Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 17
Association Between the Water Distribution in the Human Body and 25-Hydroxyvitamin D Among the Type 2 Diabetes Mellitus Population: A Possible Pathway Between Vitamin D and Diabetic Nephropathy
Authors Xu Z , Zhang J, Xiang S, Hua F, Chen L
Received 2 October 2023
Accepted for publication 18 January 2024
Published 7 February 2024 Volume 2024:17 Pages 597—610
DOI https://doi.org/10.2147/DMSO.S442789
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Konstantinos Tziomalos
Video abstract of “Water distribution and 25-hydroxyvitamin D in type 2 diabetes mellitus” [442789].
Views: 48
Zhenghui Xu,1,* Junli Zhang,1,* Shoukui Xiang,1,* Fei Hua,1 Lu Chen1,2
1Department of Endocrinology and Metabolism, The Third Affiliated Hospital of Soochow University, Changzhou, 213003, People’s Republic of China; 2Department of Clinical Nutrition, The Third Affiliated Hospital of Soochow University, Changzhou, 213003, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Fei Hua, Department of Endocrinology and Metabolism, The Third Affiliated Hospital of Soochow University, Changzhou, 213003, People’s Republic of China, Tel +86 0519-68870000, Email [email protected] Lu Chen, Department of Clinical Nutrition, The Third Affiliated Hospital of Soochow University, Changzhou, 213003, People’s Republic of China, Tel +86 0519-68870424, Email [email protected]
Purpose: To explore the association between the water distribution in the human body and 25-hydroxyvitamin D among patients with type 2 diabetes mellitus (T2DM), and to analyze whether water distribution is a mediator between vitamin D and T2DM complications.
Patients and Methods: In total, 533 T2DM inpatients were included from August 1, 2016 to April 1, 2023. Water distribution indicators, whether from the whole body, arms, trunk, or legs, were measured to calculate the association with vitamin D using linear regression analysis. Subgroups based on age, sex, body mass index, and macrovascular complications were established to clarify changes in the association between vitamin D and water distribution in different populations. Mediation analysis was applied to evaluate the correlation between vitamin D, water distribution, and T2DM complications.
Results: There was a negative correlation between 25-hydroxyvitamin D and the ratio of extracellular water (ECW)/total body water (TBW) both in the whole body (P=0.045, β=− 0.008) and in certain parts. A U-shaped restricted cubic spline curve further presented an inflection point (approximately 23 ng/mL 25-hydroxyvitamin D) in the relationship between 25-hydroxyvitamin D and the ECW/TBW of the whole body. A negative correlation was observed between ECW/TBW and vitamin D in the obese subgroup (P=0.015, β=− 0.038). In the total effect of vitamin D on diabetic nephropathy (DN), the mediation effect of ECW/TBW accounted for 15.44%.
Conclusion: A correlation between vitamin D and water distribution was observed, and a high ECW/TBW was one of the pathways through which low vitamin D levels might affect DN.
Keywords: 25-hydroxyvitamin D, ECW/TBW, type 2 diabetes mellitus, diabetic nephropathy
Introduction
Vitamin D, a long-standing fat-soluble vitamin, has been extensively studied in the past century since its use in the treatment of rickets in 1919.1 A commonly reported function of vitamin D in the endocrine field is its involvement in bone metabolism.2–4 In addition to its effects on bone improvement, vitamin D is believed to be associated with type 2 diabetes mellitus (T2DM) and some complications of diabetes.5–11 The vitamin D status in T2DM individuals has received increasing attention, with current studies suggesting that T2DM patients may suffer from relatively low levels of vitamin D.5,6 Furthermore, a study conducted in the Chinese countryside showed that a low level of 25-hydroxyvitamin D is a risk factor for T2DM occurrence.7 Meanwhile, vitamin D is also associated with some acute and chronic complications of T2DM. He et al reported a correlation between vitamin D and ketosis in their T2DM participants.8 Tang et al found that a poor vitamin D status may be related to diabetic foot in Chinese T2DM participants.9 Ahmed et al reported on the clinical value of vitamin D3 in diabetic retinopathy (DR) in the Qatari population with T2DM.10 Furthermore, Liyanage et al found that vitamin D supplementation may be an effective treatment for diabetic nephropathy (DN).11 Unfortunately, despite the crucial roles of vitamin D in human health mentioned above, the current widespread lack of vitamin D has become a serious health crisis.12,13 Over one billion children and adults have already suffered from a lack in vitamin D worldwide.12
Water distribution in the human body is an important foundation of biological metabolism and can be obtained through an analysis of the human body composition. From a micro perspective, water in the human body is the most basic environment for cells to live and function, affecting the shape of cells and determining their fate.14 The distribution of intracellular (ICW) and extracellular water (ECW) not only regulates the shape of cells, but also participates in the process of cell volume changes.15 From a macro perspective, water distribution is crucial for the health status of T2DM patients. López-Valverde et al reported a correlation between the ratio of ECW/ICW and mortality among patients suffering from diabetic foot ulcers.16 Nakajima et al found a correlation between the ratio of ECW/ICW and albuminuria in the T2DM population.17 Similarly, Low et al reported that ECW/total body water (TBW) is also associated with renal health in patients with T2DM.18 Therefore, from both perspectives of cells and diabetic individuals, water distribution in the human body is crucial for health. Identifying the clinical variables affecting the water distribution and even regulating the water distribution will greatly benefit the T2DM population.
However, while the abovementioned studies have reached a preliminary conclusion that not only vitamin D, but also the water distribution in the human body is related to T2DM complications, this has further led to two major academic gaps that have never been filled by any research yet.
Firstly, the possible direct connection between vitamin D and the water distribution in patients with T2DM has been overlooked. Since both vitamin D and the water distribution in the human body have been separately proven to be associated with T2DM complications, it is necessary to explore whether there is a direct correlation between vitamin D and the water distribution. However, to our knowledge, there is currently no study that has ever assessed the interrelationships between these two factors themselves.
Secondly, based on the first academic gap, it is currently unclear whether the possible association between vitamin D and the water distribution is one of the mechanisms by which vitamin D may affect certain complications of T2DM.
Based on the aforementioned academic gaps, we propose two clear research hypotheses. (1) There may be a direct correlation between the water distribution in the human body and 25-hydroxyvitamin D among patients with T2DM. (2) There may be a mediation role of the water distribution in the association between vitamin D and certain complications of T2DM.
Materials and Methods
Study Population
In total, 865 T2DM inpatients from the Department of Endocrinology and Metabolism of Third Affiliated Hospital of Soochow University were preliminarily recorded from August 1, 2016 to April 1, 2023, according to the American Diabetes Association (ADA) standard.19 After excluding patients with (1) diseases that may strongly affect vitamin D metabolism, (2) poor or unstable conditions (such as acute infection, a risk of death, etc.), (3) a lack of body composition parameters, or (4) severe liver or kidney dysfunction, 533 participants were ultimately included in the statistical analysis. The patients were subsequently divided into different subgroups to explore the correlation between the water distribution in the human body and 25-hydroxyvitamin D. The process of screening all participants and conducting subgroup analyses is presented in Figure 1.
Data Collection
With the patients’ consent, we obtained basic information about the participants through questionnaires or verbal inquiries, and obtained examination results through the electronic health record. The basic information included sex, age, duration of T2DM, the smoking history, and the drinking history. The examination results included 25-hydroxyvitamin D, blood pressure, blood routine examination, liver function, renal function, blood lipids, thyroid function, T2DM-related indicators, and some of the body composition parameters. All the above examination results included 25-hydroxyvitamin D, systolic blood pressure (SBP), diastolic blood pressure (DBP), leucocyte, erythrocyte, platelet, neutrophil, lymphocyte, monocyte, alanine transaminase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), total bilirubin, direct bilirubin, indirect bilirubin, urea, creatinine, uric acid (UA), total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), thyroid stimulating hormone (TSH), free triiodothyronine (FT3), free thyroxine (FT4), fasting C-peptide (FCP), glycated hemoglobin A1c (HbA1c), macrovascular complications, body mass index (BMI), ICW, ECW, and ECW/TBW.
The levels of 25-hydroxyvitamin D were measured in the same laboratory using accurate equipment (cobas 8000, Roche, Switzerland). The classification of vitamin D status in our research follows classic international standards. Vitamin D deficiency refers to a level of 25-hydroxyvitamin D <20 ng/mL. Vitamin D insufficiency refers to a level of 25-hydroxyvitamin D from 20 ng/mL to 30 ng/mL. An adequate level of 25-hydroxyvitamin D is >30 ng/mL.20
The body composition parameters of the water distribution in the human body, including the ICW, ECW, and ECW/TBW, were completed by a same physician from the Department of Clinical Nutrition with a same bioelectrical impedance analyzer (BIA) (Inbody 770, Biospace, Seoul, Korea). The BIA is an easy-to-use device that can provide patients with a wide range of accurate measurement results, including protein condition, body fat status, inorganic salt content, and water distribution. At the same time, the device can summarize a score for the nutritional status of the participants based on the above results. According to the conductivity difference of each body component, the BIA will collect and calculate the bioelectrical impedance, analyze the water, protein, inorganic salt, and other basic parameters in the human body, and integrate them to obtain indicators closely related to the nutritional status, such as muscle condition, body fat, and others. After removing all personal belongings that affect the BIA, participants with the electrodes connected only need to take a standing position and wait for an extremely short time to obtain the examination results.
We also collected information on T2DM complications, including DN, diabetic peripheral neuropathy (DPN), and macrovascular complications. DN was confirmed through the urinary albumin-to-creatinine ratio (UACR), which was assessed in the same standardization laboratory. A UACR >30 mg/g indicated DN.21 Regarding DPN, all participants were diagnosed by the same professional team through electromyography. Macrovascular complications included any one of the following situations. (1) Ultrasound indicated narrowing of the vascular lumen, including any one of carotid artery, vertebral artery, subclavian artery, or main arteries of the lower limbs. (2) Cerebral ischemic infarction was diagnosed through magnetic resonance imaging or computed tomography. (3) Patients suffered from previous or current coronary atherosclerotic heart disease. (4) Lower extremity peripheral arterial disease was diagnosed through the examination of the ankle brachial index (ABI).
Statistical Analysis
The statistical analyses were completed by R (version 4.3.0) and the Mstata software (www.mstata.com). The median and interquartile range (IQR) were used for data presentation of all numerical variables due to their non-normal distribution. Numbers (percentage) were used for all categorical variables. Three models were built to evaluate the correlation between body composition parameters of water distribution (ICW, ECW, ECW/TBW of the whole body, and ECW/TBW at different measured sites) and 25-hydroxyvitamin D using linear regression analysis. Model 0 was unadjusted. Model 1 was adjusted according to sex, age, duration of T2DM, the smoking history, and the drinking history. Model 2 was adjusted according to all variables in Table 1. Furthermore, we established different subgroups to identify potential major populations contributing to the association between the water distribution and 25-hydroxyvitamin D after all variables in Table 1 were adjusted. Finally, the mediation analysis was applied to evaluate the correlation between 25-hydroxyvitamin D, water distribution in the human body, and T2DM complications after adjusting all variables to infer the mechanism by which 25-hydroxyvitamin D might be associated with certain complications of T2DM from the perspective of water metabolism. The connection between the water distribution in the human body and 25-hydroxyvitamin D was presented through restricted cubic spline (RCS) analysis after all variables in Table 1 were adjusted. Two-tailed analyses were conducted, and a P value <0.05 was marked as statistically significant.
 |
Table 1 Basic Clinical Characteristics of the Study Population (n=533) |
Results
Basic Clinical Characteristics
Table 1 lists the basic clinical characteristics of all 533 study subjects. There were 198 (37.15%) females and 335 males (62.85%). The participants were mainly middle-aged and elderly, with a median age of 57.00 (49.00, 67.00) years old. All study subjects suffered from T2DM with a duration of 72.00 (12.00, 132.00) months.
The entire study population exhibited a state of 25-hydroxyvitamin D deficiency or insufficiency with a level of 18.17 (13.63, 22.85) ng/mL. With unstable conditions (such as acute infection) excluded, the median (IQR) of leucocytes was 6.33 (5.38, 7.06) ×109/L. With severe liver dysfunction excluded, the levels of ALT and AST were 17.60 (12.50, 28.00) U/L and 18.70 (15.10, 24.80) U/L, respectively. After excluding serious kidney dysfunction, the results of urea and creatinine were 5.40 (4.45, 6.51) mmol/L and 66.00 (57.00, 77.00) μmol/L, respectively. In terms of blood lipids, TC presented a level of 4.51 (3.90, 5.17) mmol/L, and TG showed a level of 1.64 (1.14, 2.41) mmol/L. The level of LDL-C was 2.65 (2.15, 3.17) mmol/L. The thyroid function of the study population was within the normal range. In addition, the development of T2DM was displayed by an FCP level of 583.30 (403.33, 787.70) pmol/L and an HbA1c level of 9.30 (7.70, 11.00) %. Regarding body composition parameters, the BMI was 23.93 (21.91, 26.25) kg/m2. The ECW/TBW of the whole body was 38.50 (37.90, 39.00) %, which was within an extremely narrow range.
Association Between Water Distribution in the Human Body and 25-Hydroxyvitamin D
The correlations between indicators reflecting water distribution and 25-hydroxyvitamin D are presented in Table 2. Linear regression analysis suggested that the ECW/TBW of the whole body (P=0.045, β=−0.008), rather than the ICW (P=0.796, β=−0.003) or ECW (P=0.397, β=−0.007), was statistically correlated with 25-hydroxyvitamin D after adjusting for all variables in Table 1. In the further analysis of different body parts, the ECW/TBW of the left arm (P=0.020, β=−0.007), trunk (P=0.039, β=−0.008), and right leg (P=0.047, β=−0.010) mainly contributed to this association.
 |
Table 2 Association Between Body Composition Parameters of Water Distribution (Dependent Variables) and 25-Hydroxyvitamin D (Ng/mL) According to the Linear Regression Analysis (n=533) |
The relationship between the ECW/TBW of the whole body and 25-hydroxyvitamin D was further presented by RCS analysis (Figure 2). The curve was U-shaped and approximately 23 ng/mL was the inflection point. Before the inflection point, there was a negative correlation between the ECW/TBW of the whole body and 25-hydroxyvitamin D, while the association changed to positive after this inflection point.
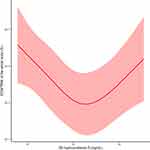 |
Figure 2 Association between the extracellular water/total body water ratio (%) of the whole body and 25-hydroxyvitamin D (ng/mL) after adjusting for all variables in Table 1 according to the restricted cubic spline analysis. Abbreviations: ECW, extracellular water; TBW, total body water. Notes: The red curve represents the trend of the water distribution in the body changing with 25-hydroxyvitamin D, and the shaded area represents the 95% confidence interval. |
Association Between ECW/TBW of the Whole Body and 25-Hydroxyvitamin D in Different Subgroups
Participants were divided into multiple subgroups based on age, sex, BMI, and macrovascular complications to further evaluate the relationship between the ECW/TBW of the whole body and 25-hydroxyvitamin D in different populations. The results in all subgroups are listed in Table 3, which confirmed a negative correlation between ECW/TBW and 25-hydroxyvitamin D only in the obese group (BMI ≥28.0 kg/m2) when all variables in Table 1 were adjusted (P=0.015, β=−0.038).
The Mediation Effect of ECW/TBW on the Association Between 25-Hydroxyvitamin D and T2DM Complications
Regarding the total effect of vitamin D on DN, the mediation effect of the water distribution accounted for 15.44%. The mediation analysis suggested that there was a statistical correlation between vitamin D and DN, whether through the total effect (P=0.020, Coefficient=−0.0111), the direct effect (P=0.040, Coefficient=−0.0094), or the indirect effect (P=0.024, Coefficient=−0.0017) of the ECW/TBW of the whole body. However, no strong evidence was found to prove that the water distribution in the human body can serve as a mediation factor in the correlation between vitamin D and other T2DM complications, including DPN and macrovascular complications (Table 4).
 |
Table 4 Mediation Analyses of the Water Distribution Between 25-Hydroxyvitamin D and Complications of Diabetes (Dependent Variables, n=418) |
Discussion
In this cross-sectional study involving 533 inpatients, for the first time, we have demonstrated a negative correlation between the ECW/TBW and 25-hydroxyvitamin D levels in the T2DM population according to linear regression analyses, proposing a preliminary theory that changing the water distribution in the human body is a potential mechanism by which low 25-hydroxyvitamin D levels may be associated with DN. Our study proved that the T2DM population is in an obvious state of vitamin D deficiency or insufficiency. Considering that the 25-hydroxyvitamin D levels of many patients with T2DM are even <10 ng/mL in severe cases, properly increasing levels of 25-hydroxyvitamin D may effectively reduce ECW/TBW, which may be beneficial for the prevention of DN. The RCS figure showed that a 25-hydroxyvitamin D level of approximately 23 ng/mL is the inflection point in the relationship between the ECW/TBW of the whole body and 25-hydroxyvitamin D.
As mentioned in the introduction, existing studies have separately discussed the effects of vitamin D5–11 and the body water distribution16–18 on T2DM complications. Based on our findings, we narrow our focus from all T2DM complications to DN. Therefore, we will briefly summarize the previous studies on the relationship between DN and vitamin D in the next paragraph. Afterwards, we will briefly summarize the previous studies on the correlation between DN and water distribution.
A considerable number of studies have shown that DN and vitamin D may be closely associated.11,22–24 The low 25-hydroxyvitamin D state in DN patients was found in a study conducted by Peng et al in Chinese T2DM participants.22 Zhou et al demonstrated that a lower 25-hydroxyvitamin D level is associated with a poor kidney status and the development of DN in the population receiving a pathological diagnosis of DN. Thus, the vitamin D levels may affect the outcome of patients with DN.23 Liyanage et al announced that 50,000 IU per month of vitamin D supplementation is beneficial for the recovery of DN. As a key evaluation indicator for the occurrence and development of DN, the situation of albuminuria has been significantly improved in their study population.11 In addition, vitamin D may benefit DN patients in a certain aspect other than the kidney condition. Barzegari et al found that the use of vitamin D in the DN population can help them control their blood lipids.24
Similarly, there have been some articles on the possible relationship between DN and the water distribution in the human body. ECW/TBW is a recently used indicator to evaluate the distribution of water in the human body. Low et al confirmed a significant correlation between high-level ECW/TBW and the development of the chronic kidney disease (CKD) in T2DM participants. They believed that matrix metalloproteinases (MMP)-2 is positively associated with the development of CKD in the T2DM population, and that ECW/TBW plays an important and mediating role in this correlation. They explained the possible mechanism through a similarity between MMP-2 and ECW/TBW. MMP-2 is an inflammatory indicator that exacerbates the interstitial fibrosis of the kidney, leading to tissue hypoxia. Similarly, an abnormal water distribution in the human body, such as high ECW/TBW, can also lead to renal damage and tissue hypoxia. Considering this similarity, they proposed a theory that inflammatory markers may affect the kidney by acting on ECW/TBW in T2DM patients,18 which is similar to our conclusion. Moh et al proved that ECW/TBW holds a significant position in the connection between a high neutrophil/lymphocyte ratio (NLR) and worse renal function in participants with T2DM. Since the NLR is an inflammatory biomarker, their results also support the fact that the water distribution in the human body is associated with both inflammation and renal health in the T2DM population.25
In summary, the above studies have separately demonstrated the relationship between vitamin D and DN, as well as the association between the water distribution in the human body and DN. However, the question of whether vitamin D is directly related to the water distribution has been overlooked. For the first time, we found a direct correlation between vitamin D levels and ECW/TBW in the T2DM population. Unfortunately, the mechanisms by which 25-hydroxyvitamin D and the water distribution interact and act on DN are not fully clear at present. We propose several possible mechanisms as follows.
Firstly, we propose a pathway between vitamin D, inflammation, water distribution, and DN, which has never been reported previously. The theoretical foundations for this new pathway mainly come from previous studies in two aspects. Previous studies have separately discussed the relationship between vitamin D, inflammation, and DN, in addition to the relationship between inflammation, water distribution, and DN, and we have combined these two relationships for the first time. On the one hand, there is a correlation between vitamin D, inflammation, and kidney health. According to a recent review published in 2023, many studies have proven that vitamin D has an obvious anti-inflammatory function.26 Moslemi et al confirmed that the correct use of vitamin D can lead to a decrease in inflammatory indicators, such as C-reactive protein, which means that vitamin D is a beneficial treatment that can improve systemic inflammation.27 Similarly, Zhou et al suggested that there is a protective effect of vitamin D against chronic inflammatory damage.28 Karonova et al found that a vitamin D therapy at a dose of 40,000 IU per week for 6 months improved the inflammatory status and microcirculation of 62 T2DM participants.29 In the case of DN, vitamin D can also utilize the vitamin D receptor to exert a similar effect, leading to a decrease in the inflammatory state of the kidney.30 Furthermore, previous evidence has proven that the use of vitamin D receptor activators can alleviate inflammation in the renal environment.31 On the other hand, there is some existing evidence to suggest an association between inflammation, the ECW/TBW, and DN. The ECW/TBW may play a mediating role in the relationship between some inflammatory markers and renal damage in the T2DM population,18,25 as mentioned earlier in our article. That is to say, inflammation represented by high levels of markers, such as MMP-2 and the NLR, may cause an increase in the ECW/TBW of the whole body. Then, higher ECW/TBW may lead to interstitial damage in the kidney and tissue hypoxia, resulting in DN. Therefore, when combining the above two parts into a new pathway, we propose a valuable theory that a low level of vitamin D is associated with a high level of inflammation, which may increase the ECW/TBW, ultimately leading to DN (Figure 3).
 |
Figure 3 The correlation between vitamin D, water distribution, and diabetic nephropathy. Abbreviations: ECW, extracellular water; TBW, total body water. |
Secondly, we believe that the ECW/TBW may reflect the anti-aging effect of vitamin D. In addition to anti-inflammatory effects, vitamin D has anti-aging effects, and the two promote each other.32 Similarly, the water distribution in the human body may also reflect the aging state of cells. A previous study reported that as aging occurs, the ECW/ICW gradually rises.33 In other words, aging leads to an imbalance between ECW and ICW, which may lead to a high level of ECW/TBW. Therefore, we propose the following theory. The aging of cells is accompanied by a decrease in the ICW,34 which may cause a relative increase in the ECW/TBW. Therefore, the ECW/TBW can be associated with vitamin D through aging (Figure 3). However, it must be pointed out that despite the above evidence, more studies are still needed to confirm the relationship between vitamin D, ECW/TBW, and aging.
In addition, we also need to explain the other three aspects of the results, including (1) why the relationship between vitamin D and the water distribution is more significant in the obese population, (2) why the water distribution is not involved in the association between vitamin D and other complications, including DPN and macrovascular complications, and (3) why the RCS result is not a straight line. We will discuss these aspects in the following three paragraphs.
A negative correlation between 25-hydroxyvitamin D and ECW/TBW was only found in the obese subgroup when all variables in Table 1 were adjusted. We hope to interpret this result from two aspects (Figure 3). Firstly, current studies have suggested that obese individuals may suffer from relatively lower levels of vitamin D.35 It is reasonable that lower levels of vitamin D can cause more obvious harms. In other words, the harmful effects caused by the lower vitamin D levels, including worse water distribution states, may become more significant as the levels of vitamin D decrease in obese individuals. Therefore, the association between vitamin D and the ECW/TBW may be more apparent in obese populations. Secondly, obesity is closely related to inflammation. A recent study found that lipids can lead to kidney disorder through an activated inflammatory state.36 Another study by Pengrattanachot et al also supported the relationship between obesity and inflammation based on the finding that atorvastatin can alleviate obesity-related renal damage by controlling the inflammatory response.37 Therefore, our explanation is that obesity can lead to an increase in inflammation. Meanwhile, the high-level inflammation may lead to a more significant correlation between vitamin D and the water distribution, which can be inferred from our aforementioned pathway between vitamin D, inflammation, water distribution, and DN. Therefore, in obese T2DM patients, the association between 25-hydroxyvitamin D and ECW/TBW may be closer due to the high-level inflammation. Furthermore, it is worth noting that more research is needed to explain why only the ECW/TBW ratios of the left arm, trunk, and right leg were shown to be related to 25-hydroxyvitamin D in our study.
The water distribution is not involved in the association between vitamin D and other complications, including DPN and macrovascular complications. We hope to explain this phenomenon from two perspectives: our results themselves and the theoretical mechanisms. Firstly, from the perspective of our results themselves, the necessary prerequisite for the water distribution to participate in the relationship between vitamin D and any other complications is the existence of a correlation between vitamin D and DPN (or macrovascular complications). However, as shown in Table 4, whether from the perspective of the total or direct effect, vitamin D was not related to DPN or macrovascular complications in our population. In fact, the relationships between vitamin D and certain complications of T2DM are still controversial. As mentioned in the introduction, although vitamin D3 and DR were found to be related by Ahmed et al, they also showed that vitamin D and neuropathy are not related in their T2DM population. Since their indicator for evaluating neuropathy was the vibration perception threshold, which is a commonly used indicator for diagnosing DPN,38 they actually demonstrated that vitamin D and DPN may not be correlated in patients with T2DM.10 Wang et al used the ABI to diagnose peripheral arterial disease, which is the same as our fourth diagnostic criterion for macrovascular complications, and no association was found between a low vitamin D status and peripheral arterial disease in the T2DM population.39 Secondly, from the perspective of the theoretical mechanisms, we would like to further explain why the water distribution is involved in the relationship between vitamin D and DN. Since the kidney is the main organ that regulates the distribution of water in the human body, changes in the water distribution in the kidneys may occur earlier and more significantly than in other parts of the human body. In this case, a high ECW/TBW may have more opportunity to affect the kidneys compared to other body parts, which may be the reason why the pathway between low vitamin D, water distribution, and complications only exists in DN. However, more research is required to prove this theory.
It is necessary to explain why the RCS result is not a straight line. Firstly, we noticed that the population after the inflection point was in a relatively better state of vitamin D and all participants with adequate 25-hydroxyvitamin D (>30 ng/mL) were included in this case. It is reasonable to believe that when the level of 25-hydroxyvitamin D reaches a relatively high value, the ECW/TBW changed by vitamin D may no longer decrease because everyone’s need for vitamin D to improve water distribution cannot be infinite. Therefore, the RCS result cannot be a straight line. Secondly, we have demonstrated an obvious state of vitamin D deficiency or insufficiency in the T2DM population, which is similar to the conclusions of previous studies.5,6 In other words, the number of participants with high levels of vitamin D in our study is relatively small. Thus, results presented after the inflection point will be affected by the small sample size of patients in a relatively sufficient state of vitamin D. The latter half of our RCS curve can only serve as a preliminary reference, and more studies are needed in the future to discuss the relationship between high levels of 25-hydroxyvitamin D and the water distribution.
Our study has some strong innovations. Firstly, to our knowledge, this is the first study to confirm the correlation between the ECW/TBW and 25-hydroxyvitamin D in the T2DM population, and to present this relationship with RCS analysis. Secondly, for the first time, we propose that changing the water distribution in the human body may be one of the mechanisms by which vitamin D levels may affect DN. We propose a groundbreaking theory that there is a pathway between vitamin D, inflammation, the water distribution, and DN. Thirdly, we believe that the ECW/TBW may reflect the anti-aging effect of vitamin D, which is also a novel perspective.
However, there are also some shortcomings in this study. Firstly, our research is cross-sectional, and studies conducted in other research designs are required. Secondly, although our study included 533 patients with T2DM, more data is required, especially for T2DM patients with high levels of vitamin D. Finally, although the 35 clinical variables listed in Table 1 have been included in our study for model adjustments, some factors that may affect vitamin D levels (such as sun exposure40 and dietary habits41) have not been considered due to a lack of data.
Conclusion
This study is the first to demonstrate a statistical correlation between vitamin D and the water distribution in the human body among participants with T2DM. Meanwhile, we found the mediation role of the ECW/TBW in the association between vitamin D and DN, which accounted for 15.44% of the total effect.
Data Sharing Statement
Raw data can be obtained upon request from the corresponding author.
Ethics Statement
Our work has been approved by the Ethics Committee of the Third Affiliated Hospital of Soochow University (2023#024). By having each participant sign an informed consent form, the rights of the study population were guaranteed. Our study complies with the Declaration of Helsinki.
Acknowledgments
We were very grateful that our study was funded by the Jiangsu Commission of Health (ZD2022017), a government department from China. This work was also supported by the Applied and Basic Research Program of Changzhou Scientific and Technological Project (CJ20190103, CJ20235089) and the Major Scientific and Technological Project of Changzhou Health Commission of China (ZD202309).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Gallagher JC, Rosen CJ. Vitamin D: 100 years of discoveries, yet controversy continues. Lancet Diabetes Endocrinol. 2023;11(5):362–374. doi:10.1016/S2213-8587(23)00060-8
2. Gharibeh N, Razaghi M, Vanstone CA, et al. Effect of Vitamin D supplementation on bone mass in infants with 25-Hydroxyvitamin D concentrations less than 50 nmol/L: a prespecified secondary analysis of a randomized clinical trial. JAMA Pediatr. 2023;177(4):353–362. doi:10.1001/jamapediatrics.2022.5837
3. Rebelos E, Tentolouris N, Jude E. The role of vitamin D in health and disease: a narrative review on the mechanisms linking vitamin d with disease and the effects of supplementation. Drugs. 2023;83(8):665–685. doi:10.1007/s40265-023-01875-8
4. Herrmann M. Assessing vitamin D metabolism - four decades of experience. Clin Chem Lab Med. 2023;61(5):880–894. doi:10.1515/cclm-2022-1267
5. Compston J. Type 2 diabetes mellitus and bone. J Intern Med. 2018;283(2):140–153. doi:10.1111/joim.12725
6. Petroni ML, Brodosi L, Marchignoli F, et al. Nutrition in patients with type 2 diabetes: present knowledge and remaining challenges. Nutrients. 2021;13(8):2748. doi:10.3390/nu13082748
7. Xiao J, Lv J, Wang S, et al. Association of serum 25-hydroxyvitamin D with metabolic syndrome and type 2 diabetes: a one sample Mendelian randomization study. BMC Geriatr. 2021;21(1):391. doi:10.1186/s12877-021-02307-6
8. He X, Luo Y, Hao J, et al. Association between serum vitamin D levels and ketosis episodes in hospitalized patients with newly diagnosed ketosis-prone type 2 diabetes. Diabetes Metab Syndr Obes. 2022;15:3821–3829. doi:10.2147/DMSO.S389609
9. Tang W, Chen L, Ma W, et al. Association between vitamin D status and diabetic foot in patients with type 2 diabetes mellitus. J Diabetes Investig. 2022;13(7):1213–1221. doi:10.1111/jdi.13776
10. Ahmed LHM, Butler AE, Dargham SR, et al. Association of vitamin D(2) and D(3) with type 2 diabetes complications. BMC Endocr Disord. 2020;20(1):65. doi:10.1186/s12902-020-00549-w
11. Liyanage P, Lekamwasam S, Weerarathna TP, Liyanage C. Effect of Vitamin D therapy on urinary albumin excretion, renal functions, and plasma renin among patients with diabetic nephropathy: a randomized, double-blind clinical trial. J Postgrad Med. 2018;64(1):10–15. doi:10.4103/jpgm.JPGM_598_16
12. Holick MF. The vitamin D deficiency pandemic: approaches for diagnosis, treatment and prevention. Rev Endocr Metab Disord. 2017;18(2):153–165. doi:10.1007/s11154-017-9424-1
13. Feehan O, Magee PJ, Pourshahidi LK, Armstrong DJ, McSorley EM. Vitamin D deficiency in nursing home residents: a systematic review. Nutr Rev. 2023;81(7):804–822. doi:10.1093/nutrit/nuac091
14. Li Y, Konstantopoulos K, Zhao R, Mori Y, Sun SX. The importance of water and hydraulic pressure in cell dynamics. J Cell Sci. 2020;133(20). doi:10.1242/jcs.240341
15. Guo M, Pegoraro AF, Mao A, et al. Cell volume change through water efflux impacts cell stiffness and stem cell fate. Proc Natl Acad Sci U S A. 2017;114(41):E8618–E8627. doi:10.1073/pnas.1705179114
16. López-Valverde ME, Aragón-Sánchez J, Víquez-Molina G, Rodríguez Ortega P. Extracellular to intracellular water ratio determined by bioimpedance is associated with mortality in patients admitted for diabetic foot ulcers. Int J Low Extr Wound. 2023. doi:10.1177/15347346231173861
17. Nakajima H, Hashimoto Y, Kaji A, et al. Impact of extracellular-to-intracellular fluid volume ratio on albuminuria in patients with type 2 diabetes: a cross-sectional and longitudinal cohort study. J Diabetes Investig. 2021;12(7):1202–1211. doi:10.1111/jdi.13459
18. Low S, Pek S, Liu YL, et al. Higher extracellular water to total body water ratio was associated with chronic kidney disease progression in type 2 diabetes. J Diabetes Complications. 2021;35(7):107930. doi:10.1016/j.jdiacomp.2021.107930
19. American Diabetes A. Classification and diagnosis of diabetes: standards of medical care in diabetes-2020. Diabetes Care. 2020;43(Suppl 1):S14–S31. doi:10.2337/dc20-S002
20. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–1930. doi:10.1210/jc.2011-0385
21. Zhang J, Hu W, Lin P, Wang R. Decreased serum myonectin concentrations in diabetic nephropathy patients. Clin Exp Med. 2020;20(4):601–607. doi:10.1007/s10238-020-00654-z
22. Peng Y, Li LJ. Serum 25-hydroxyvitamin D level and diabetic nephropathy in patients with type 2 diabetes mellitus. Int Urol Nephrol. 2015;47(6):983–989. doi:10.1007/s11255-015-0983-3
23. Zhou T, Shen L, Li Z, et al. Severe 25-Hydroxyvitamin D deficiency may predict poor renal outcomes in patients with biopsy-proven diabetic nephropathy. Front Endocrinol. 2022;13:871571. doi:10.3389/fendo.2022.871571
24. Barzegari M, Sarbakhsh P, Mobasseri M, et al. The effects of vitamin D supplementation on lipid profiles and oxidative indices among diabetic nephropathy patients with marginal vitamin D status. Diabetes Metab Syndr. 2019;13(1):542–547. doi:10.1016/j.dsx.2018.11.008
25. Moh MC, Low S, Shao Y-M, Subramaniam T, Sum CF, Lim SC. Association between neutrophil/lymphocyte ratio and kidney impairment in type 2 diabetes mellitus: a role of extracellular water/total body water ratio. Diabet Res Clin Pract. 2023;199:110634. doi:10.1016/j.diabres.2023.110634
26. Huang HY, Lin TW, Hong ZX, Lim LM. Vitamin D and diabetic kidney disease. Int J Mol Sci. 2023;24(4). doi:10.3390/ijms24043751
27. Moslemi E, Musazadeh V, Kavyani Z, Naghsh N, Shoura SMS, Dehghan P. Efficacy of vitamin D supplementation as an adjunct therapy for improving inflammatory and oxidative stress biomarkers: an umbrella meta-analysis. Pharmacol Res. 2022;186:106484. doi:10.1016/j.phrs.2022.106484
28. Zhou A, Hypponen E. Vitamin D deficiency and C-reactive protein: a bidirectional Mendelian randomization study. Int J Epidemiol. 2023;52(1):260–271. doi:10.1093/ije/dyac087
29. Karonova T, Stepanova A, Bystrova A, Jude EB. High-dose vitamin D supplementation improves microcirculation and reduces inflammation in diabetic neuropathy patients. Nutrients. 2020;12(9):2518. doi:10.3390/nu12092518
30. Sanchez-Nino MD, Bozic M, Cordoba-Lanus E, et al. Beyond proteinuria: VDR activation reduces renal inflammation in experimental diabetic nephropathy. Am J Physiol Renal Physiol. 2012;302(6):F647–657. doi:10.1152/ajprenal.00090.2011
31. Panizo S, Martinez-Arias L, Alonso-Montes C, et al. Fibrosis in chronic kidney disease: pathogenesis and consequences. Int J Mol Sci. 2021;22(1):408. doi:10.3390/ijms22010408
32. Fantini C, Corinaldesi C, Lenzi A, Migliaccio S, Crescioli C. Vitamin D as a Shield against Aging. Int J Mol Sci. 2023;24(5):4546. doi:10.3390/ijms24054546
33. Kerch G. Distribution of tightly and loosely bound water in biological macromolecules and age-related diseases. Int J Biol Macromol. 2018;118(Pt A):1310–1318. doi:10.1016/j.ijbiomac.2018.06.187
34. Minton AP. Water loss in aging erythrocytes provides a clue to a general mechanism of cellular senescence. Biophys J. 2020;119(10):2039–2044. doi:10.1016/j.bpj.2020.10.004
35. Bennour I, Haroun N, Sicard F, Mounien L, Landrier JF. Vitamin D and obesity/adiposity-A brief overview of recent studies. Nutrients. 2022;14(10):2049. doi:10.3390/nu14102049
36. Opazo-Rios L, Mas S, Marin-Royo G, et al. Lipotoxicity and diabetic nephropathy: novel mechanistic insights and therapeutic opportunities. Int J Mol Sci. 2020;21(7):2632. doi:10.3390/ijms21072632
37. Pengrattanachot N, Cherngwelling R, Jaikumkao K, et al. Atorvastatin attenuates obese-induced kidney injury and impaired renal organic anion transporter 3 function through inhibition of oxidative stress and inflammation. Biochim Biophys Acta Mol Basis Dis. 2020;1866(6):165741. doi:10.1016/j.bbadis.2020.165741
38. Yan P, Tang Q, Wu Y, et al. Serum albumin was negatively associated with diabetic peripheral neuropathy in Chinese population: a cross-sectional study. Diabetol Metab Syndr. 2021;13(1):100. doi:10.1186/s13098-021-00718-4
39. Wang Y, Feng T, Zhou H, Lu K, Bai Y, Zhang P. Vitamin D deficiency may not be an independent risk factor for peripheral arterial disease in middle-aged and elderly patients with type 2 diabetes in China. Dis Markers. 2020;2020:8854717. doi:10.1155/2020/8854717
40. Alfredsson L, Armstrong BK, Butterfield DA, et al. Insufficient sun exposure has become a real public health problem. Int J Environ Res Public Health. 2020;17(14):5014. doi:10.3390/ijerph17145014
41. Detopoulou P, Papadopoulou SK, Voulgaridou G, et al. Ketogenic diet and vitamin D metabolism: a review of evidence. Metabolites. 2022;12(12):1288. doi:10.3390/metabo12121288
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


