;22(10):1450–1456. doi:10.1016/j.hpb.2020.03.002
24. Kumar V, Chaudhary N, Garg M, Floudas CS, Soni P, Chandra AB. Current diagnosis and management of immune related adverse events (irAEs) induced by immune checkpoint inhibitor therapy. Front Pharmacol. 2017;8:49. doi:10.3389/fphar.2017.00049
25. Cuzzubbo S, Javeri F, Tissier M, et al. Neurological adverse events associated with immune checkpoint inhibitors: review of the literature. Eur J Cancer. 2017;73:1–8. doi:10.1016/j.ejca.2016.12.001
26. Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-Ll antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563–567. doi:10.1038/nature14011
27. Daud A, Wolchok JD, Robert C, et al. Programmed death-ligand 1 expression and response to the anti-programmed death 1 antibody pembrolizumab in melanoma. J Clin Oncol. 2016;34(34):4102–4109. doi:10.1200/JCO.2016.67.2477
28. Yan H, Chen Y, Wang K, et al. Identification of immune landscape signatures associated with clinical and prognostic features of hepatocellular carcinoma. Aging. 2020;12(19):19641–19659. doi:10.18632/aging.103977
29. Cristescu R, Mogg R, Ayers M, et al. Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy. Science. 2018;362(6411):eaar3593. doi:10.1126/science.aar3593
30. Rizzo A, Ricci AD, Di Federico A, et al. Predictive biomarkers for checkpoint inhibitor-based immunotherapy in hepatocellular carcinoma: where do we stand? Front Oncol. 2021;17(11):803133. doi:10.3389/fonc.2021.803133
31. Rizzo A, Cusmai A, Gadaleta-Caldarola G, Palmiotti G. Which role for predictors of response to immune checkpoint inhibitors in hepatocellular carcinoma? Expert Rev Gastroenterol Hepatol. 2022;16(4):333–339. doi:10.1080/17474124.2022.2064273
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
