Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 16
Association Between Thyroid Parameters and Subclinical Atherosclerosis in Hospitalised Euthyroid Patients with Type 2 Diabetes Mellitus
Authors Du J , Zhao X, Xu X, Zhang Z, Zhang X
Received 12 July 2023
Accepted for publication 4 October 2023
Published 12 October 2023 Volume 2023:16 Pages 3163—3171
DOI https://doi.org/10.2147/DMSO.S429941
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Konstantinos Tziomalos
Jing Du, Xin Zhao, Xiumei Xu, Zhichao Zhang, Xiaomei Zhang
Department of Endocrinology and Metabolism, Peking University International Hospital, Beijing, People’s Republic of China
Correspondence: Xiaomei Zhang, Department of Endocrinology and metabolism, Peking University International Hospital, No. 1 Life Garden Road Zhongguancun Life Science Garden Changping District, Beijing, People’s Republic of China, Tel/Fax +86-010-69006105, Email [email protected]
Purpose: To explore the association between thyroid parameters and subclinical atherosclerosis (AS) in hospitalised euthyroid patients with type 2 diabetes mellitus (T2DM).
Patients and Methods: A retrospective analysis was conducted involving 1245 inpatients with T2DM. Free triiodothyronine (FT3), free thyroxine (FT4), and thyroid stimulating hormone (TSH) levels were measured, and carotid artery ultrasonography was performed. Thyroid hormone (TH) sensitivity was evaluated using thyroid feedback quantile-based index (TFQI), TSH index (TSHI), thyrotropin thyroxine resistance index (TT4RI), and free triiodothyronine/free thyroxine ratio (FT3/FT4).
Results: In inpatients with T2DM having normal thyroid function, the incidence of subclinical AS declined with increasing levels of FT3, FT4, and FT3/FT4 (P trend < 0.05). Logistic regression analysis revealed that FT4 (OR, 0.914; 95% CI, 0.845– 0.989), FT3 (OR, 0.374; 95% CI, 0.277– 0.504), and FT3/FT4 (OR, 0.036; 95% CI, 0.013– 0.061) were independently associated with subclinical AS (P < 0.05). However, TSH, TFQI, TSHI, and TT4RI levels were not associated with subclinical AS (P > 0.05). FT3/FT4 demonstrated superior predictive accuracy for subclinical AS than that of FT3 or FT4 alone (P < 0.001), with a cutoff point of 0.25.
Conclusion: In euthyroid inpatients with T2DM, subclinical AS exhibited negative correlation with FT3, FT4, and FT3/FT4 levels, independent of other risk factors for AS. Additionally, FT3/FT4 ratio had a good predictive value for subclinical AS.
Keywords: thyroid hormone, thyroid hormone sensitivity, euthyroid, diabetes mellitus, atherosclerosis
Introduction
Type 2 diabetes mellitus (T2DM) and its associated complications have become serious public health concern worldwide.1 Previous studies have shown that the risk of macrovascular disease in patients with DM is 2–4 times higher than that in patients without DM.2 Compared with patients without DM, those with T2DM often exhibit more extensive, severe, and earlier-onset macrovascular lesions.3 The primary pathological change underlying diabetes-related macrovascular disease is atherosclerosis (AS), characterised by hardening of arteries due to plaque accumulation,4 which can worsen ischaemic events and cause damage to target organs, such as the heart, brain, and lower limbs, Macrovascular complications have become the leading cause of disability and mortality in patients with T2DM.5,6 Identifying and intervening at the subclinical stage of AS may aid in preventing the progression of this disease. One promising biomarker for identifying subclinical AS is carotid intima-media thickness (CIMT), which can be used to predict the risk of cerebral and cardiovascular diseases.7,8
Previous studies have established a strong association between thyroid hormones (THs) and AS. Subclinical or overt thyroid dysfunction, both hypothyroidism and hyperthyroidism, is positively associated with the onset and progression of AS and AS-related adverse outcomes.9 Even in euthyroid patients, some studies have suggested that high levels of free triiodothyronine (FT3) and free thyroxine (FT4) can reduce the risk of AS.10,11 Conversely, other studies have indicated that individuals with higher FT3 and FT4 levels are at high risk of developing AS.12 These paradoxical observations may result from impaired sensitivity to TH. However, only a few studies have investigated the association between impaired TH sensitivity and AS, particularly in patients with T2DM. Therefore, in this study, we aimed to explore the relationship between impaired TH sensitivity and subclinical AS in patients with T2DM who had normal thyroid function to provide clinical evidence for the prevention and treatment of AS.
Materials and Methods
Study Population
Patients admitted to the endocrinology department of Peking University International Hospital between December 2014 and September 2022 were retrospectively analysed. The inclusion criteria were as follows: (1) adults (aged ≥18 years); (2) patients diagnosed with T2DM according to the 1999 World Health Organization criteria; (3) those with normal TH, defined as having FT4, FT3 and thyroid stimulating hormone (TSH) levels within the reference ranges. (4) those with carotid color Doppler ultrasound examination results. The exclusion criteria were as follows: (1) patients with gestational diabetes mellitus (GDM), type 1 DM (T1DM), or a special type of DM; (2) those without TH test results or with abnormal TH levels; (3) those receiving drugs that could affect serum TH levels, such as methimazole, propylthiouracil, levothyroxine, and amiodarone; (4) those with serious diseases that could affect serum TH levels, such as acute diabetic complications (eg, ketoacidosis, hyperglycemic hyperosmolar state), severe infections, acute heart failure, severe liver or kidney disease, and cancer; (5) those with atherosclerotic cardiovascular disease (ASCVD), including myocardial infarction, angina, stroke, symptomatic peripheral artery disease, or cardiovascular revascularization. Finally, a total of 1245 patients were included in this study (Figure 1).
 |
Figure 1 Flow chart depicting patient selection process. |
Data Collection
We collected demographic and clinical data (age, sex, medical history, and smoking status) and anthropometric parameters, including blood pressure, body weight, and height, measured and recorded by trained nurses. Body mass index (BMI) was calculated as body weight (kg)/height squared (m2).
Venous blood was collected from each participant after a 10-hour fast, and the following parameters were examined: glycosylated haemoglobin (HbA1c), triglycerides (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and serum creatinine (sCr). Estimated glomerular filtration rate (eGFR) was calculated using the CKD-EPI equation.13 Morning urine samples were collected three times on different days to measure the urinary albumin/creatinine ratio (UACR), and the mean UACR (mUACR) was calculated. Serum levels of thyroxine (FT4), triiodothyronine (FT3), and thyroid-stimulating hormone (TSH) were measured using the Roche Cobas Elesys 601 analyzer (Roche, Basel, Switzerland). The normal reference ranges for FT4, FT3, and TSH were 12.0–22.0 pmol/L, 3.1–6.8 pmol/L, and 0.27–4.20 mIU/L, respectively.
Colour Doppler ultrasonography of the carotid artery was performed by experienced and trained sonographers using the Philips iU Elite Color Doppler Ultrasound System (Philips Healthcare, Eindhoven, the Netherlands) with a probe frequency of 3–9 MHz. Subclinical AS was confirmed by CIMT of ≥1.0 mm and/or the presence of carotid plaques. A carotid plaque was defined as CIMT of ≥1.5 mm or the presence of focal wall thickening at least 50% greater than the surrounding vessel wall.8,14
Thyroid Hormone Sensitivity Indices
Central TH sensitivity indices were employed as follows: (1) Thyroid feedback quantile-based index (TFQI) was calculated as TFQI = cdfFT4−(1−cdfTSH).15 The value of TFQI ranged from −1 to 1. Positive or negative values indicate sensitivity or insensitivity of the hypothalamic–pituitary–thyroid axis to FT4. (2) TSH index (TSHI) was calculated as TSHI = ln TSH (mIU/L) + 0.1345 × FT4 (pmol/L).16 (3) Thyrotropin–thyroxine resistance index (TT4RI) was calculated as TT4RI = FT4 (pmol/L) × TSH (mIU/L).17 Higher TSHI and TT4RI values indicate lower central sensitivity to THs.
The peripheral TH sensitivity index (FT3/FT4) was calculated as FT3 divided by FT4. Higher values indicate higher peripheral sensitivity to THs.18
Statistical Analysis
All data were analysed using SPSS software (version 24.0; IBM Corp., New York, NY, USA). Normality was assessed using the Shapiro–Wilk test. Categorical data were expressed as absolute numbers and percentages, whereas continuous data were expressed as mean ± standard deviation or median (Q1, Q3). To compare the differences between the two groups, the chi-square test, Mann–Whitney U-test, or independent t-test was employed, as appropriate. The trend in subclinical AS prevalence was assessed using the linear-by-linear association chi-square test. Univariable logistic regression (Model 1) and multivariable logistic regression (Model 2: adjusted for sex and age; Model 3: adjusted for sex, age, duration of diabetes, smoking status, BMI, SBP, LDL-C, eGFR, and mUACR) were used to analyse the associations between thyroid parameters and subclinical AS. Receiver operating characteristic (ROC) curves of different thyroid parameters for predicting subclinical AS were analysed using MedCalc statistical software. The areas under the ROC curves (AUC) were calculated and compared. A P value < 0.05 was considered statistically significant.
Results
Characteristics of Patients
Among 1245 euthyroid inpatients with T2DM, 909 cases exhibited subclinical AS. The prevalence of subclinical AS was 73.0%. Patients with subclinical AS were more likely to be older, of female sex, have a longer duration of diabetes, and exhibit higher levels of TC, LDL-C, sCr, and mUACR than those of patients without subclinical AS (P < 0.05). BMI, eGFR, and FT3, FT4, and FT3/FT4 levels were lower in patients with subclinical AS than in those without subclinical AS (P < 0.05). No significant differences in the proportion of smokers and levels of SBP, DBP, HbA1c, TG, HDL-C, TSH, TFQI, TSHI, and TT4RI were observed between the two groups (P > 0.05) (Table 1).
 |
Table 1 Comparison of Clinical Characteristics Between Patients with and without Subclinical AS |
Prevalence of Subclinical AS in Patients with Different Levels of Thyroid Parameters
The prevalence of subclinical AS was calculated across quartiles of FT3, FT4, TSH, TFQI, TSHI, TT4RI, and FT3/FT4 (Figure 2). The incidence of subclinical AS decreased with increasing FT3, FT4, and FT3/FT4 levels (P trend < 0.05). However, no significant difference in the incidence of subclinical AS were observed among patients with varying TSH, TFQI, TSHI, and TT4RI levels (P trend > 0.05).
Association Between Subclinical AS and Thyroid Parameters
Table 2 presents the results of logistic regression analysis for the association between subclinical AS and different thyroid parameters in patients with T2DM having normal thyroid function. After adjustment for sex, age, duration of diabetes, smoking status, BMI, SBP, LDL-C, eGFR, and mUACR, the logistic regression analysis revealed independent associations of subclinical atherosclerosis (AS) with FT4 (OR, 0.914; 95% CI, 0.845–0.989), FT3 (OR, 0.374; 95% CI, 0.277–0.504), and FT3/FT4 (OR, 0.036; 95% CI, 0.013–0.061) (P < 0.05). However, TSH, TFQI, TSHI, and TT4RI were not significantly associated with subclinical AS (P > 0.05).
 |
Table 2 Association Between Subclinical AS and Thyroid Parameters |
ROC Curve of Thyroid Parameters for Predicting Subclinical AS
Figure 3 displays the ROC curves for FT3, FT4, and FT3/FT4 for predicting the risk of subclinical AS. The AUCs for FT3, FT4, and FT3/FT4 were 0.621 (0.594, 0.648), 0.582 (0.554, 0.610), and 0.715 (0.689, 0.740), respectively. The predictive accuracy of FT3/FT4 was significantly higher than that of FT3 or FT4 alone (P < 0.001). No significant differences in the AUCs were observed between FT3 and FT4 levels (P = 0.212). The corresponding cutoff points for FT3, FT4 and FT3 /FT4 were 4.45 pmol/L, 17.55 pmol/L, and 0.25, respectively (Table 3).
 |
Table 3 Comparisons the AUCs for FT3, FT4 and FT3/FT4 in Predicting Subclinical AS |
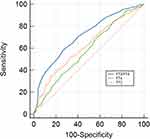 |
Figure 3 The ROC curves for FT3, FT4 and FT3/FT4 in predicting subclinical AS. Abbreviations: AS, atherosclerosis; FT3, free triiodothyronine; FT4, free thyroxine. |
Discussion
In recent years, the incidence of diabetes has increased primarily attributed to unhealthy lifestyles and dietary habits. Globally, the number of diabetes cases reached 425 million in 2017, and its prevalence is projected to increase to 629 million by 2040.19 Macrovascular complications are among the most serious complications in individuals with type 2 diabetes, significantly affecting their quality of life and imposing a substantial economic burden on both patients and the healthcare system. AS is a complex process involving multiple risk factors, including age, smoking, diabetes, hypertension, and dyslipidemia.20 The incidence of AS in patients with T2DM remains high, even if patients proactively address conventional risk factors, such as smoking cessation and management of blood pressure and blood glucose and lipid levels. This has led to a growing research interest in identifying new risk factors for AS, such as TH.
Previous studies have suggested that overt hypothyroidism is an independent risk factor of AS.9,21 Peixoto et al22 and Ning Gao et al23 have reported that subclinical hypothyroidism is associated with an increased CIMT and formation of carotid plaque. In addition, among euthyroid individuals, low levels of FT3 and FT4 increase the risk of AS.10,11 However, a prospective cohort study involving 9420 patients revealed that FT4 levels are positively associated with AS.24 Another cohort study involving 3181 euthyroid patients suggested that higher mean levels and greater variations in THs are associated with a higher risk of AS.12 These inconsistent results prompted us to conduct the present study to explore the association between AS and multiple thyroid parameters, particularly indicators of TH sensitivity, in euthyroid patients with T2DM. In this study, we observed that the incidence of AS increased with the decrease in FT3 and FT4 levels and peripheral TH sensitivity index FT3/FT4 scores; moreover, these associations remained significant even after adjusting for multiple risk factors for AS. In contrast, TSH, TFQI, TSHI, and TT4RI were not associated with the risk of AS.
The mechanism by which TH affect AS is not yet fully understood. TH deficiency is known to be associated with various metabolic abnormalities, including dyslipidemia and hypertension, which can contribute to the onset and progression of AS.25,26 Previous studies have suggested that THs stimulate the synthesis of nitric oxide (NO) in vascular endothelial cells by binding to the TH receptor (TR) on endothelial cells and activating the phosphatidylinositol 3-kinase and serine/threonine protein kinase pathways.27 NO plays a critical role in regulating vascular tone, inflammation, oxidative stress, and inhibiting proliferation of smooth muscle.28 Its production is reduced in individuals with decreased TH levels, leading to impaired endothelial function and development of AS. Furthermore, Cyrielle Billon et al29 have reported that the deletion of TRα (0/0) in the ApoE (-/-) male mice can impair cholesterol efflux and increase inflammatory reaction in macrophages, thereby accelerating plaque formation. Another study by Samia Neggazi et al30 have demonstrated that TRα deletion in ApoE-/- mice increases the expression of tissue renin-angiotensin system in the aortic wall and aggravates the dysregulation of cholesterol content in vascular smooth muscle cells. They concluded that THα has a protective role against AS. A reduction in the number of activated THα due to decreased TH levels may lead to the progression of AS. Additionally, advanced glycation end products (AGEs) are commonly present in the vascular system of patients with diabetes and contribute to the development of AS.31 HbA1c variability, which reflects the accumulation of AGEs in tissues, is positively associated with the occurrence of microvascular and macrovascular complications.32–34 Previous studies have indicated that thyroid dysfunction promotes AGE formation,35 thereby leading to vascular atherosclerosis. The two main TH in circulation are T4 and T3. FT4 is converted into its more active form, FT3, by three deiodinases. FT3/FT4 is considered an indicator of type 2 5′- deiodinase (DIO2), which is the primary and most active enzyme in human tissues.36,37 Therefore, FT3/FT4 can be used to evaluate peripheral thyroid sensitivity, potentially being a more precise and feasible indicator of TH metabolic variability than FT3 or FT4 alone.18 Previous studies have reported a significant decrease in the levels of FT3/FT4 in patients with diabetes compared to individuals with normal thyroid function, suggesting that diabetes is associated with a diminished peripheral turnover of thyroxine.38 In this study, we observed that FT3/FT4 ratio was negatively associated with subclinical AS, consistent with the findings of previous studies,37,39 and it emerged as an independent predictor of subclinical AS. The predictive accuracy of FT3/FT4 for subclinical AS was higher than that of FT3 or FT4 alone. In addition, we identified a cutoff value of 0.25 for FT3/FT4 to predict subclinical AS, with a specificity and sensitivity of 83.93% and 35.09%, respectively. A large-scale, multicentre, retrospective, cross-sectional study involving 6679 patients demonstrated that central TH sensitivity indices, including TFQI and TT4RI, were positively associated with the risk of plaque formation in the carotid artery.39 However, in this study, TFQI, TSHI, and TT4RI did not exhibit correlation with the risk of AS in the logistic regression models. This inconsistency may be attributed to differences in the study populations.
This study had some limitations. First, it was a single-centre retrospective study involving hospitalised patients, and the sample size was relatively small, which may introduce potential selection bias. Second, a causal relationship between thyroid parameters and subclinical AS could not be determined. Third, ultrasonography may not be as accurate as high-resolution computed tomography or digital subtraction angiography for assessing AS. However, ultrasound was chosen as the preferred assessment method in this study owing to its safety and noninvasiveness. Further prospective studies with larger sample sizes and different populations are required to confirm our findings and explore the underlying mechanisms.
Conclusion
In summary, this study revealed that in euthyroid patients with T2DM, the incidence of carotid atherosclerosis exhibited negative correlation with FT3 and FT4 levels, as well as the peripheral TH sensitivity index FT3/FT4, independent of other risk factors for AS. In addition, FT3/FT4 ratio was a better predictor of subclinical AS than FT3 or FT4 alone. Clinically, monitoring FT3/FT4 may hold great significance for predicting and treating subclinical AS.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics Approval and Informed Consent
This was an observational and retrospective study with no potential harm to the study participants. Moreover, no personally identifying information was included in the analysis, thereby safeguarding the safety and privacy of the individuals involved. The study protocol was approved by the Ethics Committee of Peking University International Hospital (2022-KY-0030-01) and it was sanctioned that the informed consent of the participant was not necessarily required. All the procedures were performed in accordance with the principles of the Declaration of Helsinki.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Dal Canto E, Ceriello A, Ryden L, et al. Diabetes as a cardiovascular risk factor: an overview of global trends of macro and micro vascular complications. Eur J Prev Cardiol. 2019;26:25–32. doi:10.1177/2047487319878371
2. Sarwar N, Gao P, Seshasai SR, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215–2222. doi:10.1016/S0140-6736(10)60484-9
3. Balletshofer B, Bockler D, Diener H, et al. Position paper on the diagnosis and treatment of peripheral arterial disease (PAD) in people with diabetes mellitus. Exp Clin Endocrinol Diabetes. 2022;130:S127–S136. doi:10.1055/a-1624-3631
4. Adedokun TA, Kwaghe VG, Adedokun O, et al. Prevalence and risk factors for subclinical atherosclerosis amongst adults living with HIV in university of abuja teaching hospital, Gwagwalada. Front Reprod Health. 2023;5:1092211. doi:10.3389/frph.2023.1092211
5. Ballotari P, Ranieri SC, Luberto F, et al. Sex differences in cardiovascular mortality in diabetics and nondiabetic subjects: a population-based study (Italy). Int J Endocrinol. 2015;2015:914057. doi:10.1155/2015/914057
6. Gregg EW, Cheng YJ, Srinivasan M, et al. Trends in cause-specific mortality among adults with and without diagnosed diabetes in the USA: an epidemiological analysis of linked national survey and vital statistics data. Lancet. 2018;391:2430–2440. doi:10.1016/S0140-6736(18)30314-3
7. Bauer M, Caviezel S, Teynor A, et al. Carotid intima-media thickness as a biomarker of subclinical atherosclerosis. Swiss Med Wkly. 2012;142:w13705. doi:10.4414/smw.2012.13705
8. Johri AM, Nambi V, Naqvi TZ, et al. Recommendations for the assessment of carotid arterial plaque by ultrasound for the characterization of atherosclerosis and evaluation of cardiovascular risk: from the American society of echocardiography. J Am Soc Echocardiogr. 2020;33:917–933. doi:10.1016/j.echo.2020.04.021
9. Papadopoulou AM, Bakogiannis N, Skrapari I, et al. Thyroid dysfunction and atherosclerosis: a systematic review. Vivo. 2020;34:3127–3136. doi:10.21873/invivo.12147
10. Hu Y, Hu Z, Tang W, et al. Association of thyroid hormone levels with microvascular complications in euthyroid type 2 diabetes mellitus patients. Diabetes Metab Syndr Obes. 2022;15:2467–2477. doi:10.2147/DMSO.S354872
11. Wang L, Chen T, Yu J, et al. Clinical associations of thyroid hormone levels with the risk of atherosclerosis in euthyroid type 2 diabetic patients in central China. Int J Endocrinol. 2020;2020:2172781. doi:10.1155/2020/2172781
12. Gu Y, Meng G, Zhang Q, et al. Association of longitudinal trends in thyroid function with incident carotid atherosclerosis in middle-aged and older euthyroid subjects: the Tianjin chronic low-grade systemic inflammation and health (TCLSIH) cohort study. Age Ageing. 2022;51:afab276. doi:10.1093/ageing/afab276
13. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi:10.7326/0003-4819-150-9-200905050-00006
14. Katakami N, Matsuoka TA, Shimomura I. Clinical utility of carotid ultrasonography: application for the management of patients with diabetes. J Diabetes Investig. 2019;10:883–898. doi:10.1111/jdi.13042
15. Laclaustra M, Moreno-Franco B, Lou-Bonafonte JM, et al. Impaired sensitivity to thyroid hormones is associated with diabetes and metabolic syndrome. Diabetes Care. 2019;42:303–310. doi:10.2337/dc18-1410
16. Cappelli C, Rotondi M, Pirola I, et al. TSH-lowering effect of metformin in type 2 diabetic patients: differences between euthyroid, untreated hypothyroid, and euthyroid on L-T4 therapy patients. Diabetes Care. 2009;32:1589–1590. doi:10.2337/dc09-0273
17. Yagi H, Pohlenz J, Hayashi Y, et al. Resistance to thyroid hormone caused by two mutant thyroid hormone receptors beta, R243Q and R243W, with marked impairment of function that cannot be explained by altered in vitro 3,5,3’-triiodothyroinine binding affinity. J Clin Endocrinol Metab. 1997;82:1608–1614. doi:10.1210/jcem.82.5.3945
18. Gao W, Guo W, Guo Y, et al. Thyroid hormone concentrations in severely or critically ill patients with COVID-19. J Endocrinol Invest. 2021;44:1031–1040. doi:10.1007/s40618-020-01460-w
19. Cho NH, Shaw JE, Karuranga S, et al. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–281. doi:10.1016/j.diabres.2018.02.023
20. Fan J, Watanabe T. Atherosclerosis: known and unknown. Pathol Int. 2022;72:151–160. doi:10.1111/pin.13202
21. Ning Y, Cheng YJ, Liu LJ, et al. What is the association of hypothyroidism with risks of cardiovascular events and mortality? A meta-analysis of 55 cohort studies involving 1,898,314. BMC Med. 2017;15:21. doi:10.1186/s12916-017-0777-9
22. Peixoto DME, Bittencourt MS, Pereira AC, et al. Subclinical hypothyroidism is associated with higher carotid intima-media thickness in cross-sectional analysis of the Brazilian longitudinal study of adult health (ELSA-Brasil). Nutr Metab Cardiovasc Dis. 2016;26:915–921. doi:10.1016/j.numecd.2016.06.005
23. Gao N, Zhang W, Zhang YZ, et al. Carotid intima-media thickness in patients with subclinical hypothyroidism: a meta-analysis. Atherosclerosis. 2013;227:18–25. doi:10.1016/j.atherosclerosis.2012.10.070
24. Bano A, Chaker L, Mattace-Raso F, et al. Thyroid function and the risk of atherosclerotic cardiovascular morbidity and mortality: the Rotterdam study. Circ Res. 2017;121:1392–1400. doi:10.1161/CIRCRESAHA.117.311603
25. Gluvic ZM, Zafirovic SS, Obradovic MM, et al. Hypothyroidism and risk of cardiovascular disease. Curr Pharm Des. 2022;28:2065–2072. doi:10.2174/1381612828666220620160516
26. Zhang Y, Lu P, Zhang L, et al. Association between lipids profile and thyroid parameters in euthyroid diabetic subjects: a cross-sectional study. BMC Endocr Disord. 2015;15:12. doi:10.1186/s12902-015-0008-3
27. Razvi S, Jabbar A, Pingitore A, et al. Thyroid hormones and cardiovascular function and diseases. J Am Coll Cardiol. 2018;71:1781–1796. doi:10.1016/j.jacc.2018.02.045
28. Rajendran S, Shen X, Glawe J, et al. Nitric oxide and hydrogen sulfide regulation of ischemic vascular growth and remodeling. Compr Physiol. 2019;9:1213–1247.
29. Billon C, Canaple L, Fleury S, et al. TRalpha protects against atherosclerosis in male mice: identification of a novel anti-inflammatory property for TRalpha in mice. Endocrinology. 2014;155:2735–2745. doi:10.1210/en.2014-1098
30. Neggazi S, Hamlat N, Canaple L, et al. TRalpha inhibits arterial renin-angiotensin system expression and prevents cholesterol accumulation in vascular smooth muscle cells. Ann Endocrinol. 2019;80:89–95. doi:10.1016/j.ando.2018.09.008
31. Goldin A, Beckman JA, Schmidt AM, et al. Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation. 2006;114:597–605. doi:10.1161/CIRCULATIONAHA.106.621854
32. Qu F, Shi Q, Wang Y, et al. Visit-to-visit glycated hemoglobin A1c variability in adults with type 2 diabetes: a systematic review and meta-analysis. Chin Med J. 2022;135:2294–2300. doi:10.1097/CM9.0000000000002073
33. Zhou Y, Huang H, Yan X, et al. Glycated haemoglobin A1c variability score elicits kidney function decline in Chinese people living with type 2 diabetes. J Clin Med. 2022;11:6692. doi:10.3390/jcm11226692
34. Li S, Nemeth I, Donnelly L, et al. Visit-to-Visit HbA(1c) variability is associated with cardiovascular disease and microvascular complications in patients with newly diagnosed type 2 diabetes. Diabetes Care. 2020;43:426–432. doi:10.2337/dc19-0823
35. Yavuz DG, Temizkan S, Yazici D. Serum Carboxymethyl-Lysine and Soluble Receptor for Advanced Glycation end Products in Hyperthyroid and Hypothyroid Patients. Acta Endocrinol. 2022;18:436–441. doi:10.4183/aeb.2022.436
36. Mullur R, Liu YY, Brent GA. Thyroid hormone regulation of metabolism. Physiol Rev. 2014;94:355–382. doi:10.1152/physrev.00030.2013
37. Lin J, Xiang X, Qin Y, et al. Correlation of thyroid-related hormones with vascular complications in type 2 diabetes patients with euthyroid. Front Endocrinol. 2022;13:1037969. doi:10.3389/fendo.2022.1037969
38. Qin K, Zhang F, Wu Q, et al. Thyroid hormone changes in euthyroid patients with diabetes. Diabetes Metab Syndr Obes. 2020;13:2533–2540. doi:10.2147/DMSO.S260039
39. Liu Y, Li Z, Yang T, et al. Impaired sensitivity to thyroid hormones and carotid plaque in patients with coronary heart disease: a RCSCD-TCM study in China. Front Endocrinol. 2022;13:940633. doi:10.3389/fendo.2022.940633
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

